Short-Term Post-Harvest Stress that Affects Profiles of Volatile Organic Compounds and Gene Expression in Rocket Salad during Early Post-Harvest Senescence
Abstract
1. Introduction
2. Results
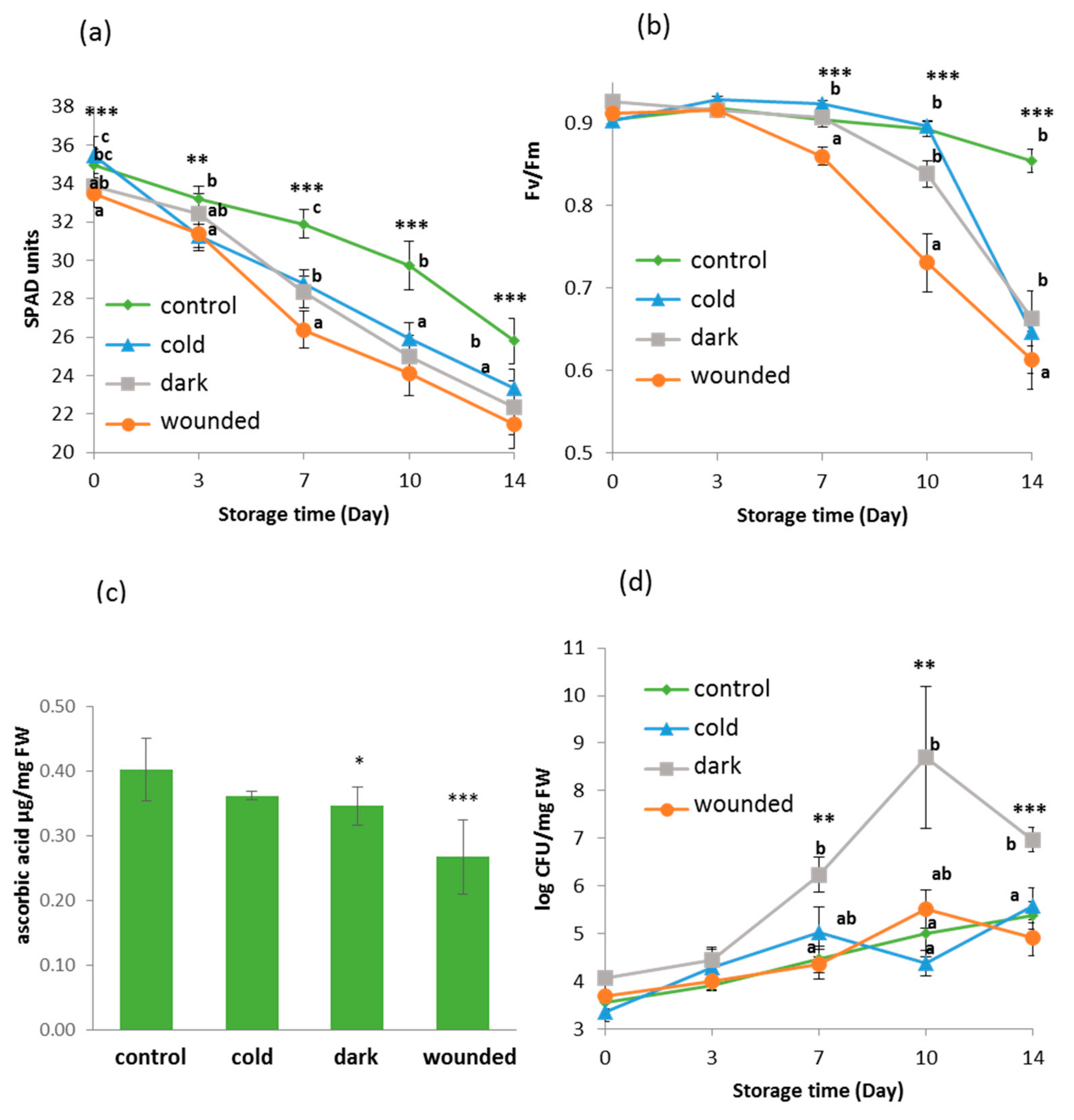
2.1. Short Term Stresses Followed by Post-Harvest Storage Affected Physiological and Biochemical Parameters of the Rocket Leaves
2.2. Microbial Growth Increased in Dark Ambient Stressed Leaves Following Storage
2.3. Gene Expression Changed During the Stress Treatment and Storage
2.4. VOC Profiles Were Affected by Short-Term Stress Treatments and Some Changes Persisted during Post-Harvest Storage
3. Discussion
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Microbiological Analyses and Assessment of Leaf Photosynthesis and Chlorophyll Content
4.3. Analysis of Vitamin C Content
4.4. Collection and Detection of Volatile Organic Compounds
4.5. Analysis of Volatile Organic Compounds
4.6. RNA Extraction and Gene Expression Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, L.; Spadafora, N.D.; Müller, C.T.; Wagstaff, C.; Rogers, H.J. Use of TD-GC – TOF-MS to assess volatile composition during post-harvest storage in seven accessions of rocket salad (Eruca sativa). Food Chem. 2016, 194, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Raimo, F.; Miccio, G. Diplotaxis tenuifolia: biology, production and properties. Eur. J. Plant Sci. Biotechnol. 2007, 1, 36–43. [Google Scholar]
- Bell, L.; Wagstaff, C. Rocket science: A review of phytochemical & health-related research in Eruca & Diplotaxis species. Food Chem. 2019, X1, 100002. [Google Scholar]
- Løkke, M.M.; Seefeldt, H.F.; Edelenbos, M. Freshness and sensory quality of packaged wild rocket. Postharvest Biol. Technol. 2012, 73, 99–106. [Google Scholar] [CrossRef]
- Amodio, M.L.; Derossi, A.; Mastrandrea, L.; Colelli, G. A study of the estimated shelf life of fresh rocket using a non-linear model. J. Food Eng. 2015, 150, 19–28. [Google Scholar] [CrossRef]
- Saini, R.K.; Ko, E.Y.; Keum, Y. Minimally processed ready-to-eat baby-leaf vegetables: Production, processing, storage, microbial safety, and nutritional potential. Food Rev. Int. 2017, 33, 644–663. [Google Scholar] [CrossRef]
- Koukounaras, A.; Siomos, A.S.; Sfakiotakis, E. Postharvest CO2 and ethylene production and quality of rocket (Eruca sativa Mill.) leaves as affected by leaf age and storage temperature. Postharvest Biol. Technol. 2007, 46, 167–173. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Bulgari, R.; Spinardi, A.; Ferrante, A. Identification of innovative potential quality markers in rocket and melon fresh-cut produce. Food Chem. 2015, 188, 225–233. [Google Scholar] [CrossRef]
