Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars
Abstract
:1. Introduction
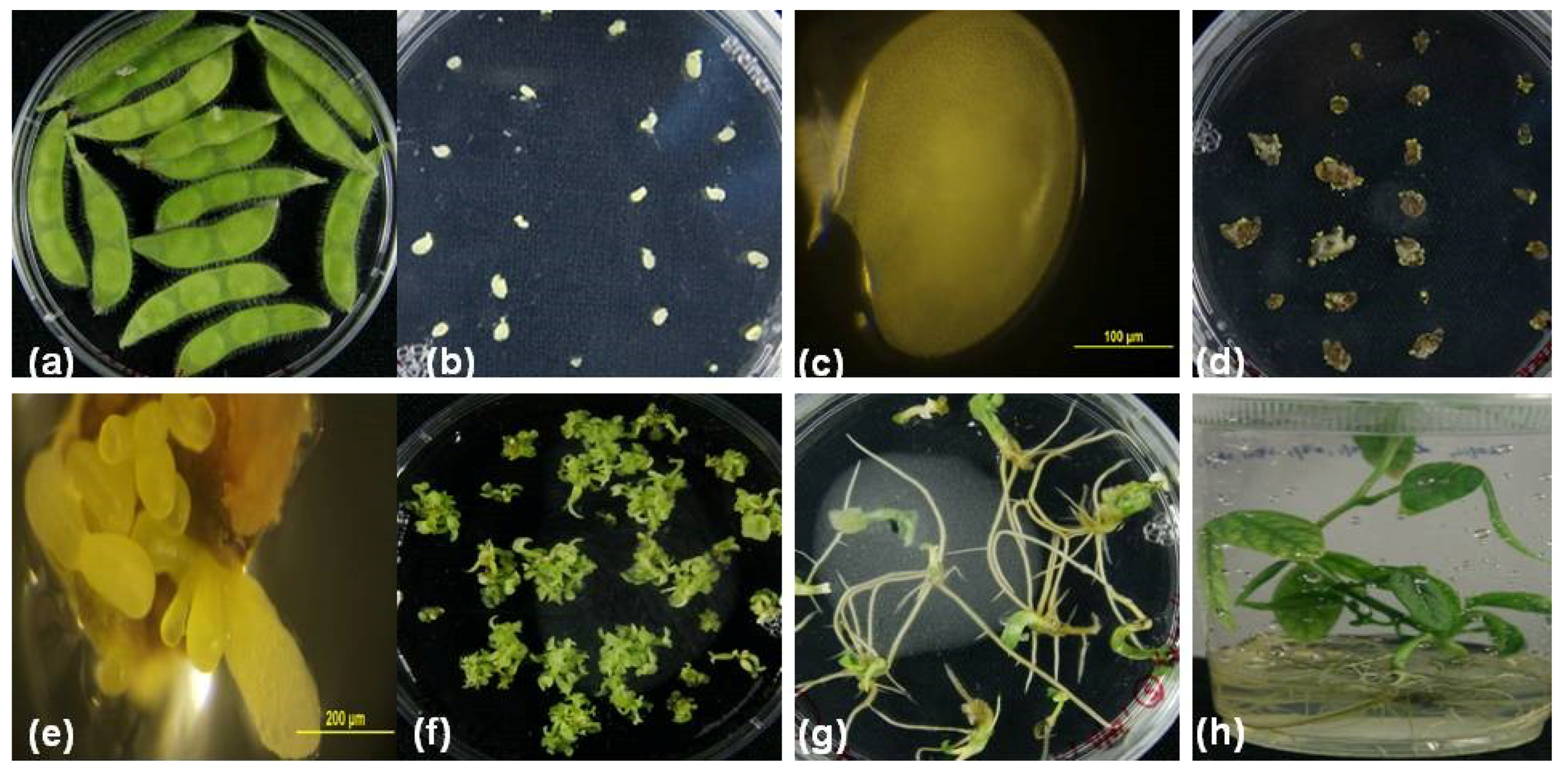
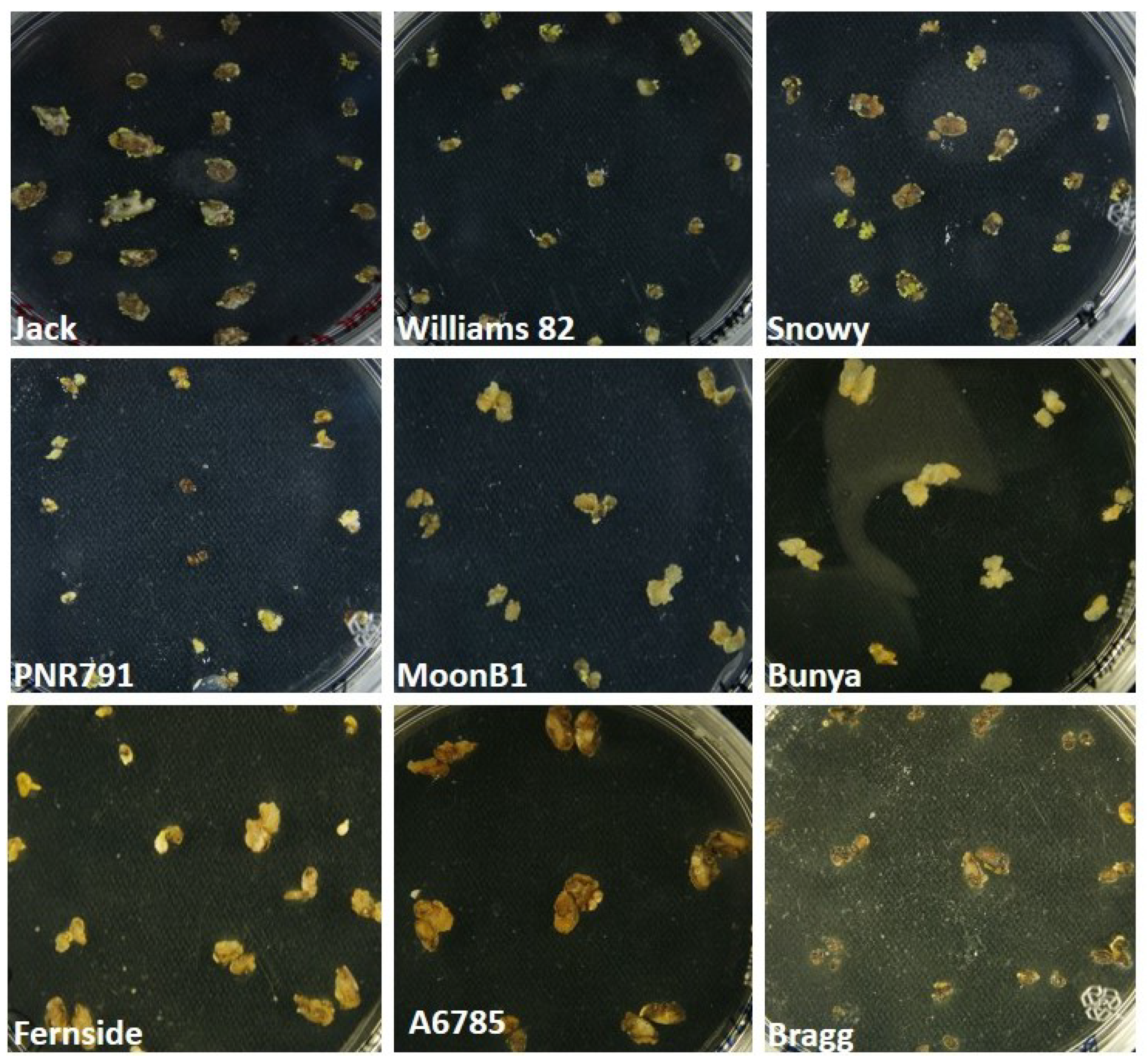
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. Statistical Yearbook; Food Agriculture Organization UN: Rome, Italy, 2016. [Google Scholar]
- Birt, D.; Hendrich, S.; Anthony, M.; Alekel, D. Soybeans and the prevention of chronic human disease. In Soybeans: Improvement, Production and Uses; Boerma, H.R., Specht, J.E., Eds.; American Society of Agronomy: Madison, WI, USA, 2004; pp. 1047–1117. [Google Scholar]
- Wilson, R. Seed composition. In Soybean: Improvement, Production and Uses; Boerma, H.R., Specht, J.E., Eds.; ASA; CSSA; SSA: Madison, WI, USA, 2004; pp. 621–677. [Google Scholar]
- Raza, G.; Ahmad, N.; Hussain, M.; Zafar, Y.; Rahman, M. Role of Genetics and Genomics in Mitigating Abiotic Stresses in Soybeans. In Environmental Stresses in Soybean Production; Elsevier: Amsterdam, The Netherlands, 2016; pp. 205–228. [Google Scholar]
- Finer, J.J. Generation of transgenic soybean (Glycine max) via particle bombardment of embryogenic cultures. Curr. Protoc. Plant Biol. 2016, 1, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Raza, G.; Singh, M.B.; Bhalla, P.L. In vitro plant regeneration from commercial cultivars of soybean. Biomed. Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droste, A.; Pasquali, G.; Bodanese-Zanettini, M. Transgenic fertile plants of soybean [Glycine max (L.) Merrill] obtained from bombarded embryogenic tissue. Euphytica 2002, 127, 367–376. [Google Scholar] [CrossRef]
