Phenotypic and Genetic Variation of an Interspecific Centaurium Hybrid (Gentianaceae) and Its Parental Species
Abstract
1. Introduction
2. Results
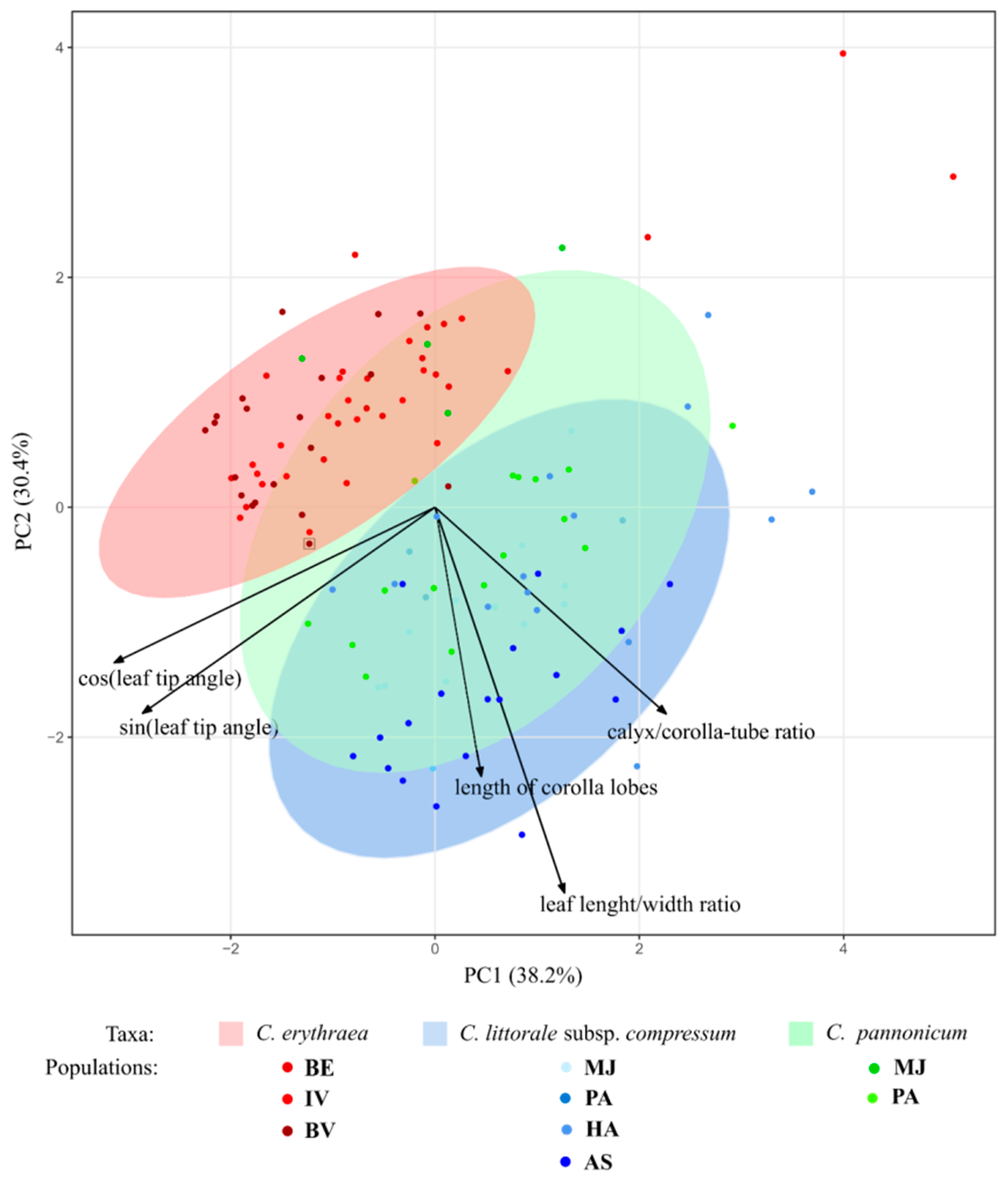
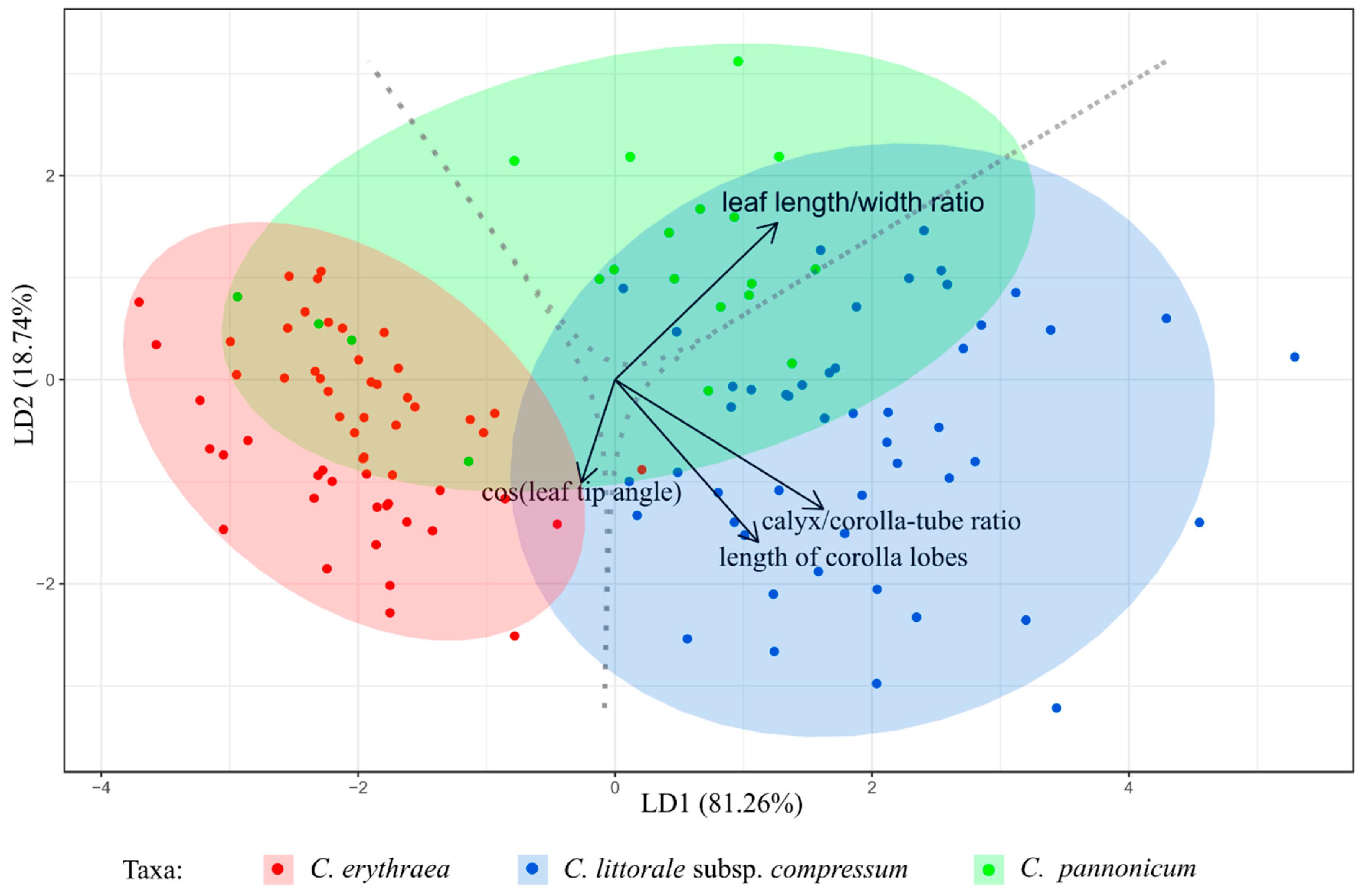
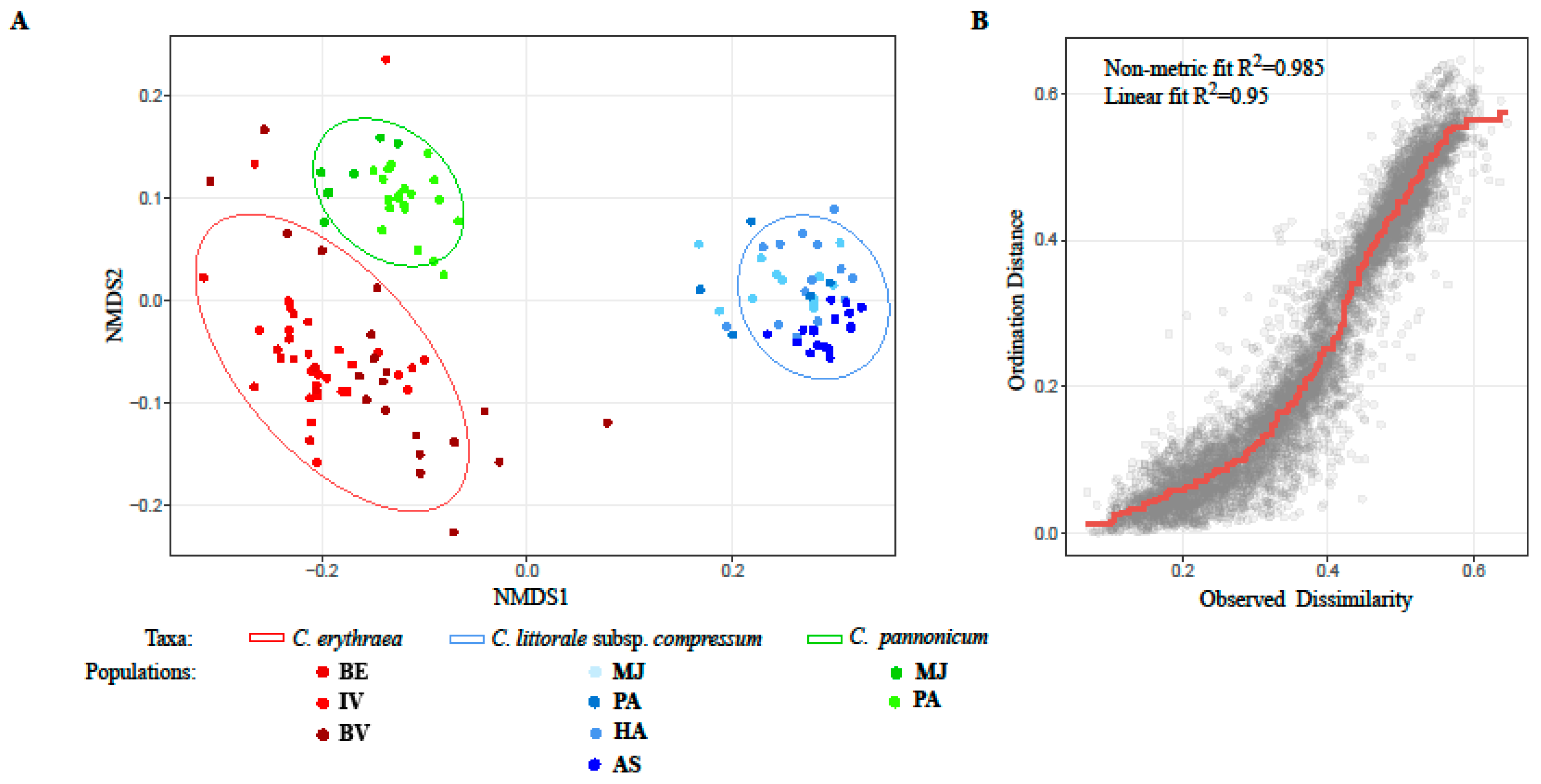
2.1. Variation of Morphological Parameters
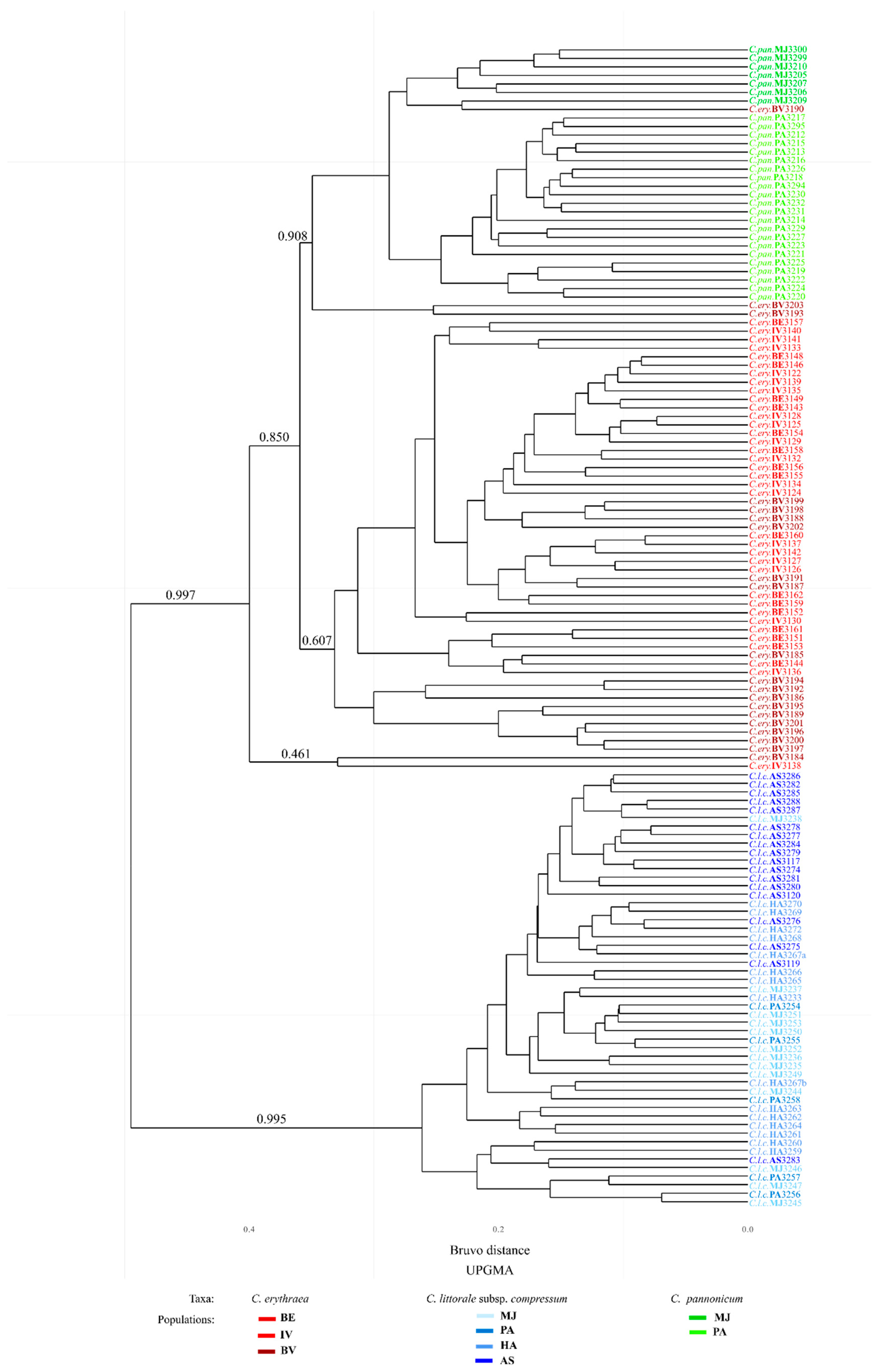
2.2. Genetic Diversity Estimation: Eight-Locus Microsatellite Analysis
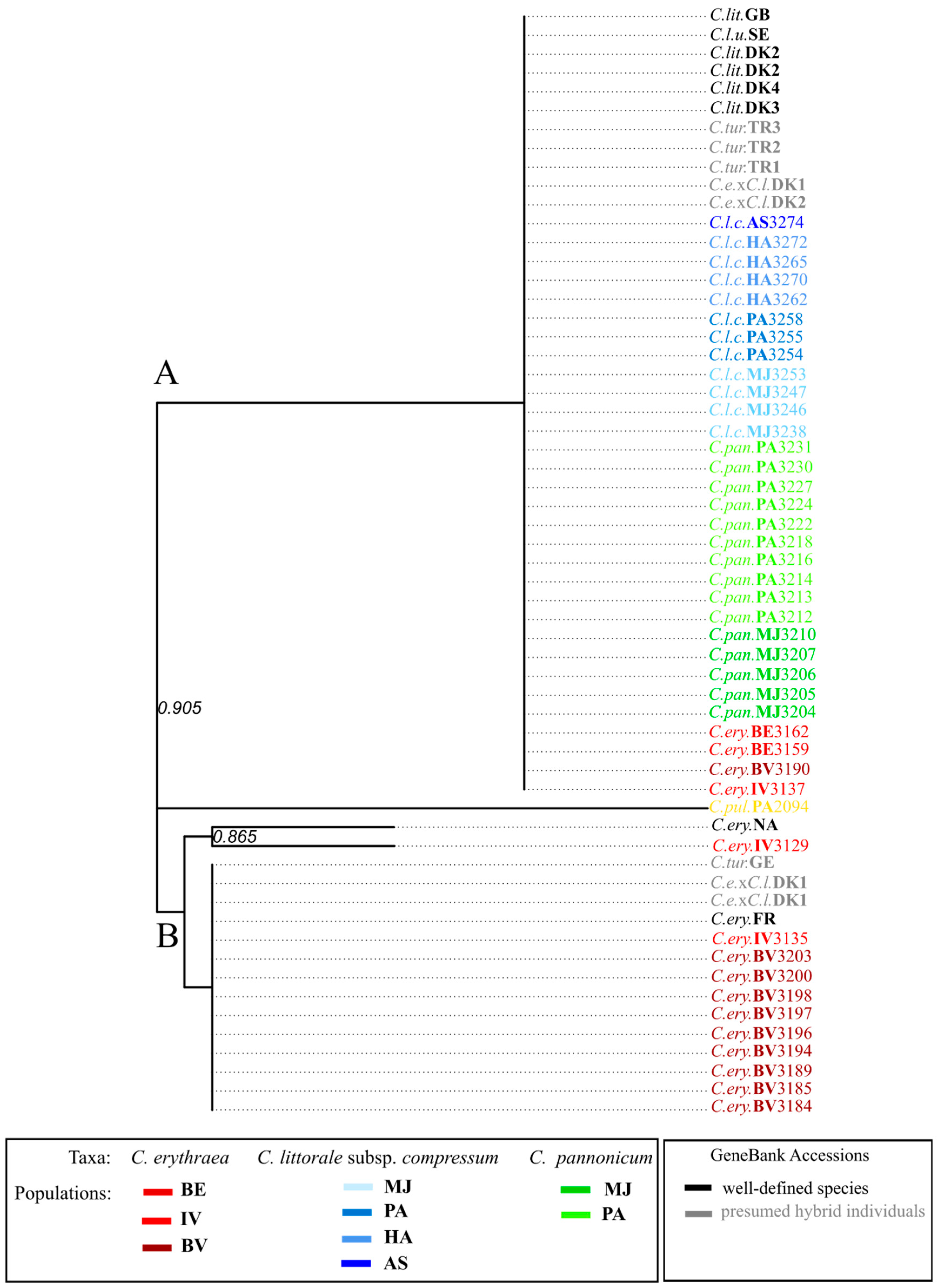
2.3. Variation in trnL-F Plastid Region
3. Discussion
3.1. About the Sample Set
3.2. Morphometrics in the Detection of Hybrids
3.3. Genetic Variation
3.4. Origin of Centaurium pannonicum
4. Materials and Methods
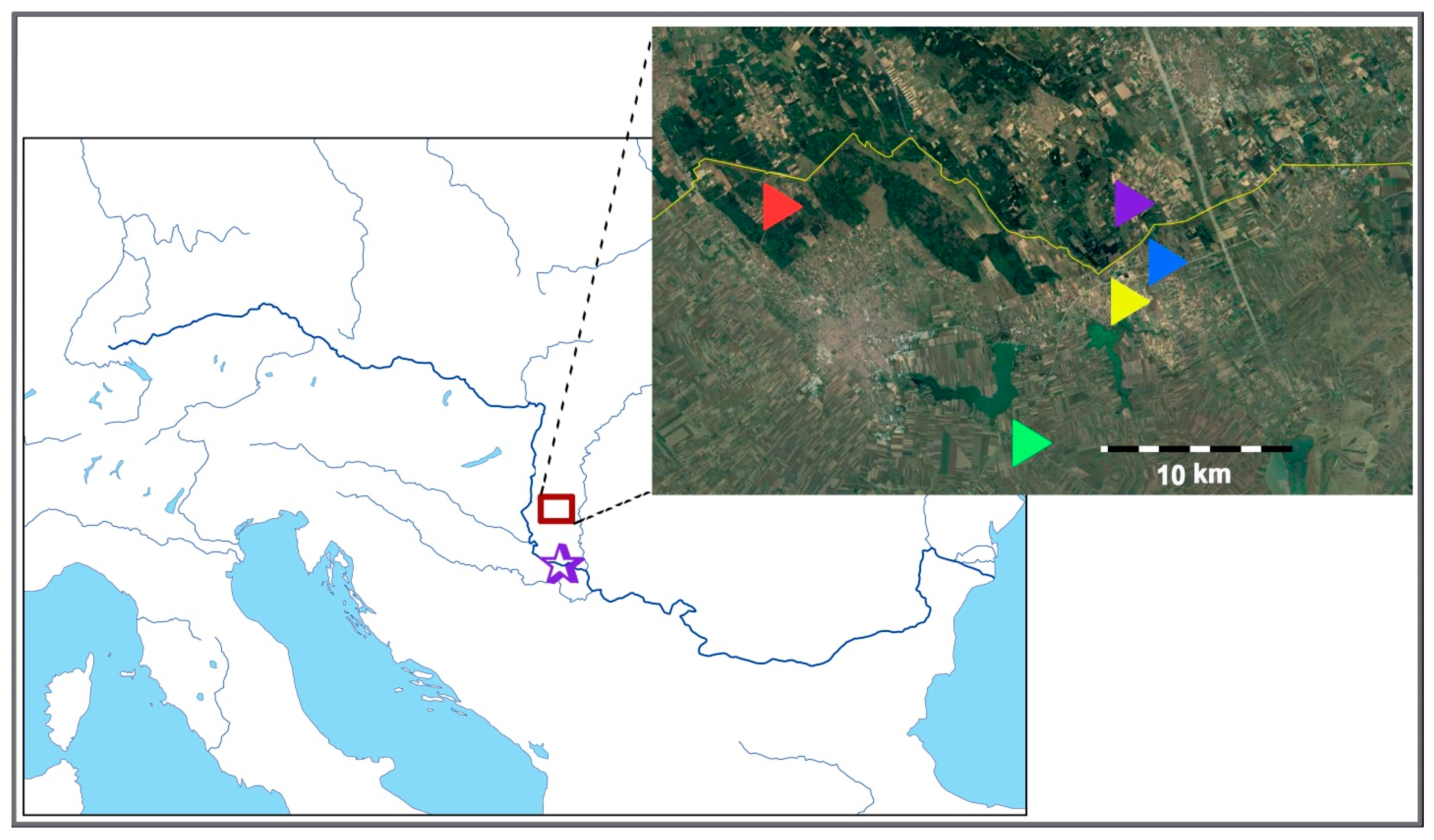
4.1. Sampling Sites
4.2. Morphometric Analysis
4.2.1. Plant Material
4.2.2. Analysis of Morphological Traits
4.2.3. Statistical Analysis of the Morphometric Data
4.3. EST-SSR Genotyping
4.3.1. Plant Material for the DNA Extraction
4.3.2. DNA Isolation and EST-SSR Amplification
4.3.3. Genetic Diversity of Populations Based on the EST-SSR Variation
4.3.4. Visualization of the Genetic Distances
4.4. trnL-F Haplotype Analysis
4.4.1. Sample Set
4.4.2. Plastid DNA Amplification and Marker Sequencing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Soltis, P.S.; Soltis, D.E. The role of hybridization in plant speciation. Annu. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef] [PubMed]
- Rieseberg, L.H.; Willis, J.H. Plant speciation. Science 2007, 317, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Barker, M.S.; Arrigo, N.; Baniaga, A.E.; Li, Z.; Levin, D.A. On the relative abundance of autopolyploids and allopolyploids. New Phytol. 2016, 210, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Mallet, J. Hybrid speciation. Nature 2007, 446, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization–diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Alix, K.; Gérard, P.R.; Schwarzacher, T.; Heslop-Harrison, J.S. Polyploidy and interspecific hybridization: Partners for adaptation; speciation and evolution in plants. Ann. Bot. 2017, 120, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Zeltner, L. Recherches de biosystématique sur les genres Blackstonia Huds. et Centaurium Hill (Gentianaceae). Bull. Soc. Neuch. Sci. Nat. 1970, 93, 1–164. [Google Scholar]
- Mansion, G.; Zeltner, L.; Bretagnolle, F. Phylogenetic Patterns and Polyploid Evolution within the Mediterranean Genus Centaurium (Gentianaceae–Chironieae). Taxon 2005, 54, 931–950. [Google Scholar] [CrossRef]
