Self-Compatibility Not Associated with Morphological or Genetic Diversity Reduction in Oil-Rewarding Calceolaria Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Species and Breeding System Characterization
2.2. Floral Traits Measurements
2.3. DNA Extraction and Genotyping
3. Results
3.1. Breeding Systems
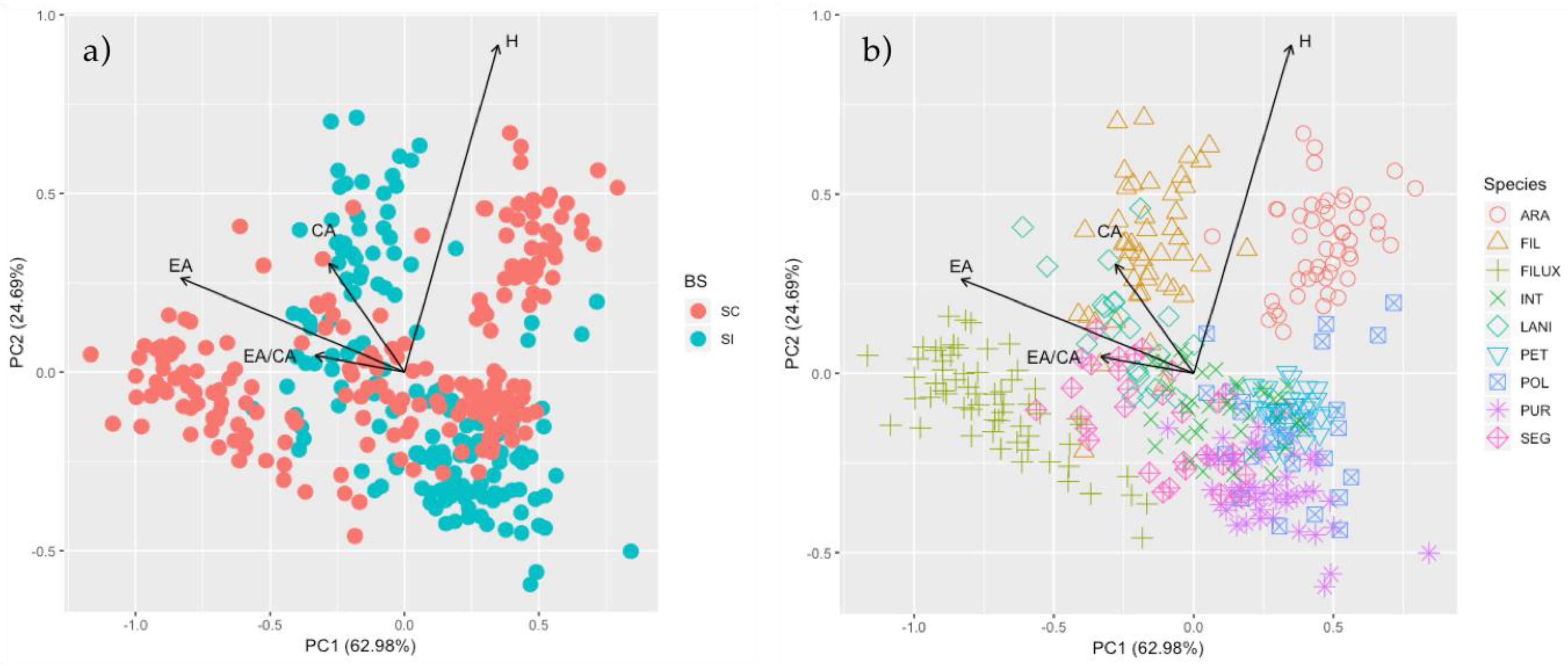
3.2. ISI and Morphological Traits
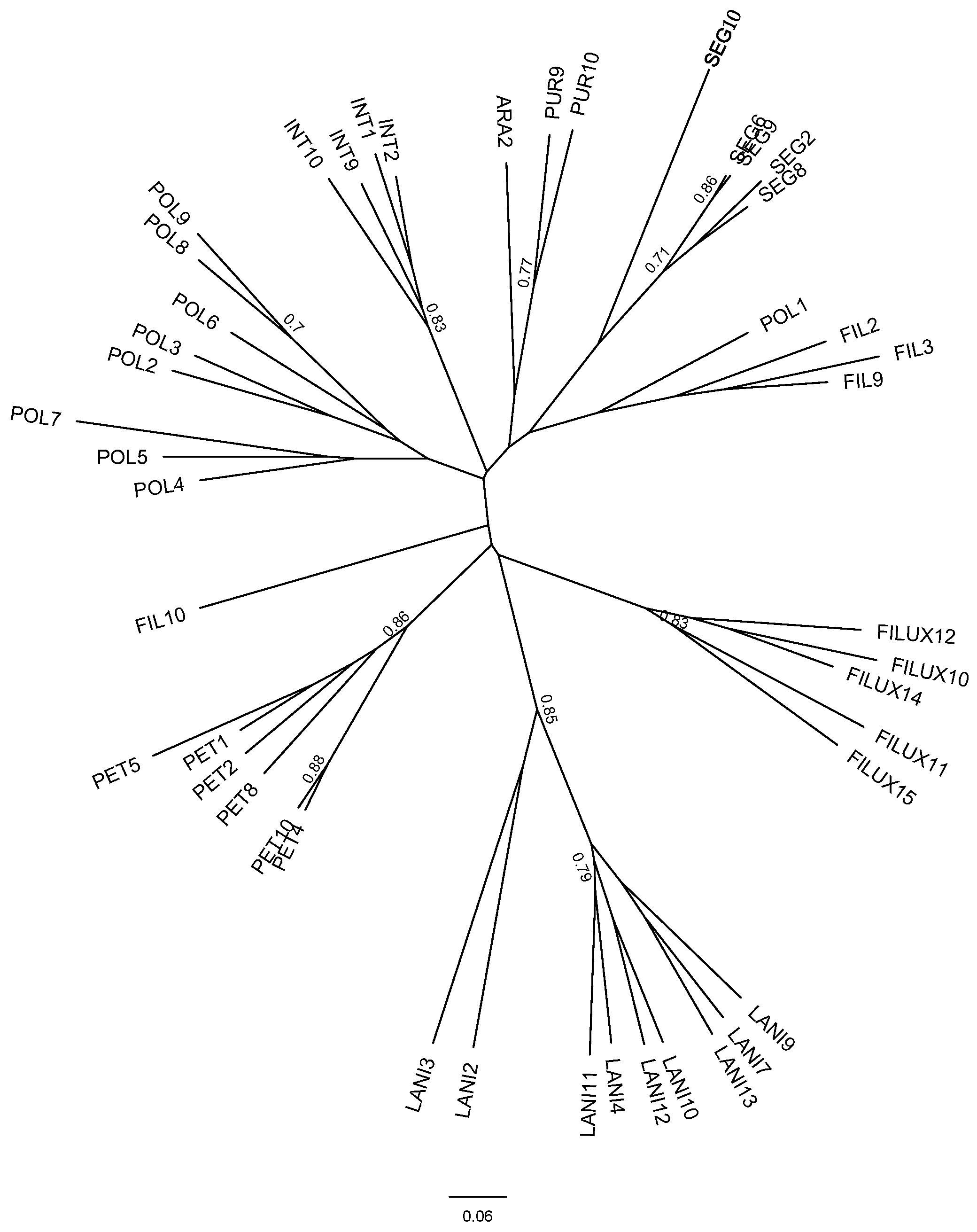
3.3. Genetic Diversity
4. Discussion
4.1. Self-Compatibility and Floral Characters
4.2. Is Selfing Associated with Lower Genetic Diversity in the Genus Calceolaria?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stebbins, G.L. Flowering Plants: Evolution above the Species Level; Harvard University Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Takebayashi, N.; Morrell, P.L. Is self-fertilization an evolutionary dead end? Revisiting an old hypothesis with genetic theories and a macroevolutionary approach. Am. J. Bot. 2001, 88, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.I.; Kalisz, S.; Slotte, T. Evolutionary consequences of self-fertilization in plants. Proc. R. Soc. B Sci. 2013, 280, 20130133. [Google Scholar] [CrossRef] [PubMed]
- Cruden, R.W. Pollen-ovule ratios: A conservative indicator of breeding systems in plants. Evolution 1977, 31, 32–46. [Google Scholar] [CrossRef]
- Bodbyl, S.A.; Kelly, J.K. Rapid evolution caused by pollinator loss in Mimulus guttatus. Evolution 2011, 65, 2541–2552. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J. Effects of floral morphology and display on outcrossing in Blue Columbine, Aquilegia caerulea (Ranunculaceae). Func. Ecol. 2002, 12, 596–606. [Google Scholar] [CrossRef]
