Leaf Gas Exchange Performance of Ten Quinoa Genotypes under a Simulated Heat Wave
Abstract
:1. Introduction
2. Results
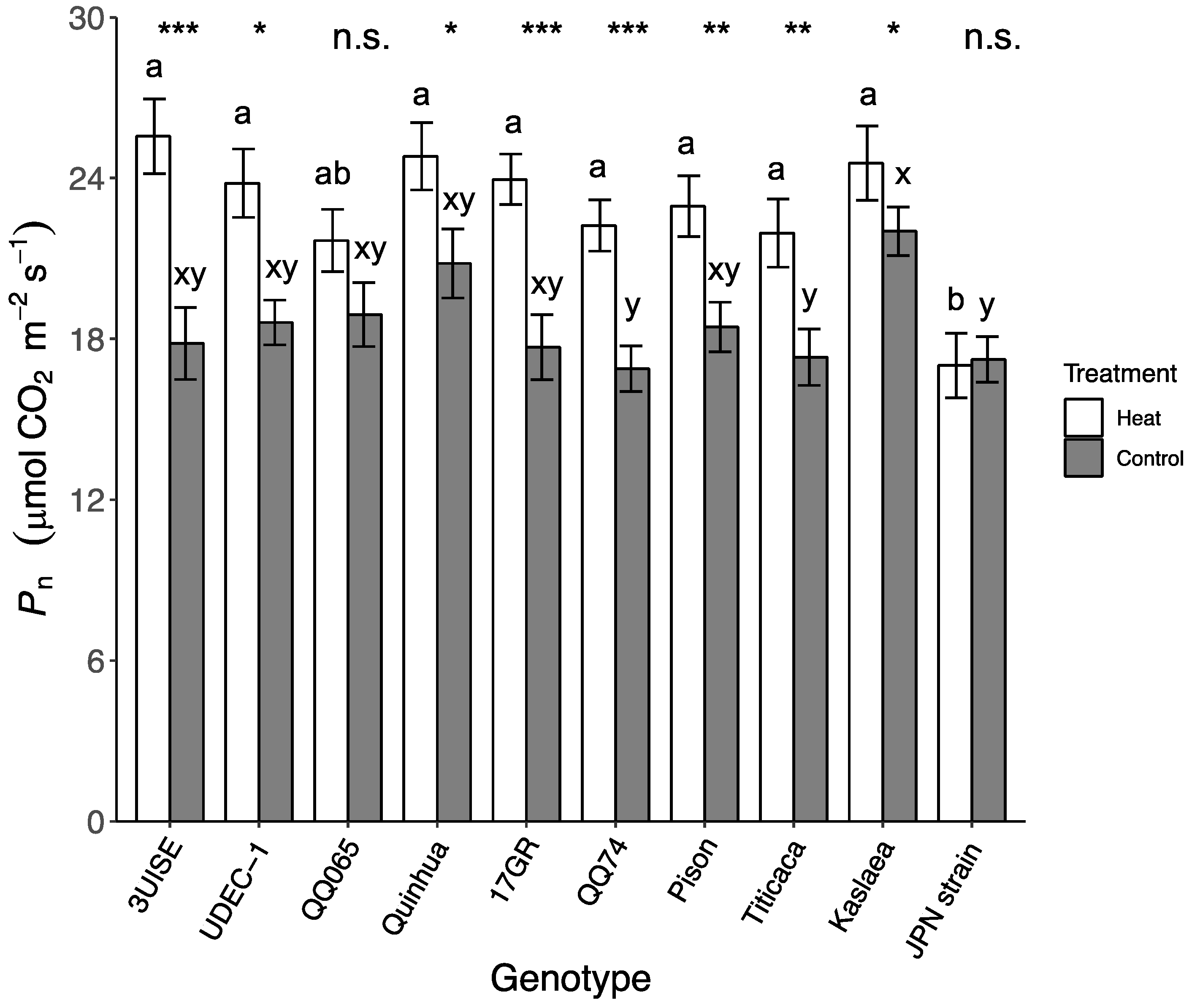
2.1. Photosynthetic Rate
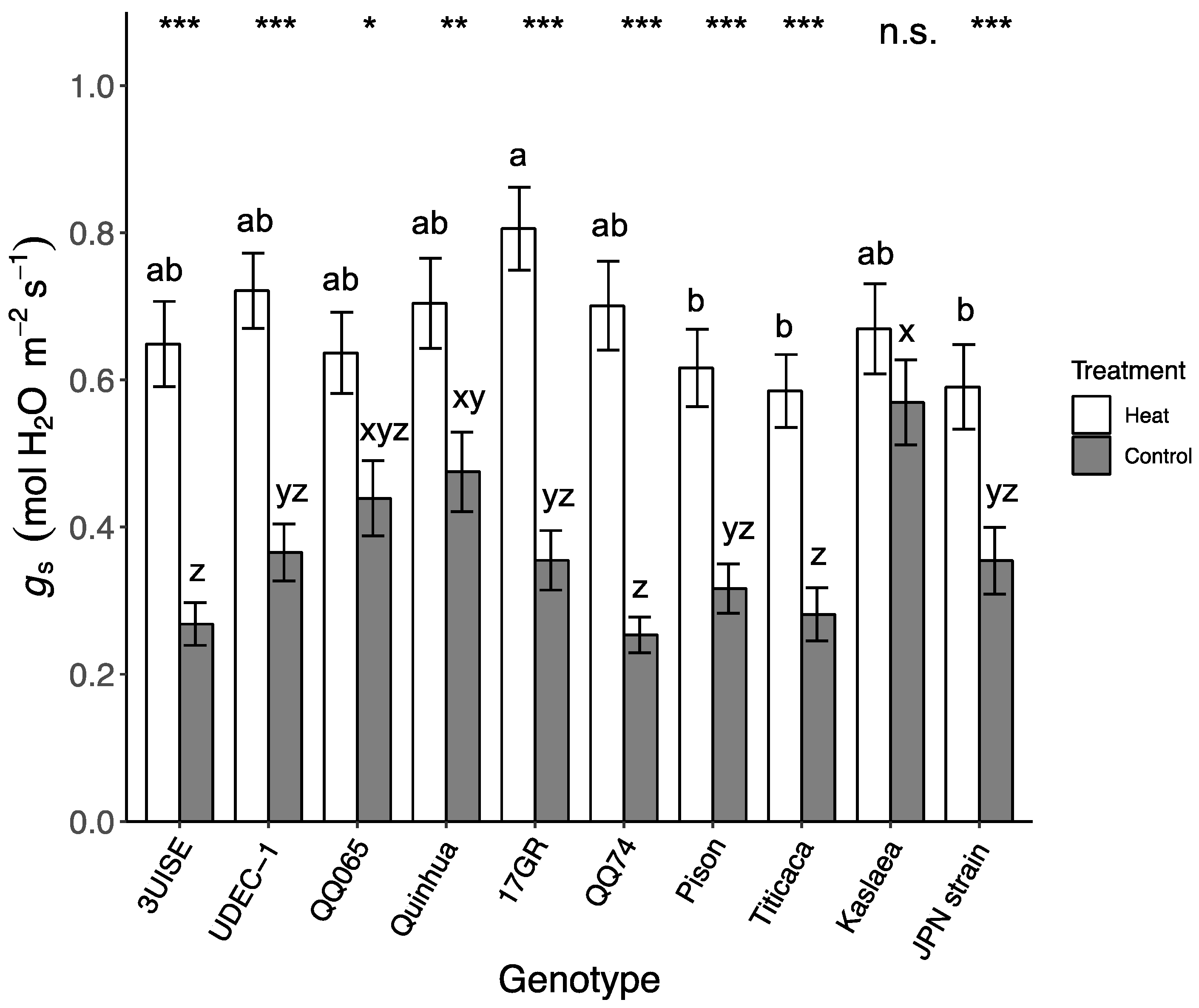
2.2. Stomatal Conductance
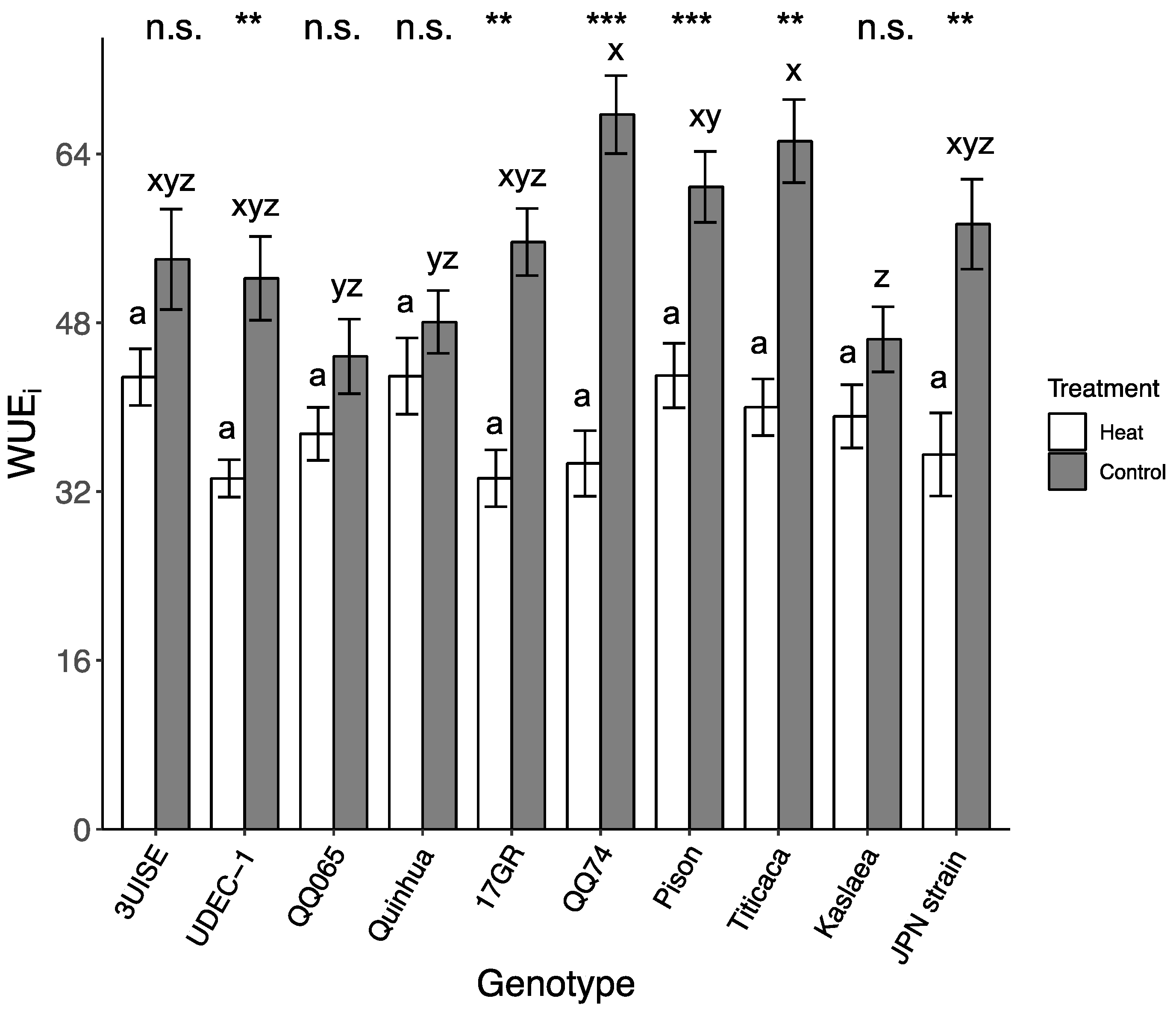
2.3. Intrinsic Water Use Efficiency
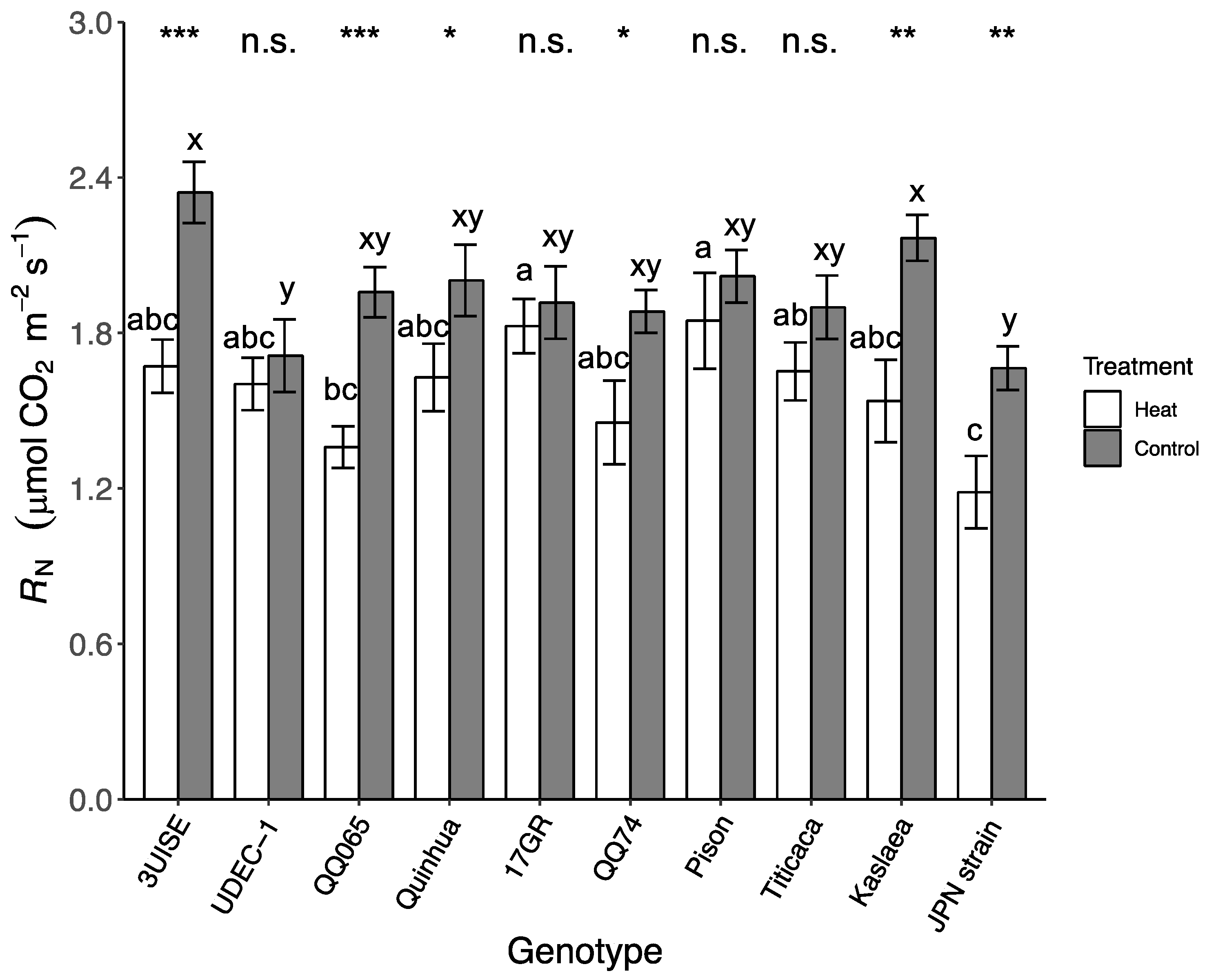
2.4. Dark Respiration
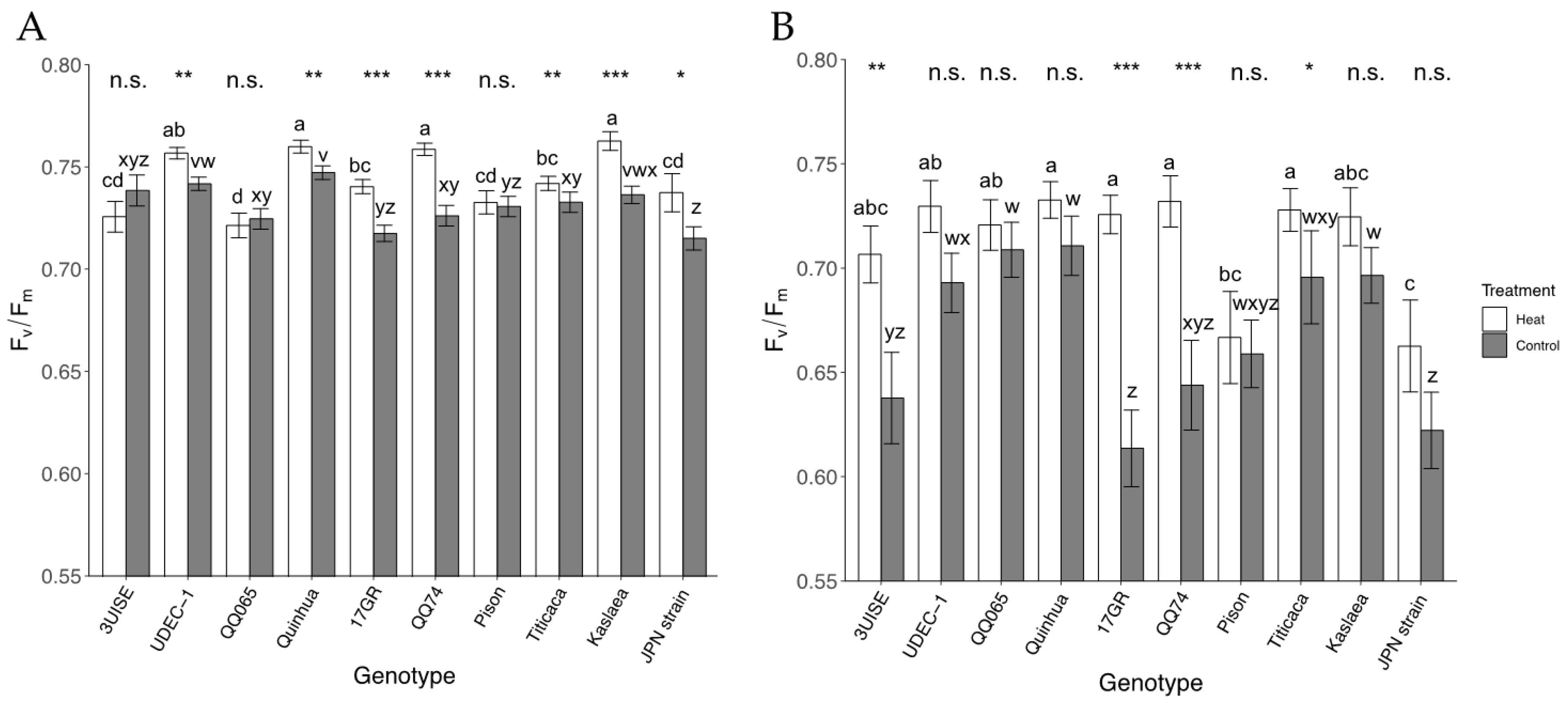
2.5. Maximum Quantum Yield of Photosystem-II
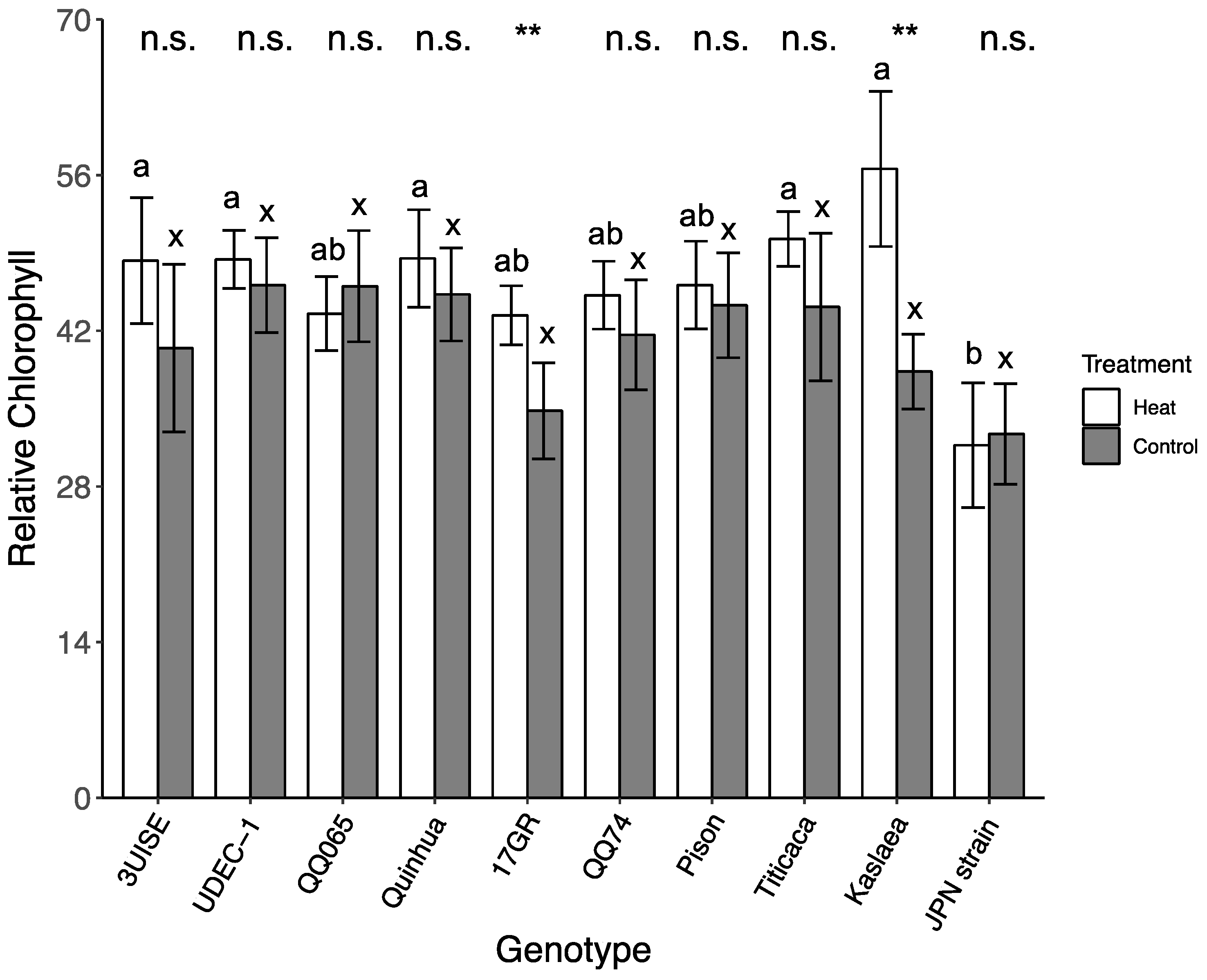
2.6. Relative Chlorophyll Content
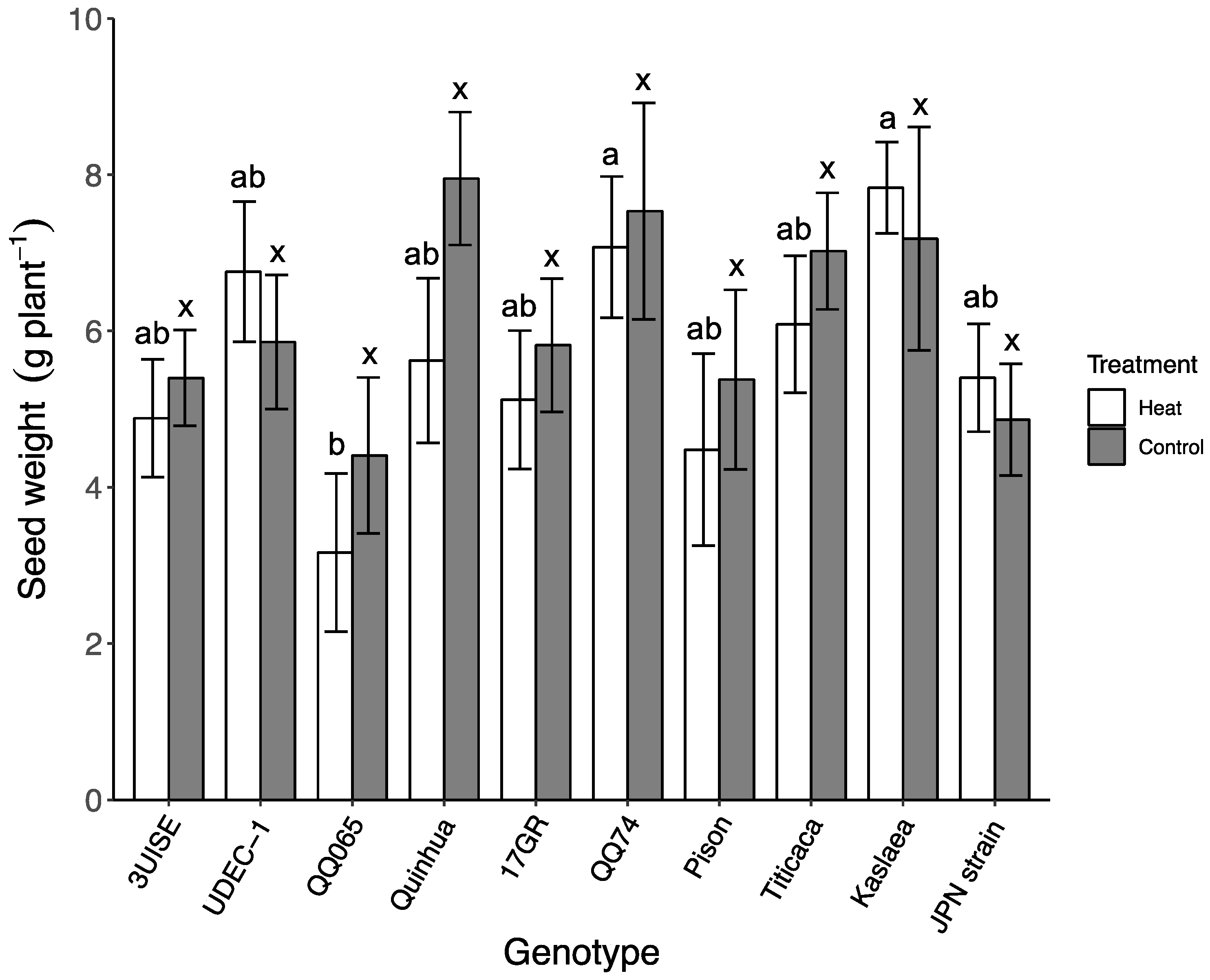
2.7. Seed and Shoot Biomass
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Leaf Gas Exchange and Chlorophyll Fluorescence
4.3. Aboveground Biomass and Seed Weight
4.4. Data Processing and Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jacobsen, S.E.; Mujica, A.; Jensen, C.R. The Resistance of Quinoa (Chenopodium Quinoa Willd.) to Adverse Abiotic Factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bazile, D.; Pulvento, C.; Verniau, A.; Al-Nusairi, M.S.; Ba, D.; Breidy, J.; Hassan, L.; Mohammed, M.I.; Mambetov, O.; Otambekova, M.; et al. Worldwide Evaluations of Quinoa: Preliminary Results from Post International Year of Quinoa FAO Projects in Nine Countries. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, K.B.; Biondi, S.; Oses, R.; Acuña-Rodríguez, I.S.; Antognoni, F.; Martinez-Mosqueira, E.A.; Coulibaly, A.; Canahua-Murillo, A.; Pinto, M.; Zurita-Silva, A.; et al. Quinoa Biodiversity and Sustainability for Food Security under Climate Change. A Review. Agron. Sustain. Dev. EDP Sci. 2014, 349–359. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, K.B.; Biondi, S.; Martínez, E.A.; Orsini, F.; Antognoni, F.; Jacobsen, S.E. Quinoa-A Model Crop for Understanding Salt-Tolerance Mechanisms in Halophytes. Plant Biosyst. 2016, 150, 357–371. [Google Scholar] [CrossRef]
- Bita, C.E.; Gerats, T. Plant Tolerance to High Temperature in a Changing Environment: Scientific Fundamentals and Production of Heat Stress-Tolerant Crops. Front. Plant Sci. Front. Res. Found. 2013. [Google Scholar] [CrossRef] [Green Version]
- Christensen, J.H.; Christensen, O.B. A Summary of the PRUDENCE Model Projections of Changes in European Climate by the End of This Century. Clim. Chang. 2007. [Google Scholar] [CrossRef]
- Porter, J.R.; Semenov, M.A. Crop Responses to Climatic Variation. Philos. Trans. R. Soc. B Biol. Soc. 2005, 360, 2021–2035. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat Tolerance in Plants: An Overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Giaveno, C.; Ferrero, J. Introduction of Tropical Maize Genotypes to Increase Silage Production in the Central Area of Santa Fe, Argentina. Crop Breed. Appl. Biotechnol. 2003, 3, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Chapin, F.S.; Pons, T.L. Plant Physiological Ecology: Second Edition; Springer: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Weis, E.; Berry, J.A. Plants and High Temperature Stress. In Symposia of the Society for Experimental Biology; Long, S.P., Woodward, F.I., Eds.; Society for Experimental Biology: London, UK, 1988; Volume 42, pp. 329–346. [Google Scholar]
