Effects of Growth Regulators and Gelling Agents on Ex Vitro Rooting of Raspberry
Abstract
1. Introduction
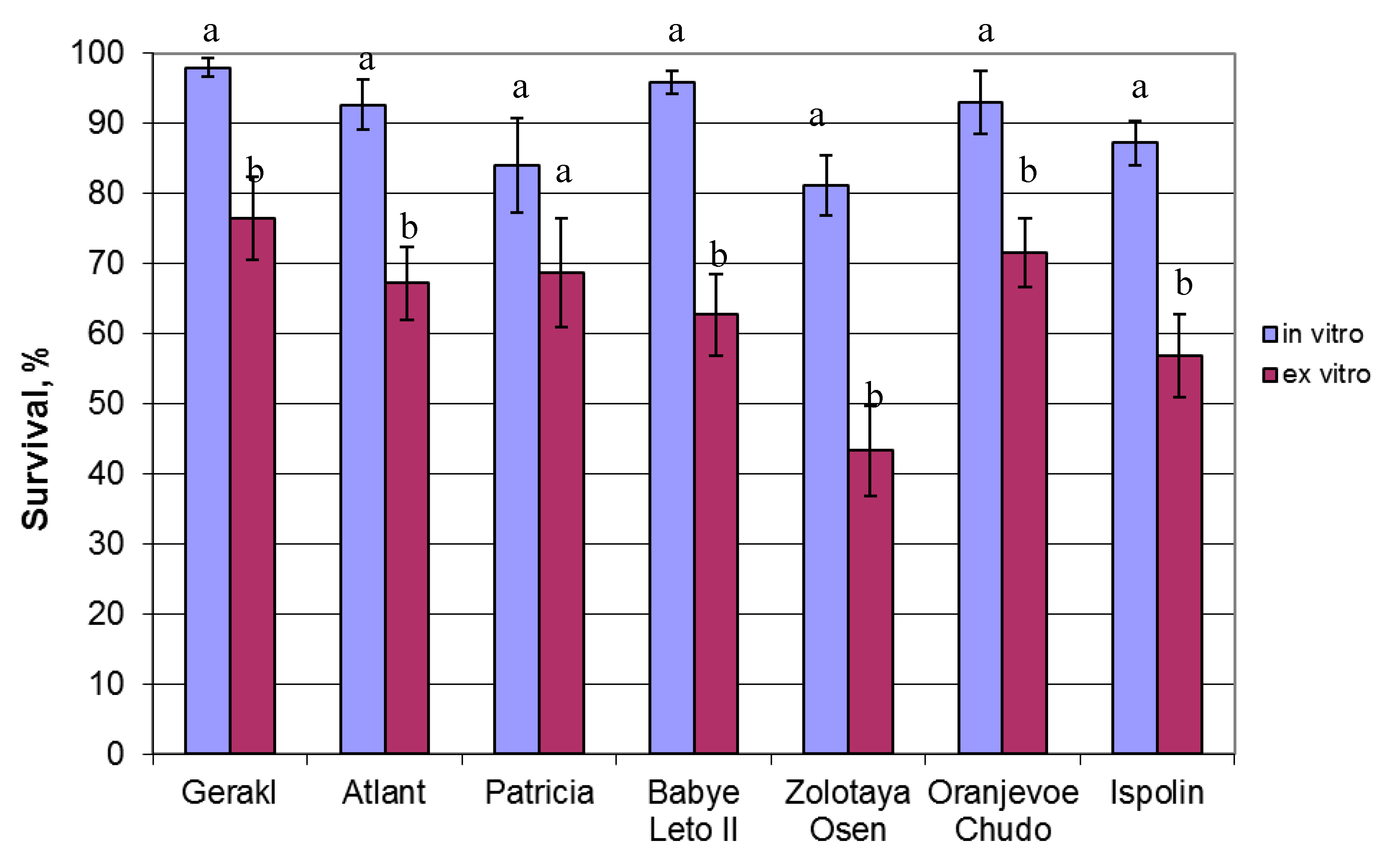
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Culture Conditions
4.2. Acclimatization
4.3. Ex Vitro Rooting
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.; Dossett, M.; Finn, C.E. Rubus fruit phenolic research: The food, the bad, and the confusing. Food Chem. 2012, 130, 785–796. [Google Scholar] [CrossRef]
- Dziedzic, E.; Jagła, J. Micropropagation of Rubus and Ribes spp. In Protocols for Micropropagation of Selected Economically Important Horticultural Plants; Lambardi, M., Ozudogru, E.A., Jain, S.M., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 149–160. ISBN 978-1-62703-073-1. [Google Scholar]
- Martin, R.R. Virus diseases of Rubus and strategies for their control. Acta Hortic. 2002, 585, 265–270. [Google Scholar] [CrossRef]
- Bite, A.; Petrevica, L. The influence of in vitro propagation on the field behaviour of red raspberry variety ‘Norna’. Acta Hortic. 2002, 585, 615–619. [Google Scholar] [CrossRef]
- Georgieva, M.; Kondakova, V.; Dragoyski, K.; Georgiev, D.; Naydenova, G. Comparative study of raspberry cv. Balgarski Rubin propagated by classical and in vitro methods. J. Pomol. 2009, 43, 81–86. [Google Scholar]
- Vujović, T.; Ružić, D.; Cerović, R.; Leposavić, A.; Karaklajić-Stajić, Z.; Mitrović, O.; Žurawicz, E. An assessment of the genetic integrity of micropropagated raspberry and blackberry plants. Sci. Hortic. 2017, 225, 454–461. [Google Scholar] [CrossRef]
- Snir, I. Red Raspberry (Rubus idaeus). In Crops II. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 6, pp. 124–141. ISBN 978-3642735226. [Google Scholar]
- Martinussen, I.; Nilsen, G.; Svenson, L.; Junttila, O.; Rapp, K. In vitro propagation of cloudberry (Rubus chamaemorus). Plant Cell Tissue Organ Cult. 2004, 78, 43–49. [Google Scholar] [CrossRef]
- Najaf-Abadi, A.; Jafari Hamidoghli, Y. Micropropagation of thornless trailing blackberry (Rubus sp.) by axillary bud explants. Aust. J. Crop Sci. 2009, 3, 191–194. [Google Scholar]
- Ismaini, L.; Destri Surya, M.I. Micropropagation of Rubus chrysophyllus Reinw. ex Miq. and Rubus fraxinifolius Poir. J. Trop. Life Sci. 2017, 7, 72–76. [Google Scholar] [CrossRef]