- Sicard, A.; Lenhard, M. The selfing syndrome: A model for studying the genetic and evolutionary basis of morphological adaptation in plants. Ann. Bot. 2011, 107, 1433–1443. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S.; Vogler, D.W. Benefits of autonomous selfing under unpredictable pollinator environments. Ecology 2003, 84, 2928–2942. [Google Scholar] [CrossRef]
- Brys, R.; Geens, B.; Beeckman, T.; Jacquemyn, H. Differences in dichogamy and hercogamy index contribute to higher selfing in contrasting environments in the annual Blackstonia perfoliata (Gentianaceae). Ann. Bot. 2013, 111, 651–661. [Google Scholar] [CrossRef]
- Lande, R.; Schemske, D.W. The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution 1985, 39, 24–40. [Google Scholar] [CrossRef]
- Goodwillie, C.; Sargent, R.D.; Eckert, C.G.; Elle, E.; Geber, M.A.; Johnston, M.O.; Kalisz, S.; Moeller, D.A.; Ree, R.H.; Vallejo-Marin, M.; et al. Correlated evolution of mating system and floral display traits in flowering plants and its implications for the distribution of mating system variation. New Phytol. 2010, 185, 311–321. [Google Scholar] [CrossRef]
- Pérez, F.; Arroyo, M.T.K.; Armesto, J.J. Evolution of autonomous selfing accompanies increased specialization in the pollination system of Schizanthus (Solanaceae). Am. J. Bot. 2009, 96, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Barrett, S.; Gao, J.-Y.; Chen, J.; Cole, W.W.; Yong, L.; Bai, Z.-L.; Li, Q.-J. Predicting mating patterns from pollination syndromes: The case of “sapromyiophily” in Tacca chantrieri (Taccaceae). Am. J. Bot. 2015, 92, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Fenster, C.B.; Marten-Rodriguez, S. Reproductive assurance and the evolution of pollination specialization, Int. J. Plant Sci. 2017, 168, 215–228. [Google Scholar] [CrossRef]
