Fumigant Toxicity in Myzus persicae Sulzer (Hemiptera: Aphididae): Controlled Release of (E)-anethole from Microspheres
Abstract
1. Introduction
2. Results
2.1. Encapsulation of (E)-Anethole
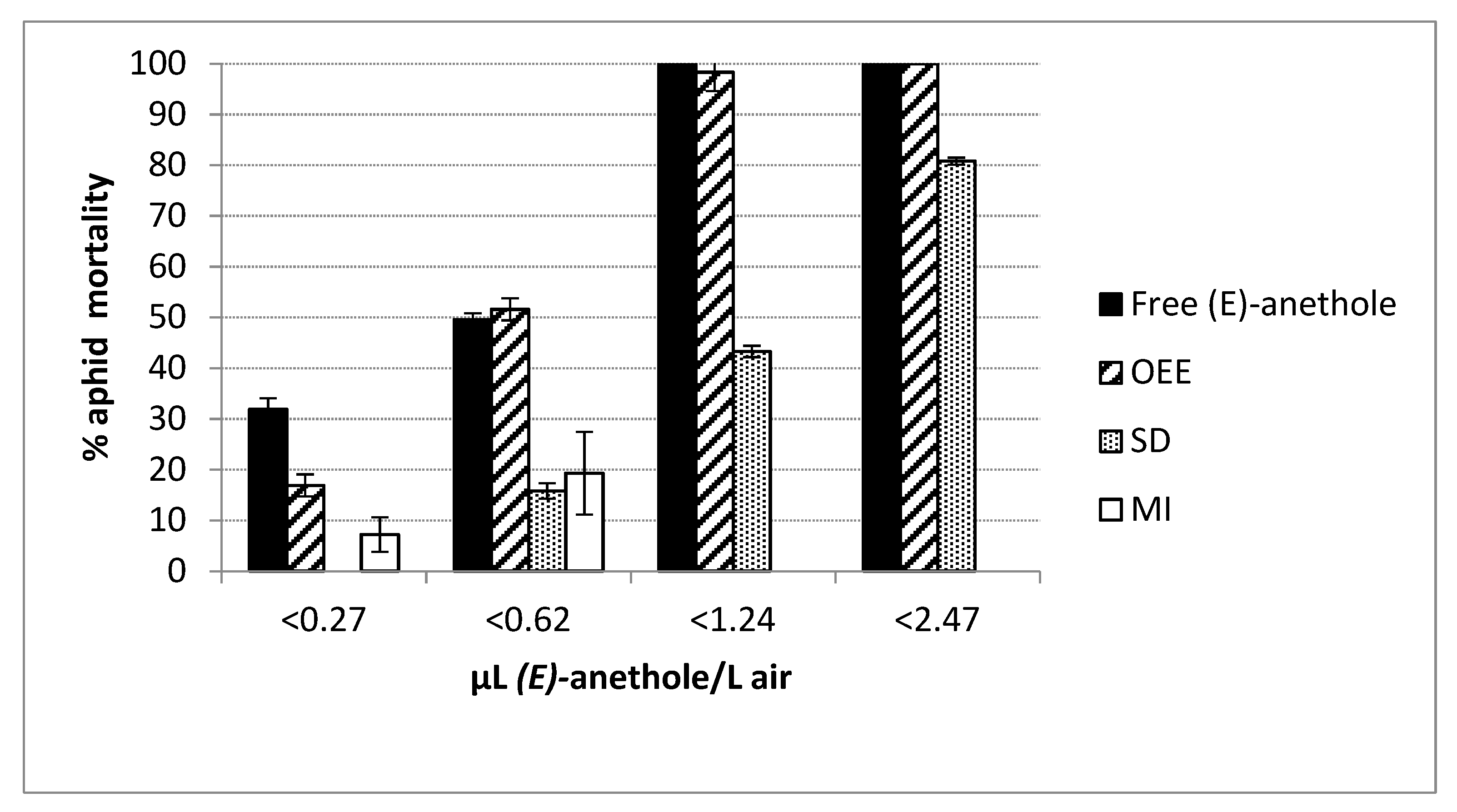
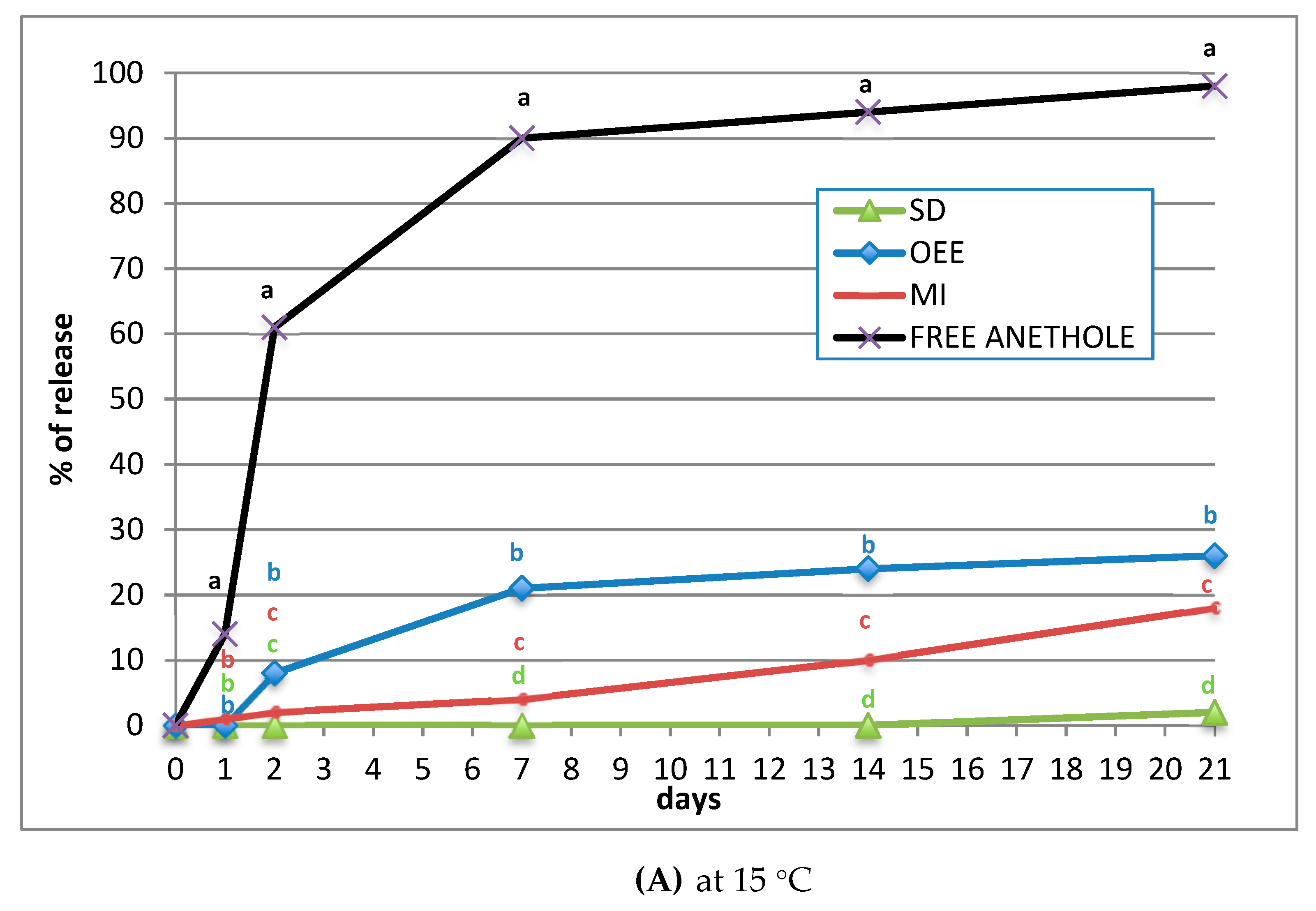
2.2. Fumigant Activity and Controlled Release of (E)-Anethole
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Microsphere Preparation
4.2.1. Beads of (E)-Anethole by Oil-Emulsion-Entrapment (OEE)
4.2.2. Preparation of β-Cyclodextrin/(E)-Anethole Molecular Inclusion (MI) Complexes
4.2.3. Spray Drying of (E)-Anethole (SD)
4.3. Encapsulation Yield and Loading
4.4. Controlled Release of (E)-anethole through Different Matrix Blends
4.5. Scanning Electron Microscopy (SEM) Analysis
4.6. Fumigation Bioassay
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Vallejo, R.; Guirao, P.; Rodríguez-Rojo, S.; Cocero, M.J. Use of nanoemulsions of plant essential oils as aphid repellents. Ind. Crops Prod. 2017, 110, 45–57. [Google Scholar] [CrossRef]
- Dwivedy, A.K.; Singh, V.K.; Prakash, B.; Dubey, N.K. Nanoencapsulated Illicium verum Hook f. essential oil as an effective novel plant-based preservative against aflatoxin B1 production and free radical generation. Food Chem. Toxicol. 2018, 111, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Addae-Mensah, I.; Asomaining, W.A.; Oteng-Yeboah, A.; Garneau, F.X.; Gagnon, H.; Jean, F.I.; Moudachirou, M.; Koumagic, K.H. (E)-anethole as a major essential oil constituent of Clausena anisata. J. Essent. Oil Res. 1996, 8, 513–516. [Google Scholar] [CrossRef]
- Blewitt, M.; Southwell, I.A. Backhousia anisata Vickery an alternative source of (E)-anethole. J. Essent. Oil Res. 2000, 12, 445–454. [Google Scholar] [CrossRef]
- Opdyke, D.L.J. Monographs in Fragance Raw Materials; Pergamon Press: Oxford, UK, 1979; p. 92. [Google Scholar]