- Banjanac, T.; Šiler, B.; Skorić, M.; Ghalawenji, N.; Milutinović, M.; Božić, D.; Mišić, D. Interspecific in vitro hybridization in genus Centaurium and identification of hybrids via flow cytometry, RAPD, and secondary metabolite profiles. Turk. J. Bot. 2014, 38, 68–79. [Google Scholar] [CrossRef]
- Guggisberg, A.; Bretagnolle, F.; Mansion, G. Allopolyploid Origin of the Mediterranean Endemic, Centaurium bianoris (Gentianaceae), Inferred by Molecular Markers. Syst. Bot. 2006, 31, 368–379. [Google Scholar] [CrossRef]
- Ubsdell, R.A.E. Studies on variation and evolution in Centaurium erythraea Rafn and C. littorale (D. Turner) Gilmour in the British Isles 1. Taxonomy and biometrical studies. Watsonia 1976, 11, 7–31. [Google Scholar]
- Ubsdell, R.A.E. Studies on variation and evolution in Centaurium erythraea Rafn and C. littorale (D. Turner) Gilmour in the British Isles 2. Cytology. Watsonia 1976, 11, 33–43. [Google Scholar]
- Ubsdell, R.A.E. Studies on variation and evolution in Centaurium erythraea Rafn and C. littorale (D. Turner) Gilmour in the British Isles 3. Breeding systems, floral biology and general discussion. Watsonia 1979, 12, 225–232. [Google Scholar]
- Grisebach, A. Genera et Species Gentianearum Adjectis Observationibus Quibusdam Phytogeographicis; J. G. Cotta: Stuttgart, Germany, 1839. [Google Scholar]
- Gilg, E. Gentianaceae. In Die Natürlichen Pflanzenfamilien; Engler, A., Prantls, K., Eds.; Verlag von Wilhelm Engelmann: Leipzig, Germany, 1895; pp. 50–108. [Google Scholar]
- Melderis, A. Genetical and taxonomical studies in the genus Erythraea Rich. Acta Horti. Bot. Univ. Latv. 1931, 6, 123–256. [Google Scholar]
- Robyns, A. Essai d’étude systématique et écologique des Centaurium de Belgique. Bull. Jard. Bot. l’Etat Brux. 1954, 24, 349–398. [Google Scholar] [CrossRef]
- Hegi, G. Gentianaceae. In Illustrierte Flora von Mittel-Europa; Carl Hanser Verlag: Munich, Germany, 1966; pp. 1953–2047. [Google Scholar]
- Euro+Med Plantbase Project. Available online: http://ww2.bgbm.org/euroPlusMed/query.asp (accessed on 8 May 2019).
- Melderis, A. Taxonomic studies on the European species of the genus Centaurium Hill. Bot. J. Linn. Soc. 1972, 65, 224–250. [Google Scholar]
- Pringle, J.S. Proposal to reject the name Centaurium minus Moench (Gentianaceae). Taxon 1987, 36, 482–483. [Google Scholar] [CrossRef]
- Pringle, J.S. The identity and nomenclature of the Pacific North American species Zeltnera muhlenbergii (Gentianaceae) and its distinction from Centaurium tenuiflorum and other species with which it has been confused. Madroño 2010, 57, 184–202. [Google Scholar] [CrossRef]