- Lloyd, D.G.; Schoen, D.J. Self- and cross-fertilization in plants. I. Functional dimensions. Int. J. Plant Sci. 1992, 153, 358–369. [Google Scholar] [CrossRef]
- Molau, U. Scrophulariaceae-Part, I. Calceolarieae. Flora Netrop. 1988, 47, 1–326. [Google Scholar]
- Ehrhart, C. Die Gattung Calceolaria (Scrophulariaceae) in Chile. Biblioth. Bot. 2000, 153, 1–283. [Google Scholar]
- Sérsic, A. Pollination biology in the genus Calceolaria L. (Calceolariaceae). Stapfia 2004, 82, 1–121. [Google Scholar]
- Cosacov, A.; Sérsic, A.N.; Sosa, V.; De-Nova, J.A.; Nylinder, S.; Cocucci, A. New insights into the phylogenetic relationships, character evolution, and phytogeographic patterns of Calceolaria (Calceolariaceae). Am. J. Bot. 2009, 96, 2240–2255. [Google Scholar] [CrossRef]
- Cisterna, J.; Murúa, M. Contrasting floral morphology and breeding systems in two subspecies of Calceolaria corymbosa in Central Chile. Gayana Bot. 2018, 75, 544–548. [Google Scholar] [CrossRef]
- Ruiz, T.; Arroyo, M.T.K. Plant reproductive ecology of a secondary deciduous forest in Venezuela. Biotropica 1978, 10, 221–230. [Google Scholar]
- Bawa, K.S. Patterns of flowering in tropical plants. In Handbook of Experimental Pollination Biology; Jones, C.E., Little, R.J., Eds.; Van Nostrand Reinhold: New York, NY, USA, 1983; pp. 394–410. [Google Scholar]
- Murúa, M.; Espíndola, A. Pollination syndromes in a specialized plant-pollinator interaction: Does floral morphology predict pollinators in Calceolaria? Plant Biol. 2015, 17, 551–557. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; RStudio Team: Boston, MA, USA, 2020; Available online: http://www.rstudio.com/ (accessed on 28 September 2020).