- Wu, J.H.; Miller, S.A.; Hall, H.K.; Mooney, P.A. Factors affecting the efficiency of micropropagation from lateral buds and shoot tips of Rubus. Plant Cell Tissus Organ Cult. 2009, 99, 17–25. [Google Scholar] [CrossRef]
- Gonzales, M.V.; Lopez, M.; Valdes, A.E.; Ordas, R.J. Micropropagation of three berry fruit species using nodal segments from field—Grown plants. Ann. Appl. Biol. 2000, 137, 73–78. [Google Scholar] [CrossRef]
- Hunkova, J.; Libiakova, G.; Gaidosova, A. Shoot proliferation ability of selected cultivars of Rubus spp. as influenced by genotype and cytokinin concentration. J. Cent. Eur. Agric. 2016, 17, 379–390. [Google Scholar] [CrossRef]
- Poothong, S.; Reed, B.M. Modeling the effects of mineral nutrition for improving growth and development of micropropagated red raspberries. Sci. Hortic. 2014, 165, 132–141. [Google Scholar] [CrossRef]
- Poothong, S.; Morré, J.; Maier, C.S.; Reed, B.M. Metabolic changes and improved growth in micropropagated red raspberry “Indian summer” are tied to improved mineral nutrition. In Vitro Cell. Dev. Biol. Plant 2017, 53, 579–590. [Google Scholar] [CrossRef]
- Clapa, D.; Fira, A.; Joshee, N. An efficient ex vitro rooting and acclimatization method for horticultural plants using float hydroculture. Hortscience 2013, 48, 1159–1167. [Google Scholar]
- Wozny, A.; Miler, N. LEDs application in ex vitro rooting and acclimatization of chrysanthemum (Chrysanthemum x grandiflorum/Ramat./Kitam). Electron. J. Pol. Agric. Univ. 2016, 19, 1–8. [Google Scholar]
- Singh, A.; Agarwal, P.K. Enhanced micropropagation protocol of ex vitro rooting of a commercially important crop plant Simmondsia chinensis (Link) Schneider. J. For. Sci. 2016, 62, 107–115. [Google Scholar] [CrossRef]
- Benmahioul, B.; Dorion, N.; Kaid-Harche, M.; Daguin, F. Micropropagation and ex vitro rooting of pistachio (Pistacia vera L.). Plant Cell Tissue Organ Cult. 2012, 108, 353–358. [Google Scholar] [CrossRef]
- Aygun, A.; Dumanoglu, H. In vitro shoot proliferation and in vitro and ex vitro root formation of Pyrus elaeagrifolia Pallas. Front. Plant Sci. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Shekafandeh, A.; Shahcheraghi, S.T. Ex vitro rooting and survival of regenerated shoots from three fig (Ficus carica L.) genotypes. Agric. Conspec. Sci. 2017, 82, 383–387. [Google Scholar]