- Homrich, M.S.; Passaglia, L.M.P.; Pereira, J.F.; Bertagnolli, P.F.; Pasquali, G.; Zaidi, M.A.; Altosaar, I.; Bodanese-Zanettini, M.H. Resistance to Anticarsia gemmatalis Hübner (Lepidoptera, Noctuidae) in transgenic soybean (Glycine max (L.) Merrill Fabales, Fabaceae) cultivar IAS5 expressing a modified Cry1Ac endotoxin. Genet. Mol. Biol. 2008, 31, 522–531. [Google Scholar] [CrossRef] [Green Version]
- Santarem, E.; Pelissier, B.; Finer, J. Effect of explant orientation, pH, solidifying agent and wounding on initiation of soybean somatic embryos. In Vitro Cell. Dev. Biol. Plant 1997, 33, 13–19. [Google Scholar] [CrossRef]
- Meurer, C.A.; Dinkins, R.D.; Redmond, C.T.; McAllister, K.P.; Tucker, D.T.; Walker, D.R.; Parrot, W.A.; Trick, H.N.; Essig, J.S.; Frantz, H.M.; et al. Embryogenic Response of multiple soybean [Glycine max(L.) Merr.] cultivars across three locations. In Vitro Cell. Dev. Biol. Plant 2001, 37, 6. [Google Scholar] [CrossRef]
- Walker, D.; Parrott, W. Effect of polyethylene glycol and sugar alcohols on soybean somatic embryo germination and conversion. Plant Cell Tissue Organ Cult. 2001, 64, 55–62. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, T.; Yu, D.; Gai, J. Somatic embryogenesis and plant regeneration in Chinese soybean (Glycine max (L.) Merr.)—Impacts of mannitol, abscisic acid, and explant age. In Vitro Cell. Dev. Biol. Plant 2009, 45, 180–188. [Google Scholar] [CrossRef]