- Isman, M.B. Plant essential oils for pest and disease management. Crop. Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- López, M.D.; Jordán, M.J.; Pascual-Villalobos, M.J. Toxic compounds in essential oils of coriander, caraway and basil active against stored rice pests. J. Agric. Food Chem. 2008, 44, 273–278. [Google Scholar] [CrossRef]
- Kim, D.-H.-; Ahn, Y.-J. Contact and fumigant activity of constituents of Foeniculum vulgare fruits against three coleopteran stored-product insects. Pest. Manag. Sci. 2001, 57, 301–306. [Google Scholar] [CrossRef]
- Koul, O.; Singh, G.; Singh, R.; Singh, J. Mortality and reproductive performance of Tribolium castaneum exposed to anethole vapours at high temperature. Biopestic. Int. 2007, 3, 126–137. [Google Scholar]
- Zarrad, K.; Pascual-Villalobos, M.J. Testing liquid formulations of essential oils against aphid pests. In Proceedings of the IX Congreso Nacional de Entomología Aplicada, XV Jornadas Científicas de la SEEA, Valencia, Spain, 19–23 October 2015; p. 234. [Google Scholar]
- Pascual-Villalobos, M.J.; Guirao, P.; Díaz-Baños, F.G.; Cantó-Tejero, M.; Villora, G. Oil in water nanoemulsions of botanical active substances. In Nano-Biopesticides Today and Future Perspectives; Koul, O., Ed.; Academic Press, Elsevier Inc.: London, UK, 2019; Volume 9, pp. 223–248. [Google Scholar]
- Pascual-Villalobos, M.J.; Zarrad, K.; Castañé, C.; Riudavets, J. Liquid formulations of monoterpenoids as space and structural treatments of store rooms. In Proceedings of the 11th International Working Conference on Stored Product Protection, Chang Mai, Thailand, 24–28 November 2014; Arthur, F.H., Kengkouponich, R., Chayaprosert, W., Suthisut, D., Eds.; 2015; pp. 1061–1070. [Google Scholar]
- Benelli, G.; Pavela, R.; Petrelli, R.; Capellaci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not just popular spices! Essential oils from Cuminum cyminum and Pimpinella anisum are toxic to insect pests and vectors without affecting non-target invertebrates. Ind. Crops Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.E.; Bakshi, M.; Abhilash, P.C.; Fernández Fraceto, L. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef]