- Zhang, M.; Barg, R.; Yin, M.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated Fatty Acid Desaturation via Overexpression of Two Distinct ω-3 Desaturases Differentially Alters Tolerance to Various Abiotic Stresses in Transgenic Tobacco Cells and Plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron Transport Is the Functional Limitation of Photosynthesis in Field-Grown Pima Cotton Plants at High Temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat Stress: An Overview of Molecular Responses in Photosynthesis. Photosynth. Res. 2008, 541–550. [Google Scholar] [CrossRef]
- Ahmad, A.; Diwan, H.; Abrol, Y.P. Global Climate Change, Stress and Plant Productivity. In Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation; Springer: Dordrecht, The Netherlands, 2010; pp. 503–521. [Google Scholar] [CrossRef]
- Karim, M.A.; Fracheboud, Y.; Stamp, P. Heat Tolerance of Maize with Reference of Some Physiological Characteristics. Ann. Bangladesh Agric. 1997, 7, 27–33. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll Fluorescence-A Practical Guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Pastenes, C.; Horton, P. Effect of High Temperature on Photosynthesis in Beans II. CO2 Assimilation and Metabolite Contents. Plant Physiol. 1996, 112, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant Cell Environ. 2008. [Google Scholar] [CrossRef]
- Sakata, T.; Higashitani, A. Male Sterility Accompanied with Abnormal Anther Development in Plants–Genes and Environmental Stresses with Special Reference to High Temperature Injury. Int. J. Plant Dev. Biol. 2008, 2, 42–51. [Google Scholar]
- Hall, A.E. Breeding for Heat Tolerance. In Plant Breeding Reviews; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 129–168. [Google Scholar] [CrossRef]
- Sumesh, K.V.; Sharma-Natu, P.; Ghildiyal, M.C. Starch Synthase Activity and Heat Shock Protein in Relation to Thermal Tolerance of Developing Wheat Grains. Biol. Plant. 2008, 52, 749–753. [Google Scholar] [CrossRef]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High Temperature Stress of Brassica Napus during Flowering Reduces Micro-and Megagametophyte Fertility, Induces Fruit Abortion, and Disrupts Seed Production. J. Exp. Bot. 2004, 55, 485–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinojosa, L.; Kumarm, N.; Gill, K.S.; Murphy, K.M. Spectral Reflectance Indices and Physiological Parameters in Quinoa under Contrasting Irrigation Regimes. Crop Sci. 2019, 59, 1927. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.L. New Grains and Pseudograins. Advances in New Crops; Timber Press: Portland, OR, USA, 1990; pp. 122–127. [Google Scholar]
- Prasad, P.V.V.; Pisipati, S.R.; Mutava, R.N.; Tuinstra, M.R. Sensitivity of Grain Sorghum to High Temperature Stress during Reproductive Development. Crop Sci. 2008, 48, 1911–1917. [Google Scholar] [CrossRef] [Green Version]
