Wheat ATI CM3, CM16 and 0.28 Allergens Produced in Pichia Pastoris Display a Different Eliciting Potential in Food Allergy to Wheat ‡
Abstract
1. Introduction
2. Results
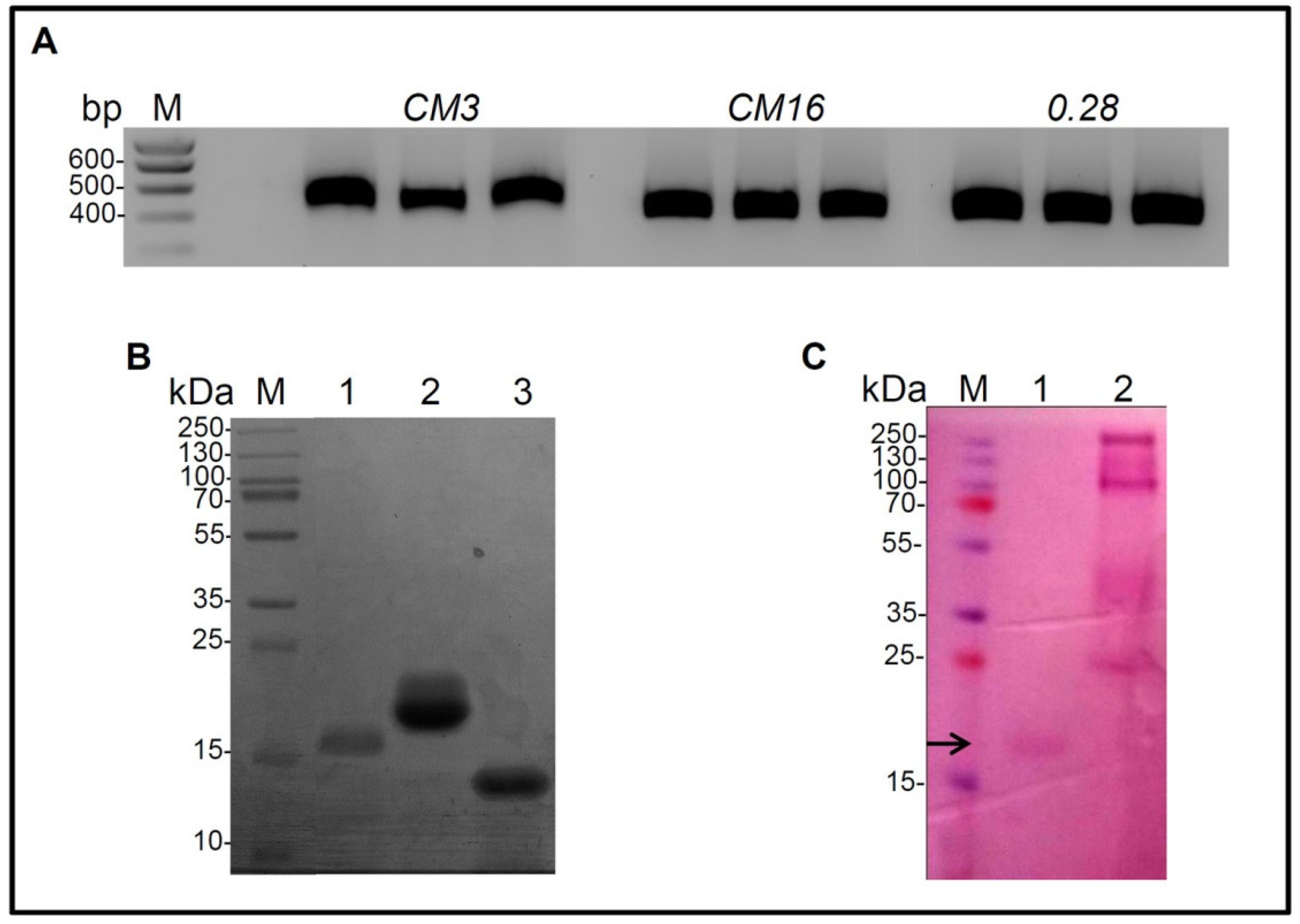
2.1. Cloning of CM3, CM16, 0.28, Expression in P. Pastoris and Purification
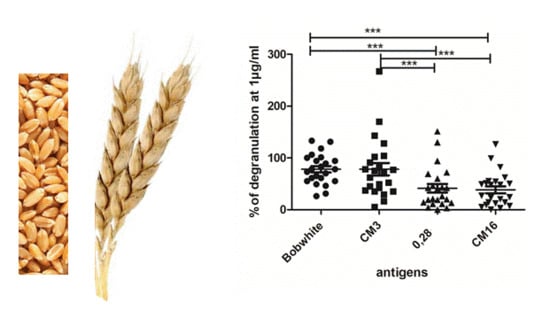
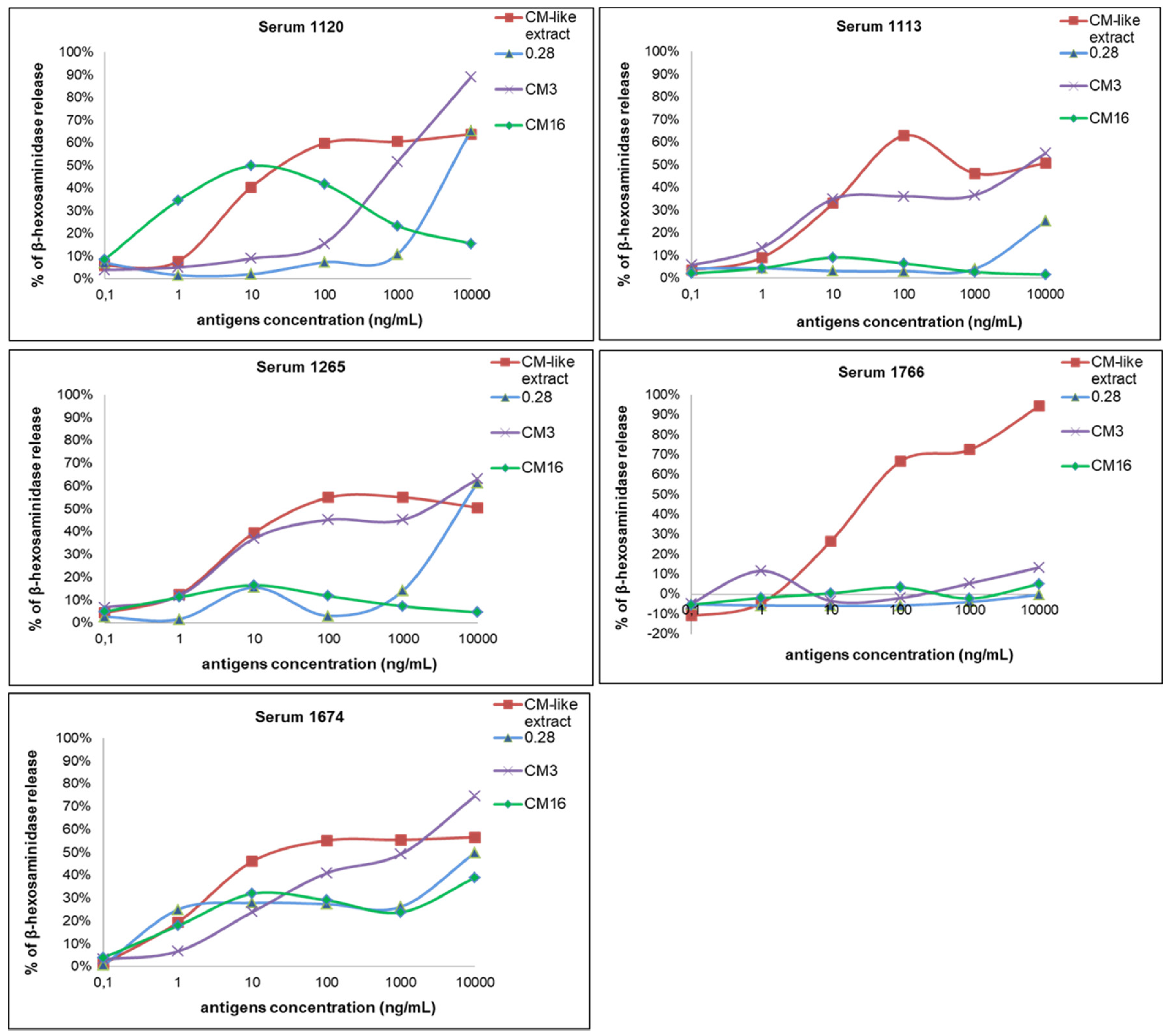
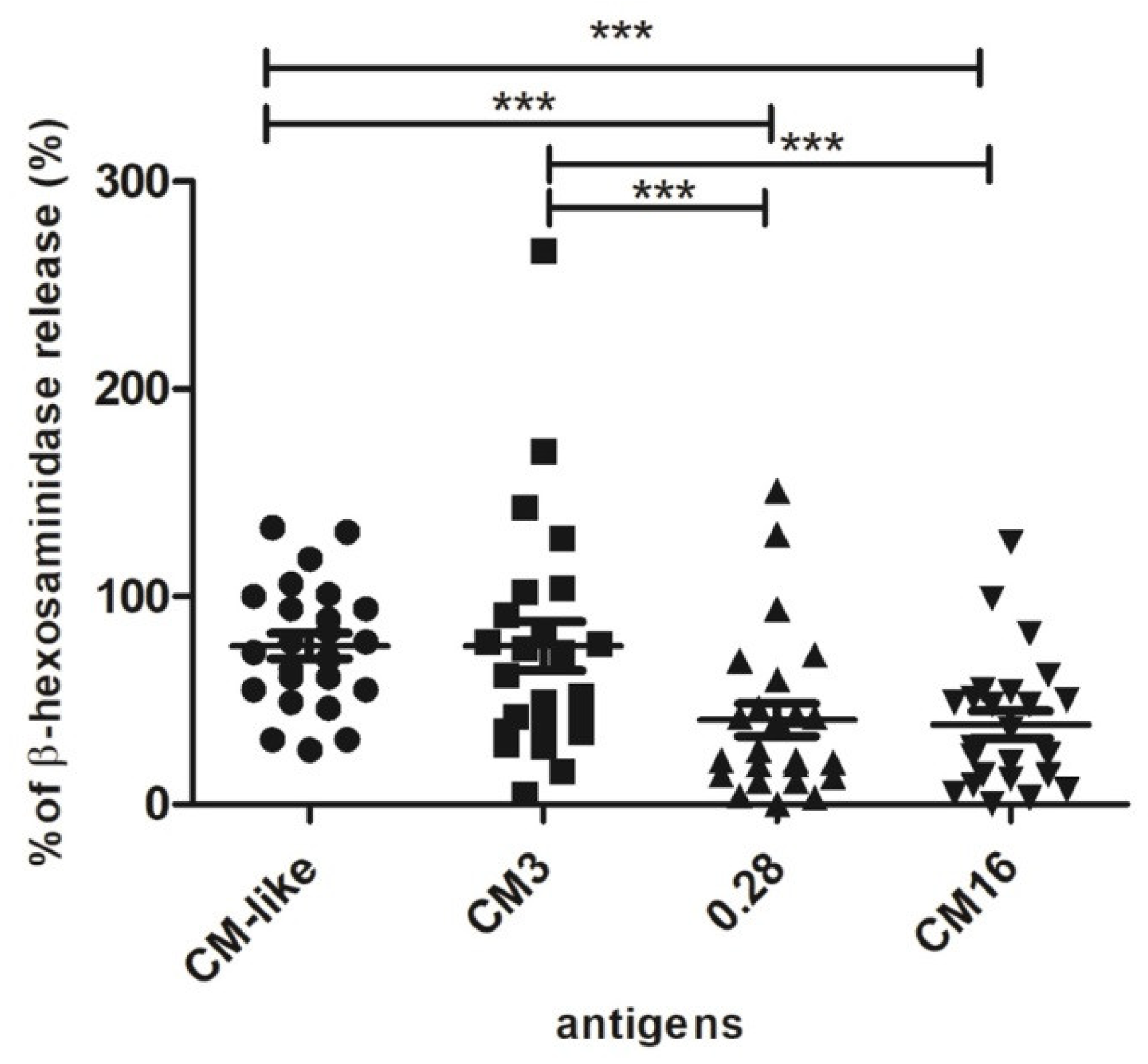
2.2. Capability of CM3, CM16 and 0.28 to Induce RBL-SX38 Cell Activation Compared to CM-Like Protein Extract
3. Discussion
4. Materials and Methods
4.1. Plant Material and Genes Isolation
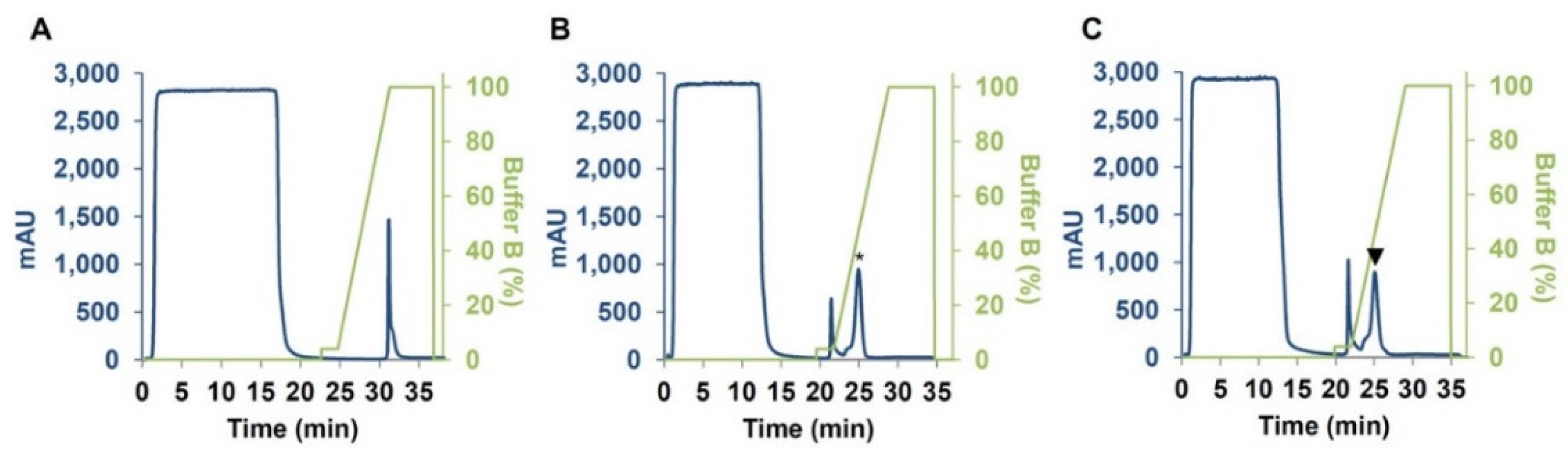
4.2. Expression and Purification of CM3, CM16 and 0.28
4.3. In Silico Prediction of N-Glycosylation
4.4. Polyacrylamide Gel Electrophoresis and Glycosylation Staining
4.5. Protein Identification by Mass Spectrometry
4.6. Extraction of Salt Soluble Proteins: CM-Like and Albumin/Globulin Fractions
4.7. Sera from Wheat Allergic Patients
4.8. RBL-SX38 Cell Degranulation Test
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reese, I.; Schäfer, C.; Kleine-Tebbe, J.; Ahrens, B.; Bachmann, O.; Ballmer-Weber, B.; Beyer, K.; Bischoff, S.C.; Blümchen, K.; Dölle, S.; et al. Non-celiac gluten/wheat sensitivity (NCGS)—A currently undefined disorder without validated diagnostic criteria and of unknown prevalence. Allergo J. Int. 2018, 27, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Sapone, A.; Bai, J.C.; Ciacci, C.; Dolinsek, J.; Green, P.H.R.; Hadjivassiliou, M.; Kaukinen, K.; Rostami, K.; Sanders, D.S.; Schumann, M.; et al. Spectrum of gluten-related disorders: Consensus on new nomenclature and classification. BMC Med. 2012, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Branchi, F.; Tomba, C.; Villalta, D.; Norsa, L.; Ferretti, F.; Roncoroni, L.; Bardella, M.T. Diagnosis of gluten related disorders: Celiac disease, wheat allergy and non-celiac gluten sensitivity. World J. Gastroenterol. 