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Murúa, M.; Peralta, G.; Carrasco, L.; Pérez, F. Isolation and cross-amplification of fifteen microsatellites for oil-rewarding Calceolaria species (Calceolariaceae). Genet. Mol. Res. 2018, 17, 16039863. [Google Scholar] [CrossRef]
- van Oosterhout, C.; Hutchinson, W.F.; Shipley, P. Micro-checker: Software for identifying and correcting genotyping errors in microsatellite data. Mol. Ecol. Notes 2004, 4, 535–538. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. 2006. Available online: http://darwin.cirad.fr/darwin (accessed on 16 September 2020).
- Harder, L.D.; Johnson, S.D. Darwin’s beautiful contrivances: Evolutionary and functional evidence for floral adaptation. New Phytol. 2009, 183, 530–545. [Google Scholar] [CrossRef]
- Cosacov, A.; Cocucci, A.A.; Sersic, A.N. Geographic differentiation in floral traits across the distribution range of the Patagonian oil-secreting Calceolaria polyrhiza: Do pollinators mater? Ann. Bot. 2014, 113, 251–266. [Google Scholar] [CrossRef]
- Murúa, M.; Cisterna, J.; Rosende, B. Pollination ecology and breeding system of two Calceolaria species in Chile. Rev. Chil. Hist. Nat. 2014, 87, 7. [Google Scholar] [CrossRef]
- Armbruster, W.S.; Mulder, C.P.; Baldwin, B.G.; Kalisz, S.; Wessa, B.; Nute, H. Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae sl). Am. J. Bot. 2002, 89, 37–49. [Google Scholar] [CrossRef]
- Kalisz, S.; Randle, A.; Chaiffetz, D.; Faigeles, M.; Butera, M.; Beight, C. Dichogamy correlates with outcrossing rate and defines the selfing syndrome in the mixed-mating genus Collinsia. Ann. Bot. 2012, 109, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Herlihy, C.R.; Eckert, C.G. Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 2002, 416, 6878. [Google Scholar] [CrossRef] [PubMed]