- Anderson, E. Hybridization of the habitat. Evolution 1948, 2, 1–9. [Google Scholar] [CrossRef]
- Mansion, G. A new classification of the polyphyletic genus Centaurium Hill (Chironiinae; Gentianaceae): Description of the New World endemic Zeltnera, and reinstatement of Gyrandra Griseb. and Schenkia Griseb. Taxon 2004, 53, 719–740. [Google Scholar] [CrossRef]
- Mészáros, S.; De Laet, J.; Goethals, V.; Smets, E.; Nilsson, S. Cladistics of Gentianaceae: A morphological approach. In Gentianaceae—Systematics and Natural History; Struwe, L., Albert, V., Eds.; Cambridge University Press: London, UK, 2002; pp. 310–376. [Google Scholar]
- Nilsson, S. Gentianaceae: A review of palynology. In Gentianaceae—Systematics and Natural History; Struwe, L., Albert, V., Eds.; Cambridge University Press: London, UK, 2002; pp. 377–497. [Google Scholar]
- Jensen, S.R.; Schripsema, J. Chemotaxonomy and pharmacology of Gentianaceae. In Gentianaceae—Systematics and Natural History; Struwe, L., Albert, V., Eds.; Cambridge University Press: London, UK, 2002; pp. 573–631. [Google Scholar]
- Šiler, B.; Mišić, D.; Nestorović, J.; Banjanac, T.; Glamočlija, J.; Soković, M.; Ćirić, A. Antibacterial and Antifungal Screening of Centaurium pulchellum Crude Extracts and Main Secoiridoid Compounds. Nat. Prod. Commun. 2010, 5, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Šiler, B.; Avramov, S.; Banjanac, T.; Cvetković, J.; Nestorović Živković, J.; Patenković, A.; Mišić, D. Secoiridoid glycosides as a marker system in chemical variability estimation and chemotype assignment of Centaurium erythraea Rafn from the Balkan Peninsula. Ind. Crops Prod. 2012, 40, 336–344. [Google Scholar] [CrossRef]
- Šiler, B. Phytochemical and Molecular Characterization of Centaury (Centaurium erythraea Rafn) Populations from the Balkan Peninsula. Ph.D. Thesis, University of Belgrade, Begrade, Serbia, 5 June 2012. [Google Scholar]
- Šiler, B.; Živković, S.; Banjanac, T.; Cvetković, J.; Nestorović Živković, J.; Ćirić, A.; Soković, M.; Mišić, D. Centauries as underestimated food additives: Antioxidant and antimicrobial potential. Food Chem. 2014, 147, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Banjanac, T.; Dragićević, M.; Šiler, B.; Gašić, U.; Bohanec, B.; Nestorović Živković, J.; Trifunović, S.; Mišić, D. Chemodiversity of two closely related tetraploid Centaurium species and their hexaploid hybrid: Metabolomic search for high-resolution taxonomic classifiers. Phytochemistry 2017, 140, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.; Ray, S.; Roy, A. Molecular Markers in Phylogenetic Studies-A Review. J. Phylogenet. Evol. Biol. 2014, 2, 131. [Google Scholar] [CrossRef]