- Clapa, D.; Fira, A.; Simu, M. The role of rooting substrate in blackberry ex vitro rooting and acclimatization stage. ProEnviron. Promediu 2015, 8, 280–284. [Google Scholar]
- Leva, A. Innovative protocol for ‘ex vitro rooting’ on olive micropropagation. Cent. Eur. J. Biol. 2011, 6, 352–358. [Google Scholar] [CrossRef]
- Hung, C.D.; Hong, C.H.; Kim, S.K.; Lee, K.H.; Park, J.Y.; Nam, M.W.; Choi, D.H.; Lee, H.I. LED light for in vitro and ex vitro efficient growth of economically important highbush blueberry (Vaccinium corymbosum L.). Acta Physiol. Plant. 2016, 38, 152. [Google Scholar] [CrossRef]
- Bohra, P.; Waman, A.A.; Sathyanarayana, B.N.; Umesha, K. Concurrent ex vitro rooting and hardening in Ney Poovan Banana (Musa AB): Effect of carbon sources and their concentrations. Erwerbs-Obstbau 2016, 58, 193–198. [Google Scholar] [CrossRef]
- Lebedev, V.; Shestibratov, K. Large-scale micropropagation of common ash. Biotechnology 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Kumar, K.; Rao, U. Morphophysiological problems in acclimatization of micropropagated plants in—ex vitro conditions—A reviews. J. Ornam. Hortic. Plants 2012, 2, 271–283. [Google Scholar]
- Lebedev, V.G.; Azarova, A.B.; Arkaev, M.S.; Nevskii, S.A.; Shestibratov, K.A. Effective mass propagation of various Betula species via in vitro culturing. Biotekhnologiya 2017, 33, 76–88. (In Russian) [Google Scholar] [CrossRef]
- Isac, V.; Popescu, A. Protocol for in vitro micropropagation of raspberry and plant regeneration by organogenesis. In A Guide to Some In Vitro Techniques—Small Fruits; Mezzetti, B., Ružić, Đ., Gajdosova, A., Eds.; COST Action 863: Brussels, Belgium, 2009; pp. 14–23. ISBN 978-86-910245-3-6. [Google Scholar]
- Sharma, U.; Kataria, V.; Shekhawat, N.S. In vitro propagation, ex vitro rooting and leaf micromorphology of Bauhinia racemosa Lam.: A leguminous tree with medicinal values. Physiol. Mol. Biol. Plants 2017, 23, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, M.S.; Manokari, M. In vitro multiplication, micromorphological studies and ex vitro rooting of Hybanthus enneaspermus (L.) F. Muell.—A rare medicinal plant. Acta Bot. Croat. 2018, 77, 80–87. [Google Scholar] [CrossRef]