- Husband, B.C.; Schemske, D.W. Evolution of the magnitude and timing of inbreeding depression in plants. Evolution 1996, 50, 54–70. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.J.; Klinkhammer, P.G.L. Evolutionary Ecology of Plant Reproductive Strategies; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
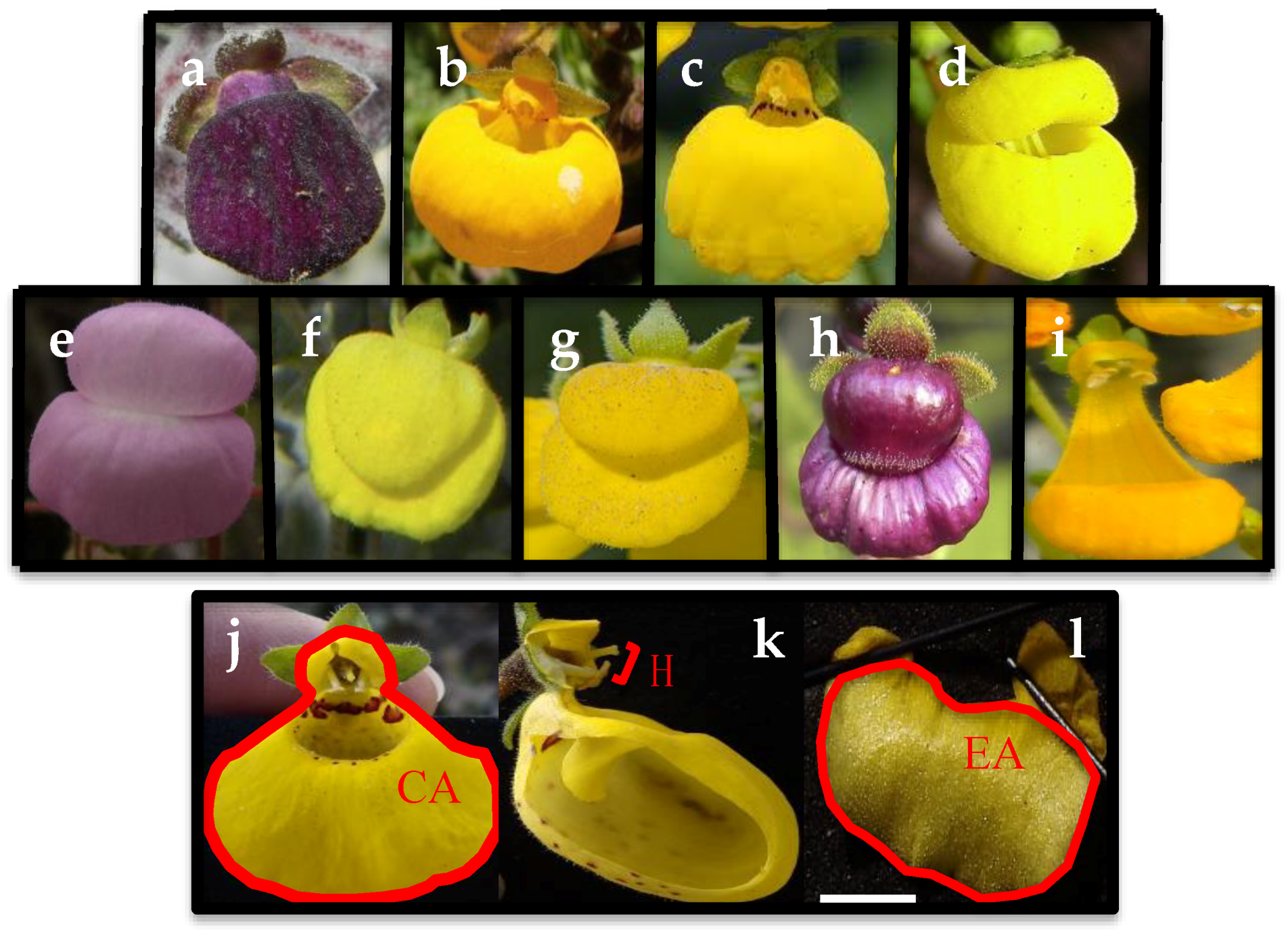
| Morphological Traits | Reproductive Traits (n° Seeds Per Fruit) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BS | Species | Coordinates | PS* | N | NF | CA (mm2) | GA (mm2) | EA/CA (mm2) | H (mm) | X | AS | ISI |
| SI | C. filicaulis spp. filicaulis | 36°36′ S/72°00′ W | <100 | 50 | 150 | 100.38 ± 3.52 | 24.63 ± 1.00 | 0.25 ± 0.01 | 5.0 ± 0.37 | 305 ± 29 | 0 | 0.14 |
| C. purpurea | 33°23′ S/70°27′ W | 0–50 | 47 | 141 | 60.61 ±3.32 | 6.54 ± 0.34 | 0.12 ± 0.01 | 0.85 ± 0.05 | 90 ± 28 | 0 | 0.01 | |
| C. polifolia | 33°00′ S/70°56′ W | 50–100 | 40 | 120 | 57.35 ± 3.99 | 5.66 ± 0.38 | 0.12 ± 0.01 | 2.04 ± 0.21 | 259 ± 7 | 0 | 0.05 | |
| C. seguetii | 33°21′ S/70°19′ W | 50–100 | 30 | 90 | 117.98 ± 8.07 | 17.28 ± 1.55 | 0.17 ± 0.02 | 0.91 ± 0.08 | 142 ± 40 | 5 ± 1 | 0.13 | |
| Mean ± SE | 84.08 ± 14.94 | 13.53 ± 4.55 | 0.17 ± 0.01 | 2.22 ±0.97 | 199 ± 26 | 1.3 ± 0.25 | 0.08 ± 0.03 | |||||
| SC | C. arachnoidea | 36°36′ S/72°00′ W | <100 | 50 | 150 | 94.70 ± 3.38 | 6.30 ± 0.24 | 0.07 ± 0.02 | 8.17 ± 0.47 | 347 ± 57 | 0 | 0.22 |
| C. lanigera | 34°14′ S/70°27′ W | 0–50 | 40 | 120 | 220.09 ± 18.02 | 22.08 ± 1.16 | 0.12 ± 0.01 | 1.42 ± 0.13 | 251 ± 62 | 3 ± 1 | 1 | |
| C. petiolaris | 33°23′ S/70°31′ W | 0–50 | 31 | 93 | 53.97 ± 1.68 | 6.42 ± 0.16 | 0.12 ± 0.004 | 2.21 ± 0.06 | 230 ± 35 | 4 ± 1 | 0.91 | |
| C. integrifolia | 33°22′ S/70°24′ W | 0–50 | 37 | 111 | 142.62 ± 8.73 | 8.86 ± 0.56 | 0.07 ± 0.001 | 1.24 ± 0.07 | 231 ± 73 | 3 ± 2 | 0.23 | |
| C. filicaulis spp. luxurians | 33°19′ S/70°16′ W | <100 | 55 | 165 | 138.56 ± 7.62 | 58.83 ± 2.94 | 0.47 ± 0.03 | 0.39 ± 0.03 | 224 ± 121 | 0 | 0.61 | |
| Mean ± SE | 129.99 ± 27.73 | 20.50 ± 10.02 | 0.17 ± 0.02 | 2.69 ± 1.40 | 257 ± 70 | 2 ± 0.8 | 0.53 ± 0.16 | |||||