2015, 21, 7110–7119. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, G.; Quirce, S.; Diaz-Perales, A. Wheat allergens associated with Baker’s asthma. J. Investig. Allergol. Clin. Immunol. 2011, 21, 81–92. [Google Scholar] [PubMed]
- Pastorello, E.A.; Farioli, L.; Conti, A.; Pravettoni, V.; Bonomi, S.; Iametti, S.; Fortunato, D.; Scibilia, J.; Bindsley-Jensen, C.; Ballmer-Weber, B.; et al. Wheat IgE-mediated food allergy in European patients: α-amylase inhibitors, lipid transfer proteins and low-molecular-weight glutenins - Allergenic molecules recognized by double-blind, placebo-controlled food challenge. Int. Arch. Allergy Immunol. 2007, 144, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Lupi, R.; Denery-Papini, S.; Rogniaux, H.; Lafiandra, D.; Rizzi, C.; De Carli, M.; Moneret-Vautrin, D.A.; Masci, S.; Larré, C. How much does transgenesis affect wheat allergenicity? Assessment in two GM lines over-expressing endogenous genes. J. Proteomics 2013, 80, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lupi, R.; Masci, S.; Rogniaux, H.; Tranquet, O.; Brossard, C.; Lafiandra, D.; Moneret-Vautrin, D.A.; Denery-Papini, S.; Larré, C. Assessment of the allergenicity of soluble fractions from GM and commercial genotypes of wheats. J. Cereal Sci. 2014, 60, 179–186. [Google Scholar] [CrossRef]
- Zapatero, L.; Martínez, M.I.; Alonso, E.; Salcedo, G.; Sánchez-Monge, R.; Barber, D.; Lombardero, M. Oral wheat flour anaphylaxis related to wheat α-amylase inhibitor subunits CM3 and CM16. Allergy 2003, 58, 956. [Google Scholar] [CrossRef] [PubMed]