- Droste, A.; Silva, A.M.D.; Souza, I.F.D.; Strohm, B.W.; Neto, L.B.; Bencke, M.; Sauner, M.V.; Zanettini, M.H.B. Screening of Brazilian soybean genotypes with high potential for somatic embryogenesis and plant regeneration. Pesqui. Agropecu. Bras. 2010, 45, 6. [Google Scholar] [CrossRef] [Green Version]
- Texeira, L.R.; Braccini, A.D.L.E.; Churata, B.G.M.; Vieira, E.S.N.; Martins, P.K.; Schuster, I. Evaluation of soybean cultivars on the embryogenic and organogenic potential. Acta Sci. Agron. 2011, 33, 67–74. [Google Scholar] [CrossRef]
- Thankaraj Salammal, M.; Vasudevan Ramesh, A.; Shu-Ye, J.; Andy, G.; Srinivasan, R. In vitro Regeneration and Genetic Transformation of Soybean: Current Status and Future Prospects. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; IntechOpen: London, UK, 2013; pp. 413–446. [Google Scholar] [CrossRef]
- Samoylov, V.M.; Tucker, D.M.; Parrott, W.A. Soybean [Glycine max (L.) merrill] embryogenic cultures: The role of sucrose and total nitrogen content on proliferation. In Vitro Cell. Dev. Biol. Plant 1998, 34, 8–13. [Google Scholar] [CrossRef]
- Samoylov, V.M.; Tucker, D.M.; Thibaud-Nissen, F.; Parrott, W.A. A liquid-medium-based protocol for rapid regeneration from embryogenic soybean cultures. Plant Cell Rep. 1998, 18, 49–54. [Google Scholar] [CrossRef]
- Schmidt, M.A.; Tucker, D.M.; Cahoon, E.B.; Parrott, W.A. Towards normalization of soybean somatic embryo maturation. Plant Cell Rep. 2005, 24, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, D.H.; Donaldson, P.A. Genotype screening for proliferative embryogenesis and biolistic transformation of short-season soybean genotypes. Plant Cell Rep. 2000, 19, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, E.; Branch, S.; Chamberlain, D.; Gabe, H.; Wright, M.; Stewart, C.N., Jr. Screening of soybean, Glycine max L.) Merrill, lines for somatic embryo induction and maturation capability from immature cotyledons. In Vitro Cell. Dev. Biol. Plant 2002, 38, 543–548. [Google Scholar] [CrossRef]
- Ko, T.-S.; Nelson, R.L.; Korban, S.S. Screening Multiple Soybean Cultivars (MG 00 to MG VIII) for Somatic Embryogenesis Following Agrobacterium-Mediated Transformation of Immature Cotyledons. Crop Sci. 2004, 44, 1825–1831. [Google Scholar] [CrossRef]
- Hiraga, S.; Minakawa, H.; Takahashi, K.; Takahashi, R.; Hajika, M.; Harada, K.; Ohtsubo, N. Evaluation of somatic embryogenesis from immature cotyledons of Japanese soybean cultivars. Plant Biotechnol. 2007, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Parrott, W.A.; Williams, E.G.; Hildebrand, D.F.; Collins, G.B. Effect of genotype on somatic embryogenesis from immature cotyledons of soybean. Plant Cell Tissue Organ Cult. 1989, 16, 15–21. [Google Scholar] [CrossRef]
- Delzer, B.W.; Somers, D.A.; Orf, J.H. Agrobacterium tumefaciens Susceptibility and Plant Regeneration of 10 Soybean Genotypes in Maturity Groups 00 to II. Crop Sci. 1990, 30, 320–322. [Google Scholar] [CrossRef]
- Bailey, M.A.; Boerma, H.R.; Parrott, W.A. Genotype-specific optimization of plant regeneration from somatic embryos of soybean. Plant Sci. 1993, 93, 117–120. [Google Scholar] [CrossRef]
- CSIRO. Snowy—The Best Australian Soybean CSIRO; CSIRO: Canberra, Australia, 2008.
