Assessment of Total Phenolic Content, In Vitro Antioxidant and Antibacterial Activity of Ruta graveolens L. Extracts Obtained by Choline Chloride Based Natural Deep Eutectic Solvents
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of NADESs
2.4. Extraction of Ruta graveolens L. with NADES and Experimental Design
2.5. Determination of Total Phenolics Content
2.6. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.7. Antibacterial Susceptibility Testing
2.7.1. Microorganisms and Growth Conditions
2.7.2. Microorganisms and Growth Conditions
2.8. Statistical Data Processing
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yazbek, P.B.; Tezoto, J.; Cassas, F.; Rodrigues, E. Plants used during maternity, menstrual cycle and other women’s health conditions among Brazilian cultures. J. Ethnopharmacol. 2016, 179, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Conway, G.A.; Slocumb, J.C. Plants used as abortifacients and emmenagogues by Spanish New Mexicans. J. Ethnopharmacol. 1979, 1, 241–261. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Khoshkam, R. Phytochemistry and pharmacological properties of Ruta graveolens L. J. Med. Plants Res. 2012, 6. [Google Scholar] [CrossRef]
- Pollio, A.; De Natale, A.; Appetiti, E.; Aliotta, G.; Touwaide, A. Continuity and change in the Mediterranean medical tradition: Ruta spp. (rutaceae) in Hippocratic medicine and present practices. J. Ethnopharmacol. 2008, 116, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Sallal, A.K.J.; Alkofahi, A. Inhibition of the haemolytic activities of snake and scorpion venoms in vitro with plant extracts. Biomed. Lett. 1996, 53, 211–215. [Google Scholar]
- Johne, S.; Härtling, S. Isolation of gamma-fagarin from Erythrochiton brasiliensis Nees et Mart. (Rutaceae). Pharmazie 1977, 32, 415. [Google Scholar] [PubMed]
- Wolters, B.; Eilert, U. Antimicrobial Substances in Callus Cultures of Ruta graveolens. Planta Med. 1981, 43, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Verzár-Petri, G.; Csedö, K.; Möllmann, K.; Szendrei, K.; Reisch, J. Fluoreszenzmikroskopische untersuchungen über die lokalisierung von acridon-alkaloiden in geweben von Ruta graveolens. Planta Med. 1976, 29, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Ratheesh, M.; Sindhu, G.; Helen, A. Anti-inflammatory effect of quinoline alkaloid skimmianine isolated from Ruta graveolens L. Inflamm. Res. 2013, 62, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Haesen, J.P.; Vörding, J.G.; Kho, K.F. Isolation and identification of xanthotoxin from the underground parts of Ruta graveolens. Planta Med. 1971, 19, 285–289. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, B.-H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Bown, D. Encyclopaedia of Herbs and Their Uses; Dorling Kindersley: London, UK, 1995; ISBN 978-0-7894-8031-6. [Google Scholar]