- Huebener, S.; Tanaka, C.K.; Uhde, M.; Zone, J.J.; Vensel, W.H.; Kasarda, D.D.; Beams, L.; Briani, C.; Green, P.H.; Altenbach, S.B.; Alaedini, A. Specific nongluten proteins of wheat are novel target antigens in celiac disease humoral response. J. Proteome Res. 2015, 14, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Zevallos, V.F.; Raker, V.; Tenzer, S.; Jimenez-Calvente, C.; Ashfaq-Khan, M.; Rüssel, N.; Pickert, G.; Schild, H.; Steinbrink, K.; Schuppan, D. Nutritional Wheat Amylase-Trypsin Inhibitors Promote Intestinal Inflammation via Activation of Myeloid Cells. Gastroenterology 2017, 152, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Junker, Y.; Zeissig, S.; Kim, S.J.; Barisani, D.; Wieser, H.; Leffler, D.A.; Zevallos, V.; Libermann, T.A.; Dillon, S.; Freitag, T.L.; et al. Wheat amylase trypsin inhibitors drive intestinal inflammation via activation of toll-like receptor 4. J. Exp. Med. 2012, 209, 2395–2408. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Elli, L.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; Cellier, C.; Cristofori, F.; de Magistris, L.; Dolinsek, J.; et al. Diagnosis of non-celiac gluten sensitivity (NCGS): The Salerno Experts’ Criteria. Nutrients 2015, 7, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Bellinghausen, I.; Weigmann, B.; Zevallos, V.; Maxeiner, J.; Reißig, S.; Waisman, A.; Schuppan, D.; Saloga, J. Wheat amylase-trypsin inhibitors exacerbate intestinal and airway allergic immune responses in humanized mice. J. Allergy Clin. Immunol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Alaedini, A.; Bojarski, C.; Bonaz, B.; Bouma, G.; Carroccio, A.; Castillejo, G.; De Magistris, L.; Dieterich, W.; Di Liberto, D.; et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients 2017, 9, 1268. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Olmedo, F.; Salcedo, G.; Sanchez-Monge, R.; Gómez, L.; Royo, J.; Carbonero, P. Plant proteinaceous, inhibitors of proteinases and 3-amylases. In Oxford Surveys of Plant Molecular and Cell Biology; Miflin, B., Ed.; Oxford University Press: Oxford, UK, 1987; Volume 4, pp. 275–334. [Google Scholar]
- Carbonero, P.; Garcia-Olmedo, F. A multigene family of trypsin/α-amylase inhibitors from cereals. In Seed Proteins; Shewry, P.R., Casey, R., Eds.; Kluwer Academic Publishers: Dordrecht, the Netherlands, 1999; pp. 617–633. [Google Scholar]
- Sanchez-Monge, R.; Gomez, L.; Garcia-Olmedo, F.; Salcedo, G. New dimeric inhibitor of heterologous α-amylases encoded by a duplicated gene in the short arm of chromosome 3B of wheat (Triticum aestivum L.). Eur. J. Biochem. 1989, 183, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Altenbach, S.B.; Vensel, W.H.; Dupont, F.M. The spectrum of low molecular weight alpha-amylase/protease inhibitor genes expressed in the US bread wheat cultivar Butte 86. BMC Res. Notes 2011, 4, 242. [Google Scholar] [CrossRef] [PubMed]
- Kreis, M.; Forde, B.G.; Rahman, S.; Miflin, B.J.; Shewry, P.R. Molecular evolution of the seed storage proteins of barley, rye and wheat. J. Mol. Biol. 1985, 183, 499–502. [Google Scholar] [CrossRef]
- Scossa, F.; Laudencia-Chingcuanco, D.; Anderson, O.D.; Vensel, W.H.; Lafiandra, D.; D’Ovidio, R.; Masci, S. Comparative proteomic and transcriptional profiling of a bread wheat cultivar and its derived transgenic line over-expressing a low molecular weight glutenin subunit gene in the endosperm. Proteomics 2008, 8, 2948–2966. [Google Scholar] [CrossRef] [PubMed]