- Hinojosa, L.; Matanguihan, J.B.; Murphy, K.M. Effect of High Temperature on Pollen Morphology, Plant Growth and Seed Yield in Quinoa (Chenopodium Quinoa Willd.). J. Agron. Crop Sci. 2019, 205, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Yang, A.; Akhtar, S.S.; Amjad, M.; Iqbal, S.; Jacobsen, S.E. Growth and Physiological Responses of Quinoa to Drought and Temperature Stress. J. Agron. Crop Sci. 2016, 202, 445–453. [Google Scholar] [CrossRef]
- Becker, V.I.; Goessling, J.W.; Duarte, B.; Caçador, I.; Liu, F.; Rosenqvist, E.; Jacobsen, S.E. Combined Effects of Soil Salinity and High Temperature on Photosynthesis and Growth of Quinoa Plants (Chenopodium Quinoa). Funct. Plant Biol. 2017, 44, 665–678. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Salvucci, M.E.; Crafts-Brandner, S.J. Inhibition of Photosynthesis by Heat Stress: The Activation State of Rubisco as a Limiting Factor in Photosynthesis. Physiol. Plant. 2004, 179–186. [Google Scholar] [CrossRef]
- Cornish, K.; Radin, J.W.; Turcotte, E.L.; Lu, Z.; Zeiger, E. Enhanced Photosynthesis and Stomatal Conductance of Pima Cotton (Gossypium Barbadense, L.) Bred for Increased Yield. Plant Physiol. 1991, 97, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High Temperature Effects on Photosynthetic Activity of Two Tomato Cultivars with Different Heat Susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Petkova, V.; Denev, I.D.; Cholakov, D.; Porjazov, I. Field Screening for Heat Tolerant Common Bean Cultivars (Phaseolus Vulgaris L.) by Measuring of Chlorophyll Fluorescence Induction Parameters. Sci. Hortic. 2007, 111, 101–106. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Phenotyping of Wheat Cultivars for Heat Tolerance Using Chlorophyll a Fluorescence. Funct. Plant Biol. 2012, 39, 936–947. [Google Scholar] [CrossRef]