- Mansion, G.; Struwe, L. Generic delimitation and phylogenetic relationships within the subtribe Chironiinae (Chironieae: Gentianaceae), with special reference to Centaurium: Evidence from nrDNA and cpDNA sequences. Mol. Phylogenet. Evol. 2004, 32, 951–977. [Google Scholar] [CrossRef] [PubMed]
- Marhold, K. Gentianaceae. In Euro+ Med Plantbase—The Information Resource for Euro-Mediterranean Plant Diversity; 2011; Available online: http://ww2.bgbm.org/EuroPlusMed/PTaxonDetail.asp?NameCache=Gentianaceae&PTRefFk=7200000 (accessed on 13 July 2019).
- Banjanac, T.; Skorić, M.; Belamarić, M.; Nestorović Živković, J.; Mišić, D.; Jelić, M.; Dmitrović, S.; Šiler, B. Pursuit for EST microsatellites in a tetraploid model from de novo transcriptome sequencing. Genetika 2018, 50, 687–703. [Google Scholar] [CrossRef]
- Mansion, G. Twenty-first Century Centauries: An Updated Review on Centaurium Hill and Allies (Gentianaceae). In The Gentianaceae-Volume 1: Characterization and Ecology; Rybczyński, J.J., Davey, M.R., Mikula, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 231–250. [Google Scholar]
- Brys, R.; Jacquemyn, H. Variation in the functioning of autonomous self-pollination, pollinator services and floral traits in three Centaurium species. Ann. Bot. 2011, 107, 917–925. [Google Scholar] [CrossRef]
- Jovanović-Dunjić, R. Fam. Gentianaceae. In Flora SR Srbije; Srpska Akademija Nauka i Umetnosti: Belgrade, Yugoslavia, 1973; pp. 403–432. [Google Scholar]
- Dawson, D.A.; Horsburgh, G.J.; Küpper, C.; Stewart, I.R.K.; Ball, A.D.; Durrant, K.L.; Hansson, B.; Bacon, I.; Bird, S.; Klein, A.; et al. New methods to identify conserved microsatellite loci and develop primer sets of high cross-species utility—As demonstrated for birds. Mol. Ecol. Resour. 2010, 10, 475–494. [Google Scholar] [CrossRef]
- Dawson, D.A.; Ball, A.D.; Spurgin, L.G.; Martín-Gálvez, D.; Stewart, I.R.K.; Horsburgh, G.J.; Potter, J.; Molina-Morales, M.; Bicknell, A.W.J.; Preston, S.A.J.; et al. High-utility conserved avian microsatellite markers enable parentage and population studies across a wide range of species. BMC Genom. 2013, 14, 176. [Google Scholar] [CrossRef]
- Chassot, P.; Nemomissa, S.; Yuan, Y.-M.; Küpfer, P. High paraphyly of Swertia L. (Gentianaceae) in the Gentianella-lineage as revealed by nuclear and chloroplast DNA sequence variation. Plant Syst. Evol. 2001, 229, 1–21. [Google Scholar] [CrossRef][Green Version]