- Ranaweera, K.K.; Gunasekara, M.T.K.; Eeswara, J.P. Ex vitro rooting: A low cost micropropagation technique for tea (Camellia sinensis (L.) O. Kuntz hybrids. Sci. Hortic. 2013, 155, 8–14. [Google Scholar] [CrossRef]
- Sisunandar, A.; Husin, A.; Julianto, T.; Yuniaty, A.; Rival, A.; Adkins, S. Ex vitro rooting using a mini growth chamber increases root induction and accelerates acclimatization of Kopyor coconut (Cocos nucifera L.) embryo culture-derived seedlings. In Vitro Cell. Dev. Biol. Plant 2018, 54, 508–517. [Google Scholar] [CrossRef]
- Hummer, K.; Hall, H.K. Raspberries. In Raspberries. Crop Production Science in Horticulture; Funt, R.C., Hall, H.K., Eds.; CAB International: Oxfordshire, UK, 2013; Volume 23, pp. 1–19. ISBN 978-1845937911. [Google Scholar]
- Valero-Aracama, C.; Kane, M.E.; Wilson, S.B.; Philman, N.L. Substitution of benzyladenine with meta-topolin during shoot multiplication increases acclimatization of difficult- and easy-to-acclimatize sea oats (Uniola paniculata L.) genotypes. Plant Growth Regul. 2010, 60, 43–49. [Google Scholar] [CrossRef]
- Lu, C.-Y. The use of thidiazuron in tissue culture. In Vitro Cell. Dev. Biol. Plant 1993, 29, 92–96. [Google Scholar] [CrossRef]
- Clapa, D.; Fira, A.; Pacurar, I. The in vitro propagation of the raspberry cultivar Citria. Bull. UASMV 2008, 65, 99–103. [Google Scholar]
- Thorpe, T.A.; Stasolla, C.; Yeung, E.C.; de Klerk, G.-J.; Roberts, A.; George, E.F. The components of plant tissue culture media II: Organic additions, osmotic and pH effects, and support systems. In Plant Propagation by Tissue Culture, 3rd ed.; George, E.F., Hall, M.A., de Klerk, G.-J., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 1, pp. 115–173. ISBN 978-1-4020-5004-6. [Google Scholar]
- Zawadzka, M.; Orlikowska, T. The influence of FeEDDHA in red raspberry cultures during shoot multiplication and adventitious regeneration from leaf explants. Plant Cell Tissue Organ Cult. 2006, 85, 145–149. [Google Scholar] [CrossRef]
- Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Bhojwani, S.S., Dantu, P.K., Eds.; Springer: New Delhi, India, 2013; pp. 245–274. ISBN 978-81-322-1025-2. [Google Scholar]