- Eickhorst, K.; DeLeo, V.; Csaposs, J. Rue the herb: Ruta graveolens—Associated phytophototoxicity. Dermatitis 2007, 18, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, I.; Bettaieb Rebey, I.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Saidani Tounsi, M. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.; Mikhova, B.; Najdenski, H.; Tsvetkova, I.; Kostova, I. Antimicrobial and cytotoxic activity of Ruta graveolens. Fitoterapia 2005, 76, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Novák, I.; Buzás, G.; Minker, E.; Koltai, M.; Szendrei, K. Isolation of some effective substance from the herb of Ruta graveolens L. Acta Pharm. Hung. 1967, 37, 130–141. [Google Scholar] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; van den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem. Int. Ed. Engl. 2013, 52, 3074–3085. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- García, A.; Rodríguez-Juan, E.; Rodríguez-Gutiérrez, G.; Rios, J.J.; Fernández-Bolaños, J. Extraction of phenolic compounds from virgin olive oil by deep eutectic solvents (DESs). Food Chem. 2016, 197, 554–561. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; van Spronsen, J.; Kroon, M.C.; Gallucci, F.; van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Choi, Y.H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Verpoorte, R. Are natural deep eutectic solvents the missing link in understanding cellular metabolism and physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef]
- Molnar, M.; Jakovljević, M.; Jokić, S. Optimization of the Process Conditions for the Extraction of Rutin from Ruta graveolens L. by Choline Chloride Based Deep Eutectic Solvents. SERDJ 2018, 25, 109–116. [Google Scholar] [CrossRef]
- Zhao, B.-Y.; Xu, P.; Yang, F.-X.; Wu, H.; Zong, M.-H.; Lou, W.-Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Baş, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Shih, M.-H.; Su, Y.-S.; Wu, C.-L. Syntheses of Aromatic Substituted Hydrazino-thiazole Derivatives to Clarify Structural Characterization and Antioxidant Activity between 3-Arylsydnonyl and Aryl Substituted Hydrazino-thiazoles. Chem. Pharm. Bull. 2007, 55, 1126–1135. [Google Scholar] [CrossRef]
- Gu, W.; Wang, S. Synthesis and antimicrobial activities of novel 1H-dibenzo[a,c]carbazoles from dehydroabietic acid. Eur. J. Med. Chem 2010, 45, 4692–4696. [Google Scholar] [CrossRef]