- Mishra, P.; Seenivasan, R.; Mukherjee, A.; Chandrasekaran, N. Polymer/layered silicate nanocomposites as matrix for bioinsecticide formulations. In Nano-Biopesticides Today and Future Perspectives; Koul, O., Ed.; Academic Press, Elsevier Inc.: London, UK, 2019; Volume 6, pp. 161–178. [Google Scholar]
- López, M.D.; Maudhuit, A.; Pascual-Villalobos, M.J.; Poncelet, D. Development of formulations to improve the controlled release of linalool to be applied as an insecticide. J. Agric. Food Chem. 2012, 60, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Manesh, M.E.; Ghaedi, Z.; Asemi, M.; Khanavi, M.; Manayi, A.; Jamalifar, H.; Atyavi, F.; Dinarvand, R. Study of antimicrobial activity of anethole and carvone loaded PLGA nanoparticles. J. Pharm. Res. 2013, 7, 290–295. [Google Scholar]
- Gharib, R.; Greige-Gerges, H.; Jraij, A.; Auezova, L.; Charcoset, C. Preparation of drug-in-cyclodextrin-in-liposomes at a large scale using a membrane contactor: Application to trans-anethole. Carbohydr. Polym. 2016, 154, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Villalobos, M.J.; López, M.D.; Castañé, C.; Soler, A.; Riudavets, J. Encapsulated essential oils as an alternative to insecticides in funnel traps. J. Econ. Entomol. 2015, 108, 2117–2120. [Google Scholar] [CrossRef]
- Telci, I.; Dermitas, I.; Schin, A. Variations in plant properties and EO composition of sweet fennel (Foeniculum vulgare Mill.) fruits during stages of maturity. Ind. Crops Prod. 2015, 30, 126–130. [Google Scholar] [CrossRef]
- Bahmani, K.; Darbandi, A.I.; Ramshini, H.A.; Moradi, N.; Akbari, A. Agro-morphological and phytochemical diversity of various Iranian fennel landraces. Ind. Crops Prod. 2015, 77, 282–294. [Google Scholar] [CrossRef]
- Arslan, N.; Gürbüz, B.; Sarihan, E.O. Variations in EO content and composition in Turkish anise (Pimpinella anisum L.) populations. Turk. J. Agric. For. 2004, 28, 173–178. [Google Scholar]