- DPI. Soybean-Growing Guide for Queensland—Variety Update the State of Queenzland; DPI: Melbourne, Australia, 2008. [Google Scholar]
- Gaynor, L.; Lawn, R.; James, A. Agronomic studies on irrigated soybean in southern New South Wales. II. Broadening options for sowing date. Crop Pasture Sci. 2012, 62, 1067–1077. [Google Scholar] [CrossRef]
- Watanabe, S.; Harada, K.; Abe, J. Genetic and molecular bases of photoperiod responses of flowering in soybean. Breed. Sci. 2012, 61, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoemaker, R.; Amberger, L.; Palmer, R.; Oglesby, L.; Ranch, J. Effect of 2,4-dichlorophenoxyacetic acid concentration on somatic embryogenesis and heritable variation in soybean [Glycine max (L) Merr.]. In Vitro Cell. Dev. Biol. 1991, 27, 84–88. [Google Scholar] [CrossRef]
- Tian, L.N.; Brown, D.C.W.; Voldeng, H.; Webb, J. In vitro response and pedigree analysis for somatic embryogenesis of long-day photoperiod adapted soybean. Plant Cell Tissue Organ Cult. 1994, 36, 269–273. [Google Scholar] [CrossRef]
- Komatsuda, T.; Ohyama, K. Genotypes of high competence for somatic embryogenesis and plant regeneration in soybean Glycine max. Theor. Appl. Genet. 1988, 75, 695–700. [Google Scholar] [CrossRef]
- Santos, K.G.B.; Mundstock, E.; Bodanese-Zanettini, M.H. Genotype-specific normalization of soybean somatic embryogenesis through the use of an ethylene inhibitor. Plant Cell Rep. 1997, 16, 859–864. [Google Scholar] [CrossRef]