- Gielly, L.; Taberlet, P. A phylogeny of the European gentians inferred from chloroplast trnL (UAA) intron sequences. Bot. J. Linn. Soc. 1996, 120, 57–75. [Google Scholar] [CrossRef]
- Struwe, L.; Thiv, M.; Kadereit, J.W.; Pepper, A.S.R.; Motley, T.J.; White, P.J.; Rova, J.H.E.; Potgieter, K.; Albert, V.A. Saccifolium (Saccifoliaceae), an endemic of Sierra de la Neblina on the Brazilian-Venezuelan border, is related to a temperate-alpine lineage of Gentianaceae. Harv. Pap. Bot. 1998, 3, 199–214. [Google Scholar]
- Yuan, Y.-M.; Wohlhauser, S.; Möller, M.; Chassot, P.; Mansion, G.; Grant, J.; Küpfer, P.; Klackenberg, J. Monophyly and relationships of the tribe Exaceae (Gentianaceae) inferred from nuclear ribosomal and chloroplast DNA sequences. Mol. Phylogenet. Evol. 2003, 28, 500–517. [Google Scholar] [CrossRef][Green Version]
- Taberlet, P.; Gielly, L.; Pautou, G.; Bouvet, J. Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Mol. Biol. 1991, 17, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Kieleczawa, J. Fundamentals of sequencing of difficult templates—An overview. J. Biomol. Tech. 2006, 17, 207–217. [Google Scholar] [PubMed]
- Corriveau, J.L.; Coleman, A.W. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. Am. J. Bot. 1988, 75, 1443–1458. [Google Scholar] [CrossRef]
- Tsitrone, A.; Kirkpatrick, M.; Levin, D.A. A model for chloroplast capture. Evolution 2003, 57, 1776–1782. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity; evolution; and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef]
- Çiçek, M.; Yaprak, A.E.; Alan, A.R. Morphometric and flow cytometric evaluations of a putative natural hybrid of Centaurium (Gentianaceae) from Turkey. Phytotaxa 2015, 204, 22–32. [Google Scholar] [CrossRef]
- Zeltner, L. Recherches sur le Centaurium bianoris (Sennen) Sennen. Rev. Biol. Ecol. Médit. 1978, 5, 51–58. [Google Scholar]
- Soltis, D.E.; Kuzoff, R.K. Discordance between nuclear and chloroplast phylogenies in the Heuchera group (Saxifragaceae). Evolution 1995, 49, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.T.; Kenton, A.Y.; Bennett, M.D. Genomic in situ hybridization reveals the allopolyploid nature of Milium montianum (Gramineae). Chromosoma 1992, 101, 420–424. [Google Scholar] [CrossRef]