- Hamraoui, A.; Regnault-Roger, C. Comparaison des activités insecticides des monoterpènes sur deux espèces d’insectes ravageurs des cultures: Ceratitis capitata et Rhopalosiphum padi. Acta Bot. Gallica 1997, 144, 414–417. [Google Scholar] [CrossRef]
- Tak, J.-H.; Jovel, E.; Isman, M.B. Contact, fumigant, and cytotoxic activities of thyme and lemongrass essential oils against larvae and an ovarian cell line of the cabbage looper, Trichoplusia ni. J. Pest. Sci. 2016, 89, 183–193. [Google Scholar] [CrossRef]
- Jahan, F.; Abbasipour, H.; Hasanshahi, G. Fumigant toxicity and nymph production deterrence effect of five essential oils on adults of the cabbage aphid, Brevicoryne brassicae L. (Hemiptera: Aphidae). J. Essent. Oil Bear. Plants 2016, 19, 140–147. [Google Scholar] [CrossRef]
- Tunc, I.; Sahinkaya, S. Sensitivity of two greenhouse pests to vapours of essential oils. Entomol. Exp. Appl. 1998, 86, 183–187. [Google Scholar] [CrossRef]
- Cantó-Tejero, M.; Pascual-Villalobos, M.J.; Guirao, P. Estudio comparativo de la actividad repelente de aceites esenciales en varias de las principales especies de pulgón de pimiento. In Proceedings of the X Congreso Nacional de Entomología Aplicada, XVI Jornadas Científicas de la SEEA, Logroño, Spain, 20–24 October 2017; p. 127. [Google Scholar]
- Cantó-Tejero, M.; Guirao, P.; Pascual-Villalobos, M.J.; Marcos-García, M.A. Aceites esenciales como repelentes frente a los pulgones Myzus persicae Sulzer y Macrosiphum euphorbiae (Thomas) (Hemiptera: Aphididae), Efectos sobre sus enemigos naturales Sphaerophoria ruepellii (Wiedemann) (Diptera: Syrphidae) y Aphidius colemani Viereck (Hymenoptera: Braconidae). In Proceedings of the XI Congreso Nacional de Entomología Aplicada, XVII Jornadas Científicas de la SEEA, Madrid, Spain, 4–8 November 2019; p. 80. [Google Scholar]