- Ismail, H.; Kumar, S.M.; Aziah, M.Y.; Hasnida, N.H.; Nor Aini, A.S. In vitro micropropagation of Acacia auriculiformis from selected juvenile sources. Dendrobiology 2016, 75, 157–165. [Google Scholar] [CrossRef]
- Tsao, C.W.V.; Reed, B.M. Gelling agents, silver nitrate, and sequestrene iron influence adventitious shoot and callus formation from Rubus leaves. In Vitro Cell. Dev. Biol. Plant 2002, 38, 29–32. [Google Scholar] [CrossRef]
- Kumar, A.; Palni, L.M.S. The effect of light source and gelling agent on micropropagation of Rosa damascena Mill. and Rhynchostylis retusa (L.) Bl. J. Hortic. Sci. Biotechnol. 2003, 78, 786–792. [Google Scholar] [CrossRef]
- Dries, N.V.; Gianni, S.; Czerednik, A.; Krens, FA.; Klerk, G.M. Flooding of the apoplast is a key factor in the development of hyperhydricity. J. Exp. Bot. 2013, 64, 5221–5230. [Google Scholar] [CrossRef]
- Christensen, B.; Sriskandarajah, S.; Serek, M.; Muller, R. In vitro culture of Hibiscus rosa-sinensis L.: Influence of iron, calcium and BAP on establishment and multiplication. Plant Cell Tissue Organ Cult. 2008, 93, 151–161. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay of tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Quoirin, M.; Lepoivre, P. Improved media for in vitro culture of Prunus species. Acta Hortic. 1977, 78, 437–442. [Google Scholar] [CrossRef]
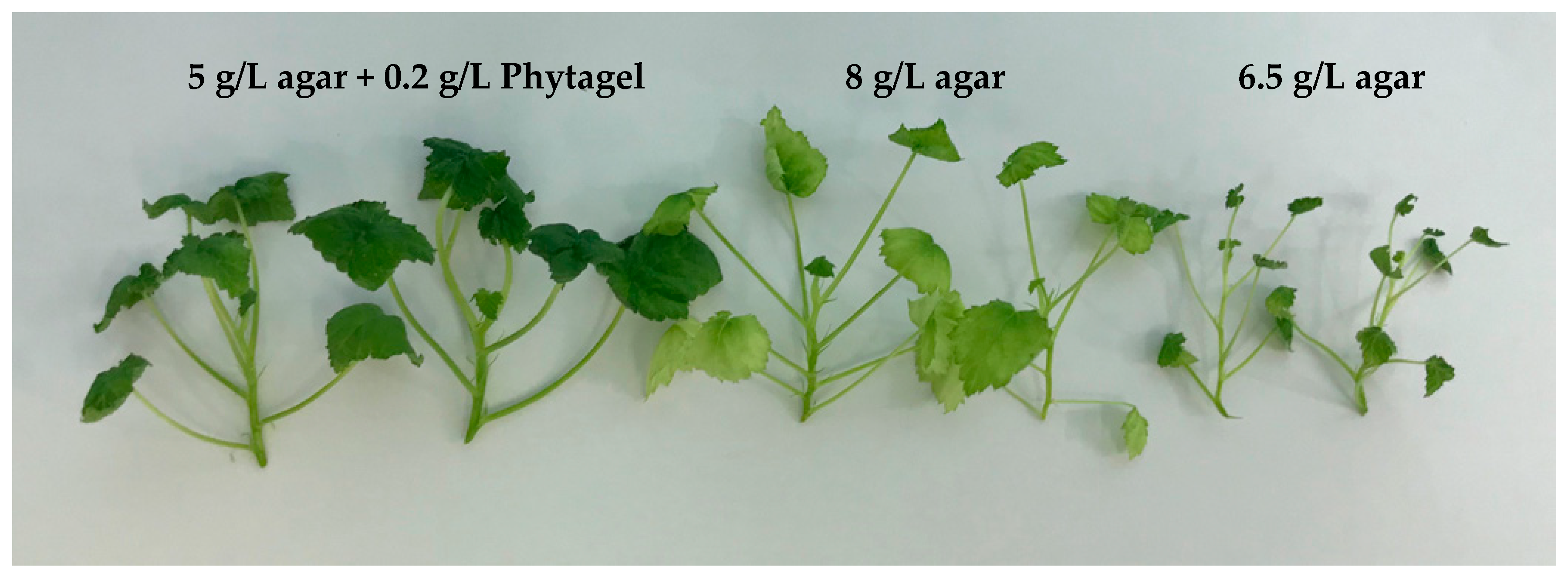

| Gelling Agent | Growth Regulators (mg/L) | Multiplication Rate | Height (mm) | Hyperhydricity | Chlorosis |
|---|---|---|---|---|---|
| Agar 8 g/L | BA 0.1 + kin 0.5 | 3.14 ± 0.17 bcd 1 | 22.5 ± 0.4 abc | - | - |
| BA 0.1 + kin 0.3 | 2.62 ± 0.19 d | 21.6 ± 0.9 abcd | - | + | |
| BA 0.2 + IBA 0.1 | 3.21 ± 0.22 bcd | 19.3 ± 1.1 cd | - | + | |
| BA 0.5 + IBA 0.1 | 2.98 ± 0.16 cd | 22.3 ± 2.3 abc | - | - | |
| Agar 6.5 g/L | BA 0.1 + kin 0.5 | 4.19 ± 0.19 a | 21.4 ± 1.8 bcd | + | - |
| BA 0.1 + kin 0.3 | 3.74 ± 0.26 ab | 18.2 ± 1.6 cd | + | - | |
| BA 0.2 + IBA 0.1 | 2.90 ± 0.15 cd | 19.1 ± 1.2 cd | + | - | |
| BA 0.5 + IBA 0.1 | 3.31 ± 0.14 bc | 25.1 ± 1.7 ab | + | - | |
| Agar 5 g/L + | BA 0.1 + kin 0.5 | 3.64 ± 0.24 ab | 20.1 ± 1.6 cd | - | - |
| Phytagel 0.2 g/L | BA 0.1 + kin 0.3 | 3.31 ± 0.20 bc | 17.0 ± 1.3 d | - | - |