- Lupi, R.; Denery-Papini, S.; Rogniaux, H.; Lafiandra, D.; Rizzi, C.; De Carli, M.; Moneret-Vautrin, A.D.; Masci, S.; Larré, C. How much does transgenesis affect wheat allergenicity? Assessment in two GM lines over-expressing endogenous genes. J. Proteomics 2013, 80, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Šotkovský, P.; Sklenář, J.; Halada, P.; Cinová, J.; Šetinová, I.; Kainarová, A.; Goliáš, J.; Pavlásková, K.; Honzová, S.; Tučková, L. A new approach to the isolation and characterization of wheat flour allergens. Clin. Exp. Allergy 2011, 41, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Kusaba-Nakayama, M.; Ki, M.; Kawada, E.; Sato, M.; Ikeda, I.; Mochizuki, T.; Imaizumi, K. Intestinal absorbability of wheat allergens, subunits of a wheat alpha-amylase inhibitor, expressed by bacteria. Biosci. Biotechnol. Biochem. 2001, 65, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Lafond, M.; Navarro, D.; Haon, M.; Couturier, M.; Berrin, J.G. Characterization of a broad-specificity β-glucanase acting on β-(1, 3)-, β-(1,4)-, and β-(1,6)-glucans that defines a new glycoside hydrolase family. Appl. Environ. Microbiol. 2012, 78, 8540–8546. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Monge, R.; Gomez, L.; Barber, D.; Lopez-Otin, C.; Armentia, A.; Salcedo, G. Wheat and barley allergens associated with baker’s asthma. Biochem. J. 1992, 281, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Tapia, A.; Kyle, R.A.; Kaplan, E.L.; Johnson, D.R.; Page, W.; Erdtmann, F.; Brantner, T.L.; Kim, W.R.; Phelps, T.K.; Lahr, B.D.; et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology 2009, 137, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Catassi, C.; Kryszak, D.; Bhatti, B.; Sturgeon, C.; Helzlsouer, K.; Clipp, S.L.; Gelfond, D.; Puppa, E.; Sferruzza, A.; Fasano, A. Natural history of celiac disease autoimmunity in a USA cohort followed since 1974. Ann. Med. 2010, 42, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Kasarda, D.D. Can an increase in celiac disease be attributed to an increase in the gluten content of wheat as a consequence of wheat breeding? J. Agric. Food Chem. 2013, 61, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Zuidmeer, L.; Goldhahn, K.; Rona, R.J.; Gislason, D.; Madsen, C.; Summers, C.; Sodergren, E.; Dahlstrom, J.; Lindner, T.; Sigurdardottir, S.T.; et al. The prevalence of plant food allergies: A systematic review. J. Allergy Clin. Immunol. 2008, 121, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Raulf, M. Allergen component analysis as a tool in the diagnosis and management of occupational allergy. Mol. Immunol. 2018, 100, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Cuccioloni, M.; Mozzicafreddo, M.; Bonfili, L.; Cecarini, V.; Giangrossi, M.; Falconi, M.; Saitoh, S.I.; Eleuteri, A.M.; Angeletti, M. Interfering with the high-affinity interaction between wheat amylase trypsin inhibitor CM3 and toll-like receptor 4: In silico and biosensor based studies. Sci. Rep. 2017, 7, 13169. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.C.; Delorme, V.; Bénarouche, A.; Martin, B.P.; Paudel, R.; Gnawali, G.R.; Madani, A.; Puppo, R.; Landry, V.; Kremer, L.; et al. Cyclipostins and Cyclophostin analogs as promising compounds in the fight against tuberculosis. Sci Rep. 2017, 7, 11751. [Google Scholar] [CrossRef] [PubMed]