- Willits, D.H.; Peet, M.M. The Effect of Night Temperature on Greenhouse Grown Tomato Yields in Warm Climates. Agric. For. Meteorol. 1998, 92, 191–202. [Google Scholar] [CrossRef]
- Fghire, R.; Anaya, F.; Ali, O.I.; Benlhabib, O.; Ragab, R.; Wahbi, S. Physiological and Photosynthetic Response of Quinoa to Drought Stress. Chil. J. Agric. Res. 2015, 75, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Buttery, B.R.; Buzzell, R.I. The Relationship between Chlorophyll Content and Rate of Photosynthesis in Soybeans. Can. J. Plant Sci. 1977, 57, 1–5. [Google Scholar] [CrossRef]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenqvist, E. Wheat Cultivars Selected for High Fv/Fm under Heat Stress Maintain High Photosynthesis, Total Chlorophyll, Stomatal Conductance, Transpiration and Dry Matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef]
- Ristic, Z.; Bukovnik, U.; Prasad, P.V.V. Correlation between Heat Stability of Thylakoid Membranes and Loss of Chlorophyll in Winter Wheat under Heat Stress. Crop Sci. 2007, 47, 2067–2073. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Impact of High Nighttime Temperature on Respiration, Membrane Stability, Antioxidant Capacity, and Yield of Rice Plants. Crop Sci. 2009, 49, 313–322. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, B.M.; Lee, C.P.; Atkin, O.K.; Cheng, R.; Brown, T.B.; Millar, A.H.; Conceived, A.H.M. Variation in Leaf Respiration Rates at Night Correlates with Carbohydrate and Amino Acid Supply 1[OPEN]. Plant Physiol. 2017, 174, 2261–2273. [Google Scholar] [CrossRef] [Green Version]