- Hegarty, M.J.; Hiscock, S.J. Hybrid speciation in plants: New insights from molecular studies. New Phytol. 2005, 165, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Kuhn, M.; Wing, J.; Weston, S.; Williams, C.A.; Keefer, C.; Engelhardt, A.; Cooper, T.; Mayer, Z.; Kenkel, B.; Benesty, M.; et al. ‘caret’: Classification and Regression Training. 2018. Available online: https://cran.r-project.org/web/packages/caret/caret.pdf (accessed on 13 July 2019).
- Horn, J.L. A rationale and test for the number of factors in factor analysis. Psychometrika 1965, 30, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Dinno, A. ‘paran’, Horn’s Test of Principal Components/Factors. R Project. 2018. Available online: https://cran.r-project.org/web/packages/paran/paran.pdf (accessed on 13 July 2019).
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from leaf tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Clark, L.V.; Jasieniuk, M. POLYSAT: An R package for polyploid microsatellite analysis. Mol. Ecol. Resour. 2011, 11, 562–566. [Google Scholar] [CrossRef] [PubMed]
- R Core Team: The R Project for Statistical Computing. 2018. Available online: https://www.r-project.org/ (accessed on 13 July 2019).
- Bruvo, R.; Michiels, N.K.; D’Souza, T.G.; Schulenburg, H. A simple method for the calculation of microsatellite genotype distances irrespective of ploidy level. Mol. Ecol. 2004, 13, 2101–2106. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. ‘vegan’: Community Ecology Package. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 13 July 2019).
- Hennig, C. ‘fpc’: Flexible Procedures for Clustering. 2019. Available online: https://cran.r-project.org/web/packages/fpc/fpc.pdf (accessed on 13 July 2019).
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]


| Populations | Number of Analyzed Individuals per Population | Total Number of Alleles in a Population (all 8 loci) | Average Number of Alleles for All Loci | Effective Number of Alleles in a Population | Average Expected Heterozygosity per Population (HE) | Average Observed Heterozygosity per Population (HO) |
|---|---|---|---|---|---|---|
| C.ery.BE | 17 | 29 | 3.6250 | 1.1400 | 0.8772 | 0.5588 |
| C.ery.IV | 19 | 31 | 3.8750 | 1.1326 | 0.8829 | 0.5658 |
| C.ery.BV | 20 | 34 | 4.2500 | 1.1198 | 0.8930 | 0.5938 |
| C.pan.MJ | 7 | 31 | 3.8750 | 1.0089 | 0.9912 | 0.7969 |
| C.pan.PA | 22 | 33 | 4.1250 | 1.0114 | 0.9888 | 0.7337 |
| C.l.c.MJ | 13 | 20 | 2.5000 | 1.1303 | 0.8847 | 0.6635 |
| C.l.c.PA | 5 | 20 | 2.5000 | 1.1482 | 0.8709 | 0.5750 |
| C.l.c.HA | 15 | 24 | 3.0000 | 1.0995 | 0.9095 | 0.7750 |
| C.l.c.AS | 18 | 24 | 3.0000 | 1.0982 | 0.9105 | 0.8125 |
| total | 136 | |||||