- Hurkman, W.J.; Tanaka, C.K. Extraction of wheat endosperm proteins for proteome analysis. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2007, 849, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Larré, C.; Lupi, R.; Gombaud, G.; Brossard, C.; Branlard, G.; Moneret-Vautrin, D.A.; Rogniaux, H.; Denery-Papini, S. Assessment of allergenicity of diploid and hexaploid wheat genotypes: Identification of allergens in the albumin/globulin fraction. J. Proteomics 2011, 74, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Lupi, R.; Denery-Papini, S.; Claude, M.; Tranquet, O.; Drouet, M.; Masci, S.; Larré, C. Thermal treatment reduces gliadin recognition by IgE, but a subsequent digestion and epithelial crossing permits recovery. Food Res. Intern. 2018, in press. [Google Scholar] [CrossRef]
- Claude, M.; Lupi, R.; Bouchaud, G.; Bodinier, M.; Brossard, C.; Denery-Papini, S. The thermal aggregation of ovalbumin as large particles decreases its allergenicity for egg allergic patients and in a murine model. Food Chem. 2016, 203, 136–144. [Google Scholar] [CrossRef] [PubMed]

| IgE Concentration (ng/mL) | |||
| Patient # | Total IgE | Specific to A/G Fraction | Specific to CM-like Protein Fraction |
| 1674 | 1468 | 208 | 250 |
| 1265 | 11486 | >500 | nd |
| 1113 | 6180 | 141 | nd |
| 1120 | 616 | 100 | nd |
| 1766 | 423 | 123 | 120 |
| 134 | 2000 | 196 | nd |
| 610 | sat | 281 | nd |
| 639 | 8150 | 333 | nd |
| 642 | 524 | 154 | 274 |
| 779 | 815 | 110 | nd |
| 1026 | 2735 | 164 | nd |
| 1157 | 11113 | 119 | nd |
| 1266 | 13098 | 140 | nd |
| 1353 | 363 | 1 | 243 |
| 1494 | 1454 | 327 | 386 |
| 1572 | 11142 | 253 | 368 |
| 1638 | 6741 | 300 | 360 |
| 1747 | 2218 | 192 | 248 |