- Prange, R.K.; McRae, K.B.; Midmore, D.J.; Deng, R. Reduction in Potato Growth at High Temperature: Role of Photosynthesis and Dark Respiration. Am. Potato J. 1990, 67, 357–369. [Google Scholar] [CrossRef]
- Sosa-Zuniga, V.; Brito, V.; Fuentes, F.; Steinfort, U. Phenological Growth Stages of Quinoa (Chenopodium Quinoa) Based on the BBCH Scale. Ann. Appl. Biol. 2017, 171, 117–124. [Google Scholar] [CrossRef]
- Douglas, B.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Linear and Nonlinear Mixed Effects Models; R Core Team: Vienna, Austria, 2018; pp. 1–137. [Google Scholar]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Core Team: Vienna, Austria, 2019; Available online: https://cran.r-project.org/web/packages/emmeans/index.html (accessed on 1 April 2019).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 2nd ed.; Sage Publication: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Filzmoser, P.; Gschwandtner, M. Mvoutlier: Multivariate Outlier Detection Based on Robust Methods [Software]; R Core Team: Vienna, Austria, 2018; Available online: https://cran.r-project.org/web/packages/mvoutlier/index.html (accessed on 8 January 2020).
- Wickham, H.; Francois, R.; Henry, L.; Muuler, K. Dplyr: A Grammar of Data Manipulation [Software]; R Core Team: Vienna, Austria, 2013; Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 8 January 2020).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer Nature: New York, NY, USA, 2016. [Google Scholar]