| average | 27.33 | 3.4167 | 1.0988 | 0.9121 | 0.6750 |
| Jost’s D | C.ery.IV | C.ery.BE | C.ery.BV | C.pan.MJ | C.pan.PA | C.l.c.HA | C.l.c.MJ | C.l.c.PA | C.l.c.AS | |
|---|---|---|---|---|---|---|---|---|---|---|
| FST | ||||||||||
| C.ery.IV | 0.015 | 0.055 | 0.106 | 0.113 | 0.200 | 0.241 | 0.173 | 0.210 | ||
| C.ery.BE | 0.019 | 0.063 | 0.111 | 0.108 | 0.200 | 0.240 | 0.180 | 0.208 | ||
| C.ery.BV | 0.126 | 0.141 | 0.072 | 0.096 | 0.144 | 0.174 | 0.114 | 0.158 | ||
| C.pan.MJ | 0.282 | 0.270 | 0.236 | 0.031 | 0.173 | 0.210 | 0.170 | 0.184 | ||
| C.pan.PA | 0.305 | 0.300 | 0.312 | 0.124 | 0.154 | 0.162 | 0.090 | 0.179 | ||
| C.l.c.HA | 0.526 | 0.497 | 0.433 | 0.553 | 0.514 | 0.033 | 0.024 | 0.013 | ||
| C.l.c.MJ | 0.553 | 0.509 | 0.437 | 0.547 | 0.501 | 0.054 | 0.007 | 0.032 | ||
| C.l.c.PA | 0.533 | 0.491 | 0.415 | 0.507 | 0.467 | 0.039 | -0.021 | 0.030 | ||
| C.l.c.AS | 0.552 | 0.519 | 0.466 | 0.609 | 0.573 | 0.019 | 0.065 | 0.077 | ||
| Taxon | Locality | Population Accession Code | Geographic Latitude and Longitude | Estimated Ploidy Level * | No. of Studied Individuals | ||
|---|---|---|---|---|---|---|---|
| EST-SSR | Morphometrics | Plastid DNA | |||||
| C. erythraea | Beočin | C.ery.BE | 45°10′35.24″ N 19°43′16.32″ E | 4x | 17 | 17 | 2 |
| C. erythraea | Iriški Venac | C.ery.IV | 45°09′27.65″ N 19°51′43.64″ E | 4x | 19 | 19 | 3 |
| C. erythraea | Bački Vinogradi | C.ery.BV | 46°07′29.19″ N 19°50′56.02″ E | 4x | 20 | 20 | 10 |
| C. littorale subsp. compressum | Majdan | C.l.c.MJ | 46°09′29.34″ N 19°36′20.53″ E | 4x | 13 | 13 | 4 |
| C. littorale subsp. compressum | Palić | C.l.c.PA | 46°03′08.04″ N 19°46′34.89″ E | 4x | 5 | 4 | 3 |
| C. littorale subsp. compressum | Hajdukovo | C.l.c.HA | 46°06′52.27″ N 19°49′53.58″ E | 4x | 15 | 15 | 4 |
| C. littorale subsp. compressum | Ásotthalom | C.l.c.AS | 46°11′04.50″ N 19°50′47.62″ E | 4x | 18 | 18 | 1 |
| C. pannonicum | Majdan | C.pan.MJ | 46°09′29.34″ N 19°36′20.53″ E | 6x | 7 | 5 | 5 |
| C. pannonicum | Palić | C.pan.PA | 46°03′08.04″ N 19°46′34.89″ E | 6x | 22 | 16 | 10 |
| C. pulchellum | Palić | C.pul.PA | 46°03′08.04″ N 19°46′34.89″ E | NA | / | / | 1 |
| in total | 136 | 127 | 43 | ||||
| Taxon | GenBank Accession No. | Accession Code Used in Manuscript | References | Ploidy Level | Country, Region, Locality |
|---|---|---|---|---|---|
| Centaurium erythraea | AY315697 | C.ery.NA | Mansion, G. and Zeltner, L.; Unpublished | NA | NA |
| Centaurium erythraea | AY879973 | C.ery.FR | [8] | 4x | France, Gard, Espiguette |