| BS | Species | Na | Ho | He | FIS |
|---|---|---|---|---|---|
| SI | C. filicaulis spp. filicaulis | 3.00 ± 0.5 | 0.18 ± 0.08 | 0.35 ± 0.08 | 0.63 ± 0.20 |
| C. purpurea | 3.44 ± 0.5 | 0.19 ± 0.09 | 0.51 ± 0.07 | 0.53 ± 0.20 | |
| C. polifolia | 3.78 ± 0.3 | 0.31 ± 0.08 | 0.55 ± 0.06 | 0.41 ± 0.14 | |
| C. segethii | 2.56 ± 0.4 | 0.08 ± 0.06 | 0.30 ± 0.08 | 0.71 ± 0.14 | |
| Mean ± SE | 3.20 ± 0.27 | 0.19 ± 0.04 | 0.43 ± 0.06 | 0.57 ± 0.06 | |
| SC | C. arachnoidea | 3.78 ± 0.8 | 0.34 ± 0.13 | 0.46 ± 0.09 | 0.38 ± 0.24 |
| C. lanigera | 5.00 ± 0.6 | 0.34 ± 0.09 | 0.57 ± 0.03 | 0.35 ± 0.18 | |
| C. petiolaris | 2.56 ± 0.4 | 0.13 ± 0.06 | 0.35 ± 0.08 | 0.56 ± 0.17 | |
| C. integrifolia | 2.89 ± 0.4 | 0.20 ± 0.05 | 0.36 ± 0.08 | 0.35 ± 0.12 | |
| C. filicaulis spp. luxurians | 6.67 ± 0.9 | 0.32 ± 0.08 | 0.65 ± 0.06 | 0.51 ± 0.09 | |
| Mean ± SE | 4.18 ± 0.75 | 0.27 ± 0.04 | 0.48 ± 0.06 | 0.43 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murúa, M.; Espíndola, A.; Pérez, F. Self-Compatibility Not Associated with Morphological or Genetic Diversity Reduction in Oil-Rewarding Calceolaria Species. Plants 2020, 9, 1377. https://doi.org/10.3390/plants9101377
Murúa M, Espíndola A, Pérez F. Self-Compatibility Not Associated with Morphological or Genetic Diversity Reduction in Oil-Rewarding Calceolaria Species. Plants. 2020; 9(10):1377. https://doi.org/10.3390/plants9101377
Chicago/Turabian StyleMurúa, Maureen, Anahí Espíndola, and Fernanda Pérez. 2020. "Self-Compatibility Not Associated with Morphological or Genetic Diversity Reduction in Oil-Rewarding Calceolaria Species" Plants 9, no. 10: 1377. https://doi.org/10.3390/plants9101377
APA StyleMurúa, M., Espíndola, A., & Pérez, F. (2020). Self-Compatibility Not Associated with Morphological or Genetic Diversity Reduction in Oil-Rewarding Calceolaria Species. Plants, 9(10), 1377. https://doi.org/10.3390/plants9101377