| Genotype | Plant Introduction # | Location |
|---|---|---|
| 3UISE | AMES 13756 | New Mexico, USA |
| UDEC-1 | PI 634923 | Bucalemu, Chile |
| QQ065 | PI 614880 | Los Lagos, Chile |
| Quinhua | N/A | Chile |
| 17GR | AMES 13735 | New Mexico, USA |
| QQ74 | PI 614886 | Maule, Chile |
| Pison | AMES 13746 | New Mexico, USA |
| Titicaca | N/A | Denmark |
| Kaslaea | AMES 13745 | New Mexico, USA |
| Japanese Strain | PI 677100 | Washington, USA |
| Time of Day | Treatment (°C) | |
|---|---|---|
| Heat | Control | |
| 00:00 | 30 | 14 |
| 06:00 | 32 | 16 |
| 08:00 | 35 | 18 |
| 10:00 | 40 | 18 |
| 12:00 | 45 | 20 |
| 16:00 | 40 | 18 |
| 18:00 | 35 | 16 |
| 20:00 | 32 | 16 |
| 22:00 | 30 | 14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eustis, A.; Murphy, K.M.; Barrios-Masias, F.H. Leaf Gas Exchange Performance of Ten Quinoa Genotypes under a Simulated Heat Wave. Plants 2020, 9, 81. https://doi.org/10.3390/plants9010081
Eustis A, Murphy KM, Barrios-Masias FH. Leaf Gas Exchange Performance of Ten Quinoa Genotypes under a Simulated Heat Wave. Plants. 2020; 9(1):81. https://doi.org/10.3390/plants9010081
Chicago/Turabian StyleEustis, Ashley, Kevin M. Murphy, and Felipe H. Barrios-Masias. 2020. "Leaf Gas Exchange Performance of Ten Quinoa Genotypes under a Simulated Heat Wave" Plants 9, no. 1: 81. https://doi.org/10.3390/plants9010081
APA StyleEustis, A., Murphy, K. M., & Barrios-Masias, F. H. (2020). Leaf Gas Exchange Performance of Ten Quinoa Genotypes under a Simulated Heat Wave. Plants, 9(1), 81. https://doi.org/10.3390/plants9010081