| C. erythraea × C. littorale | AY879975 | C.e.×C.l.DK1 | [8] | 4x | Denmark, Jutland, Sand Milen |
| C. erythraea × C. littorale | AY879976 | C.e.×C.l.DK2 | [8] | 4x | Denmark, Jutland, Skallingen |
| C. erythraea × C. littorale | AY879977 | C.e.×C.l.DK1 | [8] | 4x | Denmark, Jutland, Sand Milen |
| C. erythraea × C. littorale | AY879978 | C.e.×C.l.DK1 | [8] | 4x | Denmark, Jutland, Sand Milen |
| Centaurium littorale | AY879979 | C.lit.DK3 | [8] | 4x | Denmark, Jutland, Grenen |
| Centaurium littorale | AY879980 | C.lit.DK4 | [8] | 4x | Danemark, Lakolk, Romo Island |
| Centaurium littorale | AY879981 | C.lit.DK2 | [8] | 4x | Denmark, Jutland, Skallingen |
| Centaurium littorale | AY879982 | C.lit.DK2 | [8] | 4x | Denmark, Jutland, Skallingen |
| Centaurium littorale | AY251756 | C.lit.GB | [34] | 4x | England, Holy Island |
| Centaurium turcicum | AY880009 | C.tur.GE | [8] | 6x | Georgia, Agara |
| Centaurium turcicum | AY880010 | C.tur.TR1 | [8] | 6x | Turkey, Phrygie, Pamukkale |
| Centaurium turcicum | AY880011 | C.tur.TR2 | [8] | 6x | Turkey, Phrygie, Beysehir |
| Centaurium turcicum | AY880012 | C.tur.TR3 | [8] | 6x | Turkey, Pamphylie, Aksu Isparta |
| C. littorale subsp. uliginosum | AY880013 | C.l.u.SE | [8] | 4x | Sweden, Gotland, Herrvik |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banjanac, T.; Đurović, S.; Jelić, M.; Dragićević, M.; Mišić, D.; Skorić, M.; Nestorović Živković, J.; Šiler, B. Phenotypic and Genetic Variation of an Interspecific Centaurium Hybrid (Gentianaceae) and Its Parental Species. Plants 2019, 8, 224. https://doi.org/10.3390/plants8070224
Banjanac T, Đurović S, Jelić M, Dragićević M, Mišić D, Skorić M, Nestorović Živković J, Šiler B. Phenotypic and Genetic Variation of an Interspecific Centaurium Hybrid (Gentianaceae) and Its Parental Species. Plants. 2019; 8(7):224. https://doi.org/10.3390/plants8070224
Chicago/Turabian StyleBanjanac, Tijana, Sanja Đurović, Mihailo Jelić, Milan Dragićević, Danijela Mišić, Marijana Skorić, Jasmina Nestorović Živković, and Branislav Šiler. 2019. "Phenotypic and Genetic Variation of an Interspecific Centaurium Hybrid (Gentianaceae) and Its Parental Species" Plants 8, no. 7: 224. https://doi.org/10.3390/plants8070224
APA StyleBanjanac, T., Đurović, S., Jelić, M., Dragićević, M., Mišić, D., Skorić, M., Nestorović Živković, J., & Šiler, B. (2019). Phenotypic and Genetic Variation of an Interspecific Centaurium Hybrid (Gentianaceae) and Its Parental Species. Plants, 8(7), 224. https://doi.org/10.3390/plants8070224







