Primary Structure Analysis of Antifungal Peptides from Cultivated and Wild Cereals
Abstract
1. Introduction
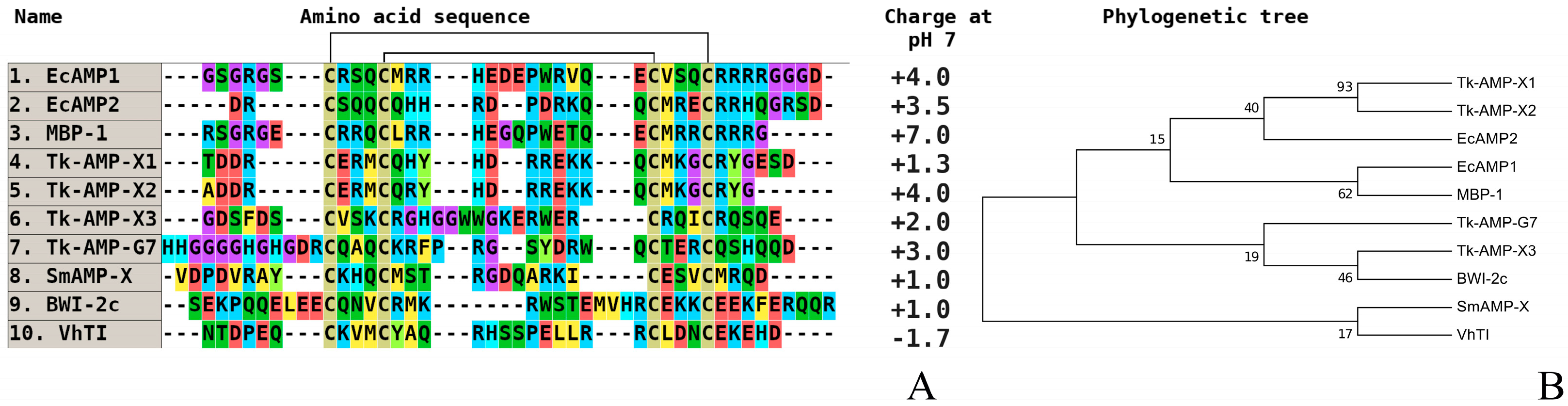
2. Comparative Analysis of the Primary Structure of Defensins Isolated from Wild and Cultivated Cereals
3. Investigation of Structural Determinants of Other AMPs (Thionins, Hevein-Like Peptides, and Alpha-Hairpinins), Which Provide Higher Antifungal Activity to Wild Cereals
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, F.; Cheng, Z. Research Progress on the use of Plant Allelopathy in Agriculture and the Physiological and Ecological Mechanisms of Allelopathy. Front. Plant Sci. 2015, 6, 1020. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.N.; Iqbal, J.; Ullah, A.; Yang, G.; Yousaf, M.; Fahad, S.; Tanveer, M.; Hassan, W.; Tung, S.A.; Wang, L.; et al. Allelopathic potential of oil seed crops in production of crops: A review. Environ. Sci. Pollut. Res. Int. 2016, 15, 14854–14867. [Google Scholar] [CrossRef] [PubMed]
- Hanin, M.; Ebel, C.; Ngom, M.; Laplaze, L.; Masmoudi, K. New Insights on Plant Salt Tolerance Mechanisms and Their Potential Use for Breeding. Front. Plant Sci. 2016, 7, 1787. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Baek, K.H. Physiological and biochemical perspectives of non-salt tolerant plants during bacterial interaction against soil salinity. Plant Physiol. Biochem. 2017, 116, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Bohra, A.; Jha, R. Breeding approaches and genomics technologies to increase crop yield under low-temperature stress. Plant Cell Rep. 2017, 36, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Mesihovic, A.; Iannacone, R.; Firon, N.; Fragkostefanakis, S. Heat stress regimes for the investigation of pollen thermotolerance in crop plants. Plant Reprod. 2016, 29, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; El-Sayed, M.; Jogaiah, S.; Burrit, D.J.; Tran, L.P. The “STAY-GREEN” trait and phytohormone signaling networks in plants under heat stress. Plant Cell Rep. 2017, 36, 1009–1025. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cândido, E.; Sousa, D.A.; Viana, J.C.; de Oliveira-Júnior, N.G.; Miranda, V.; Franco, O.L. The use of versatile plant antimicrobial peptides in agribusiness and human health. Peptides 2014, 55, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Yuan, S.S.; Jiang, L.L.; Ye, X.J.; Ng, T.B.; Wu, Z.J. Plant antifungal proteins and their applications in agriculture. Appl. Microbiol. Biotechnol. 2015, 99, 4961–4981. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.S.; Prodhan, Z.H.; Biswas, S.K.; Le, C.F.; Sekaran, S.D. Antimicrobial peptides from different plant sources: Isolation, characterisation