| 1826 | 35024 | 432 | >500 |
| 1829 | 14661 | 123 | 290 |
| 1830 | 1854 | 137 | 171 |
| 1862 | 4724 | 216 | 221 |
| 1875 | 5609 | >500 | >500 |
| Atopic Serum (Allergic to Grasses Pollen) | |||
| # | Total IgE | ||
| 319 | 2800 | ||
| 322 | 3123 | ||
| 997 | 1471 | ||
| 1240 | 1253 | ||
| 1246 | 1112 | ||
| 1617 | 940 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tundo, S.; Lupi, R.; Lafond, M.; Giardina, T.; Larré, C.; Denery-Papini, S.; Morisset, M.; Kalunke, R.; Sestili, F.; Masci, S. Wheat ATI CM3, CM16 and 0.28 Allergens Produced in Pichia Pastoris Display a Different Eliciting Potential in Food Allergy to Wheat ‡. Plants 2018, 7, 101. https://doi.org/10.3390/plants7040101
Tundo S, Lupi R, Lafond M, Giardina T, Larré C, Denery-Papini S, Morisset M, Kalunke R, Sestili F, Masci S. Wheat ATI CM3, CM16 and 0.28 Allergens Produced in Pichia Pastoris Display a Different Eliciting Potential in Food Allergy to Wheat ‡. Plants. 2018; 7(4):101. https://doi.org/10.3390/plants7040101
Chicago/Turabian StyleTundo, Silvio, Roberta Lupi, Mickael Lafond, Thierry Giardina, Colette Larré, Sandra Denery-Papini, Martine Morisset, Raviraj Kalunke, Francesco Sestili, and Stefania Masci. 2018. "Wheat ATI CM3, CM16 and 0.28 Allergens Produced in Pichia Pastoris Display a Different Eliciting Potential in Food Allergy to Wheat ‡" Plants 7, no. 4: 101. https://doi.org/10.3390/plants7040101
APA StyleTundo, S., Lupi, R., Lafond, M., Giardina, T., Larré, C., Denery-Papini, S., Morisset, M., Kalunke, R., Sestili, F., & Masci, S. (2018). Wheat ATI CM3, CM16 and 0.28 Allergens Produced in Pichia Pastoris Display a Different Eliciting Potential in Food Allergy to Wheat ‡. Plants, 7(4), 101. https://doi.org/10.3390/plants7040101