- Hofmann, N.; Nelson, R.; Korban, S. nfluence of Media Components and pH on Somatic Embryo Induction in Three Genotypes of Soybean. Plant Cell Tissue Organ Cult. 2004, 77, 157–163. [Google Scholar] [CrossRef]
- Ko, T.-S.; Korban, S. Enhancing the frequency of somatic embryogenesis following Agrobacterium-mediated transformation of immature cotyledons of soybean [Glycine max (L.) Merrill.]. In Vitro Cell. Dev. Biol. Plant 2004, 40, 552–558. [Google Scholar] [CrossRef]
- Bailey, M.A.; Boerma, H.R.; Parrott, W.A. Genotype effects on proliferative embryogenesis and plant regeneration of soybean. In Vitro Cell. Dev. Biol. Plant 1993, 29, 102–108. [Google Scholar] [CrossRef]
- Lazzeri, P.; Hildebrand, D.; Collins, G. A procedure for plant regeneration from immature cotyledon tissue of soybean. Plant Mol. Biol. Rep. 1985, 3, 160–168. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Payne, R.W. GenStat. Wiley Interdisciplinary Reviews: Computational Statistics. WIREs 2009, 1, 255–258. [Google Scholar]
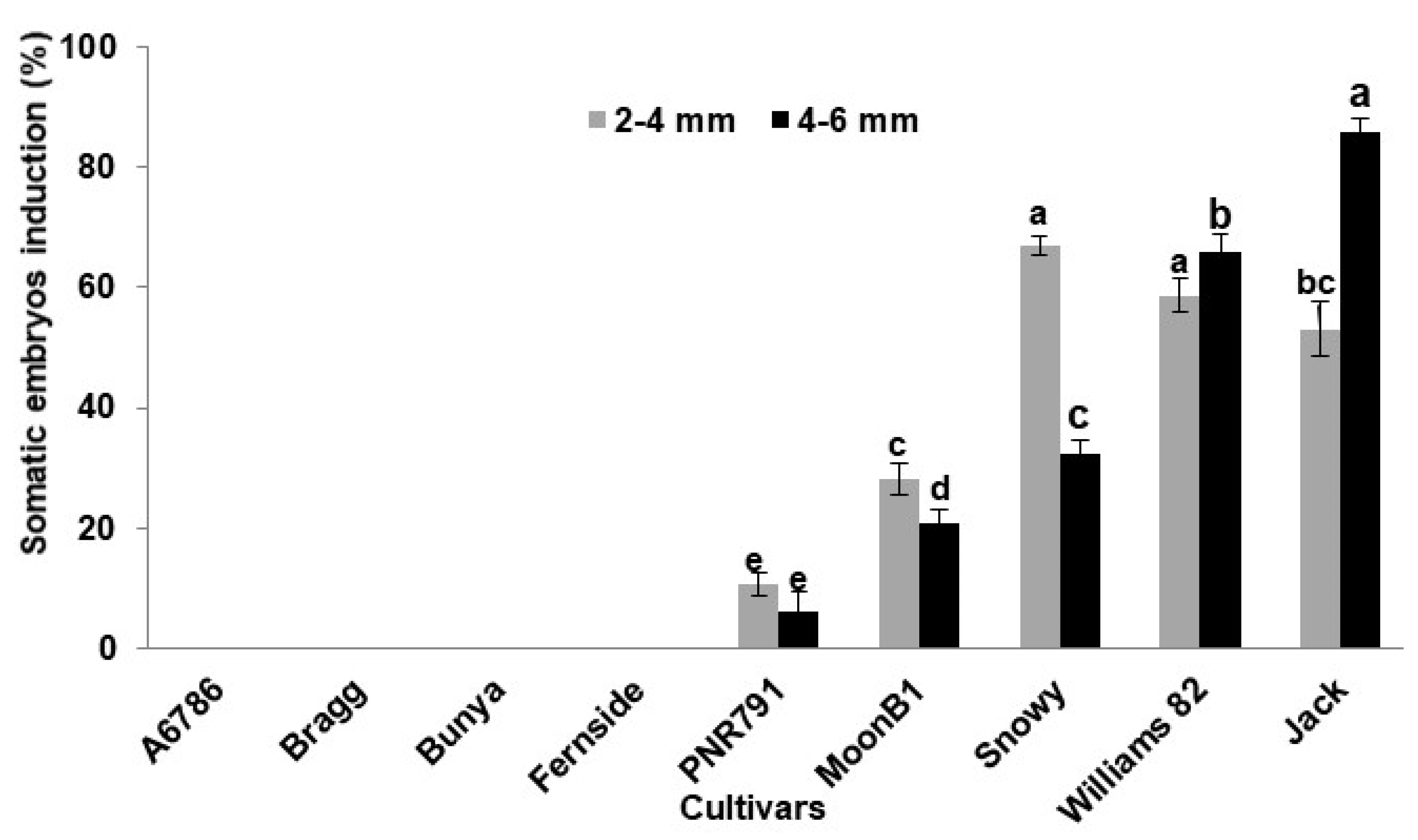
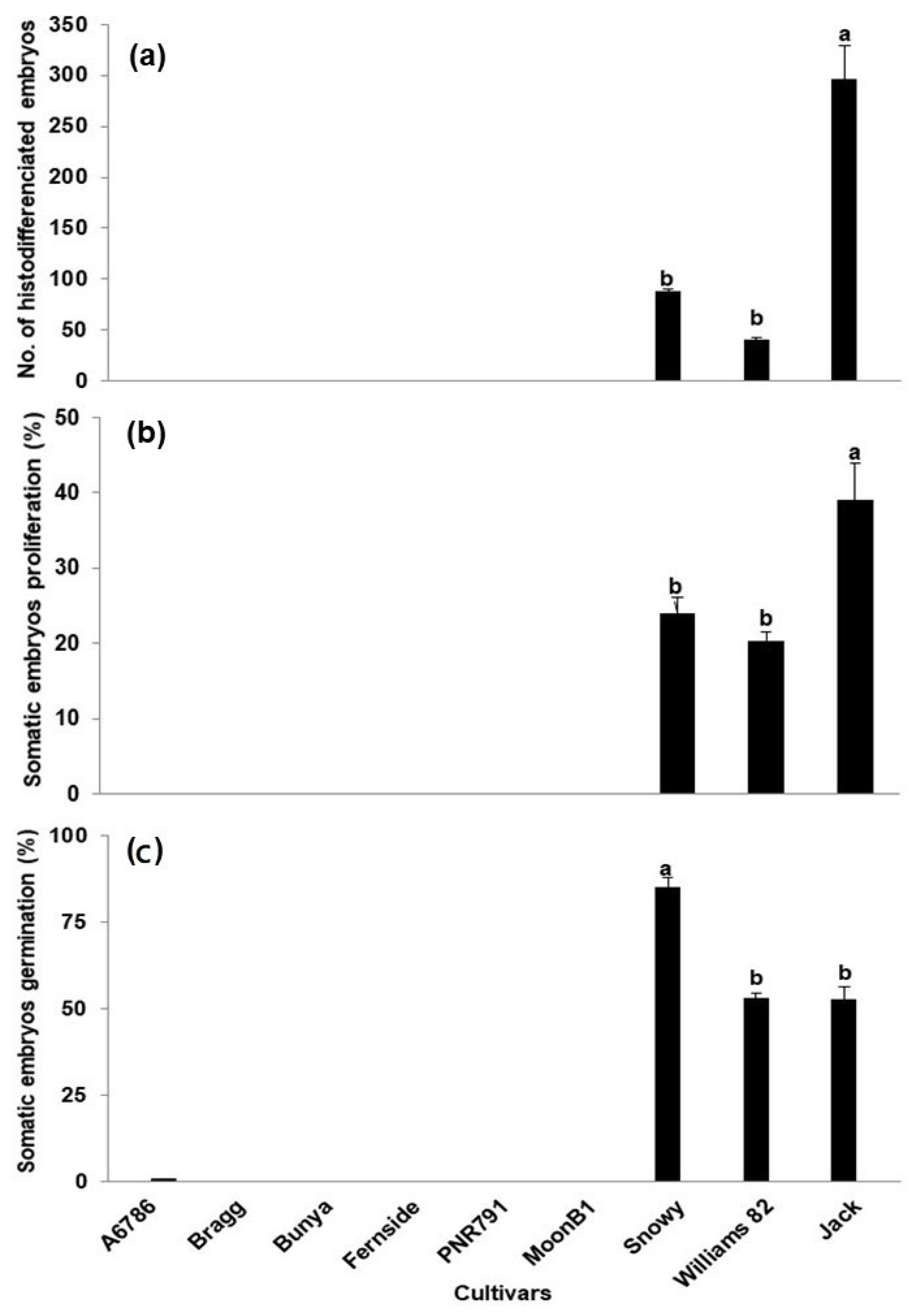

| Soybean Cultivars | Maturity Group | Total no. of Explants (Both Sizes) | Somatic Embryos Induction (%) (Mean ± S.E) | Proliferated Embryos (Mean ± S.E) | Embryos on Germination Medium | Gemination (%) (Mean ± S.E) | Plants Obtained |