- Molnar, M.; Pavić, V.; Šarkanj, B.; Čačić, M.; Vuković, D.; Klenkar, J. Mono-and bis-dipicolinic acid heterocyclic derivatives–thiosemicarbazides, triazoles, oxadiazoles and thiazolidinones as antifungal and antioxidant agents. Heterocycl. Commun. 2017, 23, 35–42. [Google Scholar] [CrossRef]
- Popescu, A.-M.; Constantin, V. Synthesis, characterization and thermophysical properties of three neoteric solvents-ionic liquids based on choline chloride. Chem. Res. Chin. Univ. 2014, 30, 119–124. [Google Scholar] [CrossRef]
- Masuda, T.; Inaba, Y.; Maekawa, T.; Takeda, Y.; Tamura, H.; Yamaguchi, H. Recovery mechanism of the antioxidant activity from carnosic acid quinone, an oxidized sage and rosemary antioxidant. J. Agric. Food Chem. 2002, 50, 5863–5869. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.-J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Benazir, J.F. Phytochemical profiling, antimicrobial and cytotoxicity studies of methanolic extracts from Ruta graveolens. J. Pharm. Res. 2011, 44, 1407–1409. [Google Scholar]
- Arunkumar, S.; Muthuselvam, M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. WJAS 2009, 5, 572–576. [Google Scholar]
- Sousa, E.T.; da Sorryilva, M.M.; de Andrade, S.J.; Cardoso, M.P.; Silva, L.A.; de Andrade, J.B. Evaluation of thermal stability of quinones by thermal analysis techniques. Thermochim. Acta 2012, 529, 1–5. [Google Scholar] [CrossRef]
- Vázquez, M.F.B.; Comini, L.R.; Milanesio, J.M.; Montoya, S.C.N.; Cabrera, J.L.; Bottini, S.; Martini, R.E. Pressurized hot water extraction of anthraquinones from Heterophyllaea pustulata Hook f. (Rubiaceae). J. Supercrit. Fluids 2015, 101, 170–175. [Google Scholar] [CrossRef]
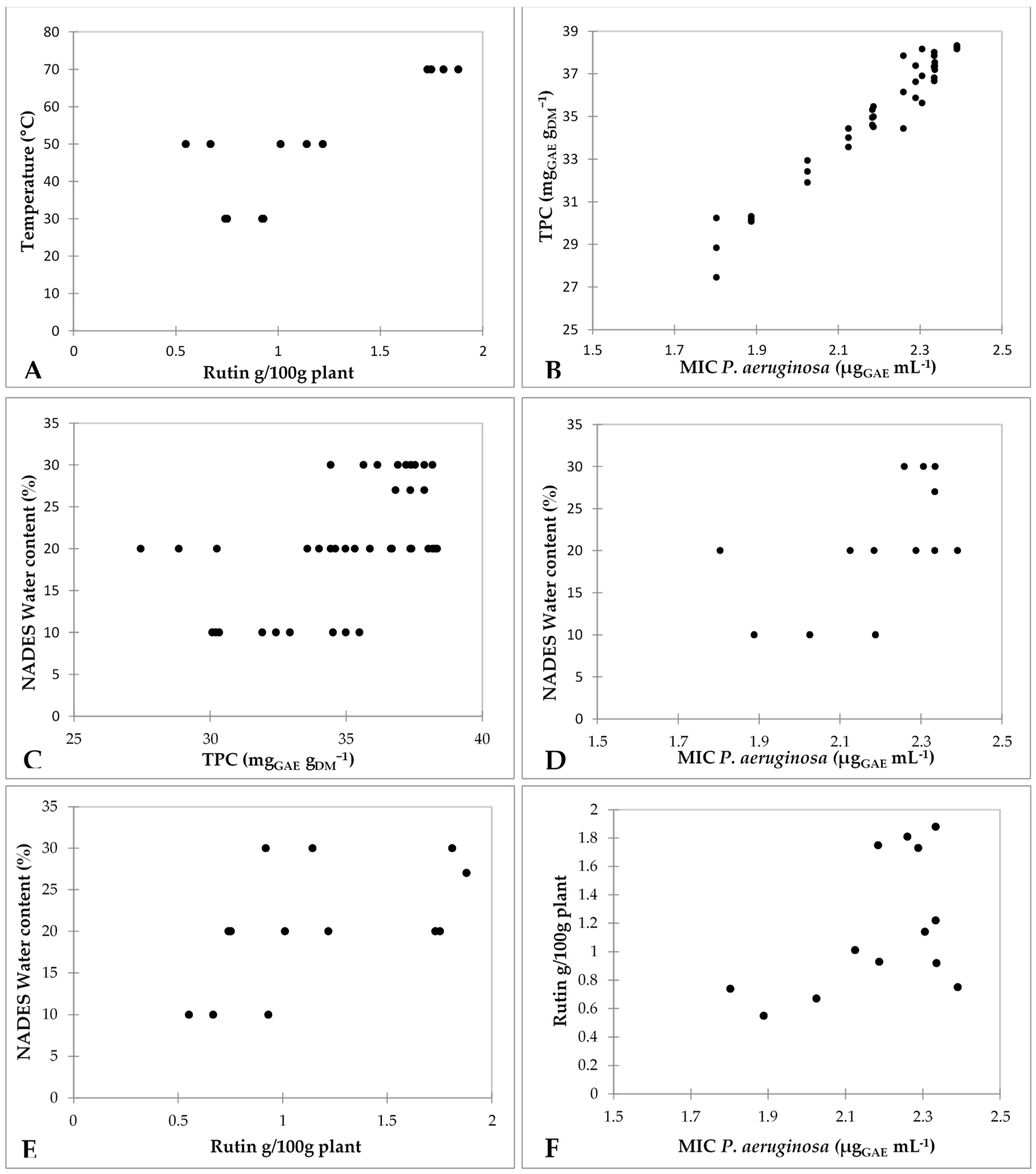
| Run | NADES Water Content (%) * | Time (min) * | Temp. (°C) * | Rutin g/100g Plant * | TPC (mgGAE gDM−1) | DPPH Radical Scavenging Activity (%) |
|---|---|---|---|---|---|---|
| 1 | 10 | 30 | 50 | 0.55 | 30.19 ± 0.16 | 67.59 ± 0.98 |
| 2 | 20 | 60 | 50 | 1.01 | 34.02 ± 0.61 | 65.79 ± 0.15 |
| 3 | 20 | 30 | 70 | 1.75 | 34.95 ± 0.51 | 65.61 ± 0.15 |
| 4 | 20 | 60 | 50 | 1.19 | ND | ND |
| 5 | 20 | 30 | 30 | 0.74 | 28.84 ± 1.96 | 66.25 ± 0.15 |
| 6 | 10 | 60 | 30 | 0.93 | 34.99 ± 0.68 | 57.63 ± 0.363 |
| 7 | 20 | 60 | 50 | 1.08 | ND | ND |
| 8 | 30 | 60 | 30 | 0.92 | 37.36 ± 0.24 | 70.91 ± 0.31 |
| 9 | 30 | 60 | 70 | 1.81 | 36.14 ± 2.41 | 68.04 ± 0.47 |
| 10 | 20 | 90 | 30 | 0.75 | 38.24 ± 0.11 | 72.53 ± 0.31 |
| 11 | 20 | 60 | 50 | 1.22 | 37.33 ± 0.95 | 70.02 ± 0.26 |
| 12 | 10 | 90 | 50 | 0.67 | 32.42 ± 0.73 | 62.29 ± 0.05 |
| 13 | 20 | 90 | 70 | 1.73 | 36.62 ± 1.06 | 68.31 ± 0.11 |
| 14 | 30 | 90 | 50 | 1.67 | ND | ND |
| 15 | 10 | 60 | 70 | 1.07 | ND | ND |
| 16 | 30 | 30 | 50 | 1.14 | 36.89 ± 1.79 | 64.09 ± 0.15 |