- Rico, D.; Martín-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut fruit and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Allende, A.; Bennett, R.N.; Ferreres, F.; Gil, M.I. Microbial, nutritional and sensory quality of rocket leaves as affected by different sanitizers. Postharvest Biol. Technol. 2006, 42, 86–97. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Amaro, A.L.; Pereira, M.J.; Müller, C.T.; Pintado, M.; Rogers, H.J. Multi-trait analysis of post-harvest storage in rocket salad (Diplotaxis tenuifolia) links sensorial, volatile and nutritional data. Food Chem. 2016, 211, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.K.D.; Jobling, J.J.; Rogers, G.S. Variations in the most abundant types of glucosinolates found in the leaves of baby leaf rocket under typical commercial conditions. J. Sci. Food Agric. 2015, 95, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Oruna-Concha, M.J.; Wagstaff, C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC – MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chem. 2015, 172, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Bennett, R.N.; Rosa, E.A.S.; Mellon, F.A.; Kroon, P.A. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket). J. Agric. Food Chem. 2006, 54, 4005–4015. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Moreno, D.C.D.A.; Carvajal, M. The physiological importance of glucosinolates on plant response to abiotic stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef]
- Verkerk, R.; Dekker, M.; Jongen, W.M.F. Post-harvest increase of indolyl glucosinolates in response to chopping and storage of Brassica vegetables. J. Sci. Food Agr. 2001, 81, 953–958. [Google Scholar] [CrossRef]
- Deng, Q.; Zinoviadou, K.G.; Galanakis, C.M.; Lebovka, N.; Barba, F.J. The effects of conventional and non-conventional processing on glucosinolates and its derived forms, isothiocyanates: extraction, degradation, and applications. Food Eng. Rev. 2015, 7, 357–381. [Google Scholar] [CrossRef]
- Force, L.E.; O’Hare, T.J.; Wong, L.S.; Irving, D.E. Impact of cold storage on glucosinolate levels in seed-sprouts of broccoli, rocket, white radish and kohl-rabi. Postharvest Biol. Tech. 2007, 44, 175–178. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Spadafora, N.D.; Müller, C.T. Gene expression analysis of rocket salad under pre-harvest and postharvest stresses: A transcriptomic resource for Diplotaxis tenuifolia. PLoS ONE 2017, 12, e0178119. [Google Scholar] [CrossRef]
- Luca, A.; Kjær, A.; Edelenbos, M. Volatile organic compounds as markers for quality changes during the storage of wild rocket. Food Chem. 2017, 232, 579–586. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Cammarisano, L.; Rogers, H.J.; Müller, C.T. Using volatile organic compounds to monitor shelf-life in rocket salad. Acta Hortic. 2018, 1194, 1299–1306. [Google Scholar] [CrossRef]
- Mastrandrea, L.; Amodio, M.L.; Pati, S.; Colelli, G. Effect of modified atmosphere packaging and temperature abuse on flavor related volatile compounds of rocket leaves (Diplotaxis tenuifolia L.). J. Food Sci. Technol. 2017, 54, 2433–2442. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Barah, P.; Suarez-Rodriguez, M.C.; Bressendorff, S.; Friis, P.; Costantino, P.; Mundy, J. Transcriptome responses to combinations of stresses in Arabidopsis. Plant Physiol. 2013, 161, 1783–1794. [Google Scholar] [CrossRef]
- Humbeck, K. Senescence in plants. J. Plant Growth Regul. 2014, 33, 1–3. [Google Scholar] [CrossRef]