| BA 0.2 + IBA 0.1 | 3.36 ± 0.17 bc | 26.1 ± 1.4 a | - | - | |
| BA 0.5 + IBA 0.1 | 3.00 ± 0.13 cd | 22.4 ± 0.7 abc | - | - |
| Gelling Agent | Growth Regulators (mg/L) | Survival (%) | Height (mm) |
|---|---|---|---|
| Agar 8 g/L | BA 0.1 + kin 0.5 | 74.3 ± 3.7 de 1 | 125.5 ± 8.9 a |
| BA 0.1 + kin 0.3 | 89.9 ± 3.8 bc | 76.1 ± 6.0 cde | |
| BA 0.2 + IBA 0.1 | 80.2 ± 3.1 cde | 73.1 ± 5.6 de | |
| BA 0.5 + IBA 0.1 | 77.5 ± 4.7 cde | 118.0 ± 7.9 a | |
| Agar 6.5 g/L | BA 0.1 + kin 0.5 | 83.9 ± 3.5 bcd | 65.6 ± 3.5 ef |
| BA 0.1 + kin 0.3 | 85.4 ± 3.5 bcd | 88.2 ± 4.7 bcd | |
| BA 0.2 + IBA 0.1 | 65.6 ± 4.7 ef | 70.3 ± 4.2 def | |
| BA 0.5 + IBA 0.1 | 60.9 ± 2.9 f | 54.1 ± 4.5 f | |
| Agar 5 g/L + | BA 0.1 + kin 0.5 | 91.6 ± 3.6 ab | 117.4 ± 7.2 a |
| Phytagel 0.2 g/L | BA 0.1 + kin. 0.3 | 78.8 ± 4.4 cde | 99.5 ± 2.9 b |
| BA 0.2 + IBA 0.1 | 97.2 ± 2.0 a | 85.8 ± 3.4 bcd | |
| BA 0.5 + IBA 0.1 | 82.2 ± 5.4 bcd | 93.6 ± 6.9 bc |
| Factors | Degrees of | Survival | Shoot Height | ||
|---|---|---|---|---|---|
| Freedom | F-Value | p | F-Value | p | |
| Growth regulators | 3 | 2.805 | 0.053471 | 10.543 | 0.000041 |
| Gelling agent | 2 | 12.376 | 0.000081 | 33.845 | 0.000000 |
| Growth regulator x gelling agent | 6 | 6.926 | 0.000060 | 11.771 | 0.000000 |
| Error | 36 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebedev, V.; Arkaev, M.; Dremova, M.; Pozdniakov, I.; Shestibratov, K. Effects of Growth Regulators and Gelling Agents on Ex Vitro Rooting of Raspberry. Plants 2019, 8, 3. https://doi.org/10.3390/plants8010003
Lebedev V, Arkaev M, Dremova M, Pozdniakov I, Shestibratov K. Effects of Growth Regulators and Gelling Agents on Ex Vitro Rooting of Raspberry. Plants. 2019; 8(1):3. https://doi.org/10.3390/plants8010003
Chicago/Turabian StyleLebedev, Vadim, Mikhail Arkaev, Mariya Dremova, Ivan Pozdniakov, and Konstantin Shestibratov. 2019. "Effects of Growth Regulators and Gelling Agents on Ex Vitro Rooting of Raspberry" Plants 8, no. 1: 3. https://doi.org/10.3390/plants8010003
APA StyleLebedev, V., Arkaev, M., Dremova, M., Pozdniakov, I., & Shestibratov, K. (2019). Effects of Growth Regulators and Gelling Agents on Ex Vitro Rooting of Raspberry. Plants, 8(1), 3. https://doi.org/10.3390/plants8010003