|---|---|---|---|---|---|---|---|
| Snowy | III | 145 | 36 ± 2.59 c | 88 ± 2.31 b | 50 | 85 ± 2.64 a | 42 |
| Jack | II | 141 | 69 ± 2.38 a | 297 ± 32.05 a | 50 | 53 ± 3.54 b | 27 |
| Williams | III | 131 | 62 ± 2.91 b | 41 ± 2.08 c | 50 | 53 ± 1.61 b | 26 |
| MoonB1 | V | 130 | 24 ± 2.38 d | 0 | 0 | 0 | 0 |
| PNR791 | V | 120 | 8 ± 2.60 e | 0 | 0 | 0 | 0 |
| A6785 | VI | 154 | 0 | 0 | 0 | 0 | 0 |
| Bunya | VI | 168 | 0 | 0 | 0 | 0 | 0 |
| Bragg | VII | 70 | 0 | 0 | 0 | 0 | 0 |
| Fernside | VII | 134 | 0 | 0 | 0 | 0 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, G.; Singh, M.B.; Bhalla, P.L. Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars. Plants 2020, 9, 38. https://doi.org/10.3390/plants9010038
Raza G, Singh MB, Bhalla PL. Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars. Plants. 2020; 9(1):38. https://doi.org/10.3390/plants9010038
Chicago/Turabian StyleRaza, Ghulam, Mohan B. Singh, and Prem L. Bhalla. 2020. "Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars" Plants 9, no. 1: 38. https://doi.org/10.3390/plants9010038
APA StyleRaza, G., Singh, M. B., & Bhalla, P. L. (2020). Somatic Embryogenesis and Plant Regeneration from Commercial Soybean Cultivars. Plants, 9(1), 38. https://doi.org/10.3390/plants9010038