- Kreps, J.A.; Wu, Y.; Chang, H.-S.; Zhu, T.; Wang, X.; Harper, J.F. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Phys. 2002, 130, 2129–2141. [Google Scholar] [CrossRef]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef]
- Nakashima, K.; Takasaki, H.; Mizoi, J.; Shinozaki, K.; Yamaguchi-Shinozaki, K. NAC transcription factors in plant abiotic stress responses. BBA Gen. Regul. Mech. 2012, 1819, 97–103. [Google Scholar] [CrossRef]
- Tran, L.-S.P.; Nakashima, K.; Sakuma, Y.; Simpson, S.D.; Fujita, Y.; Maruyama, K.; Yamaguchi-Shinozaki, K. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. Plant Cell 2004, 16, 2481–2498. [Google Scholar] [CrossRef]
- Bu, Q.; Jiang, H.; Li, C.; Zhai, Q.; Zhang, J.; Wu, X.; Sun, J. Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Res. 2008, 18, 756–767. [Google Scholar] [CrossRef]
- Hickman, R.; Hill, C.; Penfold, C.A.; Breeze, E.; Bowden, L.; Moore, J.D.; Zhang, P.; Jackson, A.; Cooke, E.; Bewicke-Copley, F.; et al. A local regulatory network around three NAC transcription factors in stress responses and senescence in Arabidopsis leaves. Plant J. 2013, 75, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Balazadeh, S.; Kwasniewski, M.; Caldana, C.; Mehrnia, M. ORS1, an H2O2-responsive NAC transcription factor, controls senescence in Arabidopsis thaliana. Mol. Plant. 2011, 4, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Liebsch, D.; Keech, O. Dark-induced leaf senescence:new insights into a complex light-dependent regulatory pathway. New Phytol. 2016, 212, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Harpaz-Saad, S.; Yoon, G.M.; Mattoo, A.K.; Kieber, J.J. The formation of ACC and competition between polyamines and ethylene for SAM. Annl. Plant Rev. 2012, 44, 53–81. [Google Scholar]
- Morker, K.H.; Roberts, M.R. Light as both an input and an output of wound-induced reactive oxygen formation in Arabidopsis leaves. Plant Signal. Behav. 2011, 6, 1087–1089. [Google Scholar] [CrossRef]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Cocetta, G.; Baldassarre, V.; Spinardi, A.; Ferrante, A. Effect of cutting on ascorbic acid oxidation and recycling in fresh-cut baby spinach (Spinacia oleracea L.) leaves. Postharvest Biol. Technol. 2014, 88, 8–16. [Google Scholar] [CrossRef]
- Bell, L.; Yahya, H.N.; Oloyede, O.O.; Methven, L.; Wagstaff, C. Changes in rocket salad phytochemicals within the commercial supply chain: glucosinolates, isothiocyanates, amino acids and bacterial load increase significantly after processing. Food Chem. 2017, 221, 521–534. [Google Scholar] [CrossRef]
- Fall, R.; Karl, T.; Hansel, A.; Jordan, A.; Lindinger, W. Volatile organic compounds emitted after leaf wounding: on-line analysis by proton-transfer- reaction mass spectrometry. J. Geophys. Res. 1999, 104, 15963–15974. [Google Scholar] [CrossRef]
- Cocetta, G.; Mishra, S.; Raffaelli, A.; Ferrante, A. Effect of heat root stress and high salinity on glucosinolates metabolism in wild rocket. J. Plant Physiol. 2018, 231, 261–270. [Google Scholar] [CrossRef]
- Winter, D.; Vinegar, B.; Nahal, H.; Ammar, R.; Wilson, G.V.; Provart, N.J. An “Electronic Fluorescent Pictograph” browser for exploring and analyzing large-scale biological data sets. PLoS ONE 2007, 2, e718. [Google Scholar] [CrossRef] [PubMed]