- Al-Antary, T.M.; Belghasem, I.H.; Araj, S.A. Toxicity of anise oil against the green peach aphid Myzus persicae Sulzer using four solvents (Homoptera: Aphididae). Fresenius Environ. Bull. 2017, 36, 3705–3710. [Google Scholar]
- González, J.O.W.; Stefanazzi, N.; Murray, A.P.; Ferrero, A.A.; Fernández-Band, B. Novel nanoinsecticides based on essential oils to control the German cockroach. J. Pest. Sci. 2015, 88, 393–404. [Google Scholar] [CrossRef]



| Formulation Method 1 | Dry Sphere Size (µm) | Loading (g Monoterpene 100 g−1 Powder) | Encapsulation Yield (%) |
|---|---|---|---|
| SD | 4.00 a | 6.15 a | 73 a |
| OEE | 1.70 b | 5.20 a | 26 b |
| MI | 3.52 a | 1.33 b | 14 c |
| Formulation Method 2 | LC50 | 95% CI | LC90 | 95% CI | χ2 |
|---|---|---|---|---|---|
| SD | 1.292 | 1.169–1.476 | 3.383 | 2.706–4.305 | 0.487 ns |
| OEE | 0.415 | 0.416–0.468 | 0.780 | 0.675–0.832 | 23.850 * |
| Free (E)-anethole | 0.336 | 0.306–0.369 | 1.043 | 0.867–1.255 | 8.572 ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascual-Villalobos, M.J.; Cantó-Tejero, M.; Guirao, P.; López, M.D. Fumigant Toxicity in Myzus persicae Sulzer (Hemiptera: Aphididae): Controlled Release of (E)-anethole from Microspheres. Plants 2020, 9, 124. https://doi.org/10.3390/plants9010124
Pascual-Villalobos MJ, Cantó-Tejero M, Guirao P, López MD. Fumigant Toxicity in Myzus persicae Sulzer (Hemiptera: Aphididae): Controlled Release of (E)-anethole from Microspheres. Plants. 2020; 9(1):124. https://doi.org/10.3390/plants9010124
Chicago/Turabian StylePascual-Villalobos, María J., Manuel Cantó-Tejero, Pedro Guirao, and María D. López. 2020. "Fumigant Toxicity in Myzus persicae Sulzer (Hemiptera: Aphididae): Controlled Release of (E)-anethole from Microspheres" Plants 9, no. 1: 124. https://doi.org/10.3390/plants9010124
APA StylePascual-Villalobos, M. J., Cantó-Tejero, M., Guirao, P., & López, M. D. (2020). Fumigant Toxicity in Myzus persicae Sulzer (Hemiptera: Aphididae): Controlled Release of (E)-anethole from Microspheres. Plants, 9(1), 124. https://doi.org/10.3390/plants9010124