| 17 | 27 | 52 | 70 | 1.88 | 37.33 ± 0.72 | 57.54 ± 0.15 |
| Run | Minimum Inhibitory Concentration | |||||||
|---|---|---|---|---|---|---|---|---|
| E. coli | P. aeruginosa | B. subtilis | S. aureus | |||||
| μgGAE mL−1 | μg mL−1 | μgGAE mL−1 | μg mL−1 | μgGAE mL−1 | μg mL−1 | μgGAE mL−1 | μg mL−1 | |
| 1 | 3.77 | 125 | 1.89 | 62.5 | 3.77 | 125 | 3.77 | 125 |
| 2 | 4.25 | 125 | 2.13 | 62.5 | 4.25 | 125 | 4.25 | 125 |
| 3 | 4.37 | 125 | 2.18 | 62.5 | 4.37 | 125 | 4.37 | 125 |
| 4 | ND | ND | ND | ND | ND | ND | ND | ND |
| 5 | 3.61 | 125 | 1.80 | 62.5 | 3.61 | 125 | 3.61 | 125 |
| 6 | 4.37 | 125 | 2.19 | 62.5 | 4.37 | 125 | 4.37 | 125 |
| 7 | ND | ND | ND | ND | ND | ND | ND | ND |
| 8 | 4.67 | 125 | 2.34 | 62.5 | 4.67 | 125 | 4.67 | 125 |
| 9 | 4.52 | 125 | 2.26 | 62.5 | 4.52 | 125 | 4.52 | 125 |
| 10 | 4.78 | 125 | 2.39 | 62.5 | 4.78 | 125 | 4.78 | 125 |
| 11 | 4.67 | 125 | 2.33 | 62.5 | 4.67 | 125 | 4.67 | 125 |
| 12 | 4.05 | 125 | 2.03 | 62.5 | 4.05 | 125 | 4.05 | 125 |
| 13 | 4.58 | 125 | 2.29 | 62.5 | 4.58 | 125 | 4.58 | 125 |
| 14 | ND | ND | ND | ND | ND | ND | ND | ND |
| 15 | ND | ND | ND | ND | ND | ND | ND | ND |
| 16 | 4.61 | 125 | 2.31 | 62.5 | 4.61 | 125 | 4.61 | 125 |
| 17 | 4.67 | 125 | 2.33 | 62.5 | 4.67 | 125 | 4.67 | 125 |
| G | ND | 0.976 | ND | 0.976 | ND | 1.95 | ND | 3.91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavić, V.; Flačer, D.; Jakovljević, M.; Molnar, M.; Jokić, S. Assessment of Total Phenolic Content, In Vitro Antioxidant and Antibacterial Activity of Ruta graveolens L. Extracts Obtained by Choline Chloride Based Natural Deep Eutectic Solvents. Plants 2019, 8, 69. https://doi.org/10.3390/plants8030069
Pavić V, Flačer D, Jakovljević M, Molnar M, Jokić S. Assessment of Total Phenolic Content, In Vitro Antioxidant and Antibacterial Activity of Ruta graveolens L. Extracts Obtained by Choline Chloride Based Natural Deep Eutectic Solvents. Plants. 2019; 8(3):69. https://doi.org/10.3390/plants8030069
Chicago/Turabian StylePavić, Valentina, Dora Flačer, Martina Jakovljević, Maja Molnar, and Stela Jokić. 2019. "Assessment of Total Phenolic Content, In Vitro Antioxidant and Antibacterial Activity of Ruta graveolens L. Extracts Obtained by Choline Chloride Based Natural Deep Eutectic Solvents" Plants 8, no. 3: 69. https://doi.org/10.3390/plants8030069
APA StylePavić, V., Flačer, D., Jakovljević, M., Molnar, M., & Jokić, S. (2019). Assessment of Total Phenolic Content, In Vitro Antioxidant and Antibacterial Activity of Ruta graveolens L. Extracts Obtained by Choline Chloride Based Natural Deep Eutectic Solvents. Plants, 8(3), 69. https://doi.org/10.3390/plants8030069