- Cheong, Y.H.; Chang, H.S.; Gupta, R.; Wang, X.; Zhu, T.; Luan, S. Transcriptional profiling reveals novel interactions between wounding, pathogen, abiotic stress, and hormonal responses in Arabidopsis. Plant Physiol. 2002, 129, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Caldana, C.; Degenkolbe, T.; Cuadros-Inostroza, A.; Klie, S.; Sulpice, R.; Leisse, A.; Steinhauser, D.; Fernie, A.R.; Willmitzer, L.; Hannah, M.A. High-density kinetic analysis of the metabolomic and transcriptomic response of Arabidopsis to eight environmental conditions. Plant J. 2011, 67, 869–884. [Google Scholar] [CrossRef] [PubMed]
- Nojavan, S.; Khalilian, F.; Kiaie, F.; Rahimi, A.; Arabanian, A.; Chalavi, S. Extraction and quantitative determination of ascorbic acid during different maturity stages of Rosa canina L. fruit. J. Food Compos. Anal. 2008, 21, 300–305. [Google Scholar] [CrossRef]
- Anderson, M.J.; Willis, T.J. Canonical analysis of principal coordinates: A useful method of constrained ordination for ecology. Ecology 2003, 84, 511–525. [Google Scholar] [CrossRef]
- Oksanen, J.; Guillaume Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H.; et al. Vegan: Community ecology package. R package version 2.0-8. 2013. [Google Scholar]
- Kindt, R.; Coe, R. Tree diversity analysis. In A Manual and Software for Common Statistical Methods for Ecological and Biodiversity Studies; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2005. [Google Scholar]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucl. Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadafora, N.D.; Cocetta, G.; Ferrante, A.; Herbert, R.J.; Dimitrova, S.; Davoli, D.; Fernández, M.; Patterson, V.; Vozel, T.; Amarysti, C.; et al. Short-Term Post-Harvest Stress that Affects Profiles of Volatile Organic Compounds and Gene Expression in Rocket Salad during Early Post-Harvest Senescence. Plants 2020, 9, 4. https://doi.org/10.3390/plants9010004
Spadafora ND, Cocetta G, Ferrante A, Herbert RJ, Dimitrova S, Davoli D, Fernández M, Patterson V, Vozel T, Amarysti C, et al. Short-Term Post-Harvest Stress that Affects Profiles of Volatile Organic Compounds and Gene Expression in Rocket Salad during Early Post-Harvest Senescence. Plants. 2020; 9(1):4. https://doi.org/10.3390/plants9010004
Chicago/Turabian StyleSpadafora, Natasha D., Giacomo Cocetta, Antonio Ferrante, Robert J. Herbert, Simone Dimitrova, Daniela Davoli, Marta Fernández, Valentine Patterson, Tinkara Vozel, Canesia Amarysti, and et al. 2020. "Short-Term Post-Harvest Stress that Affects Profiles of Volatile Organic Compounds and Gene Expression in Rocket Salad during Early Post-Harvest Senescence" Plants 9, no. 1: 4. https://doi.org/10.3390/plants9010004
APA StyleSpadafora, N. D., Cocetta, G., Ferrante, A., Herbert, R. J., Dimitrova, S., Davoli, D., Fernández, M., Patterson, V., Vozel, T., Amarysti, C., Rogers, H. J., & Müller, C. T. (2020). Short-Term Post-Harvest Stress that Affects Profiles of Volatile Organic Compounds and Gene Expression in Rocket Salad during Early Post-Harvest Senescence. Plants, 9(1), 4. https://doi.org/10.3390/plants9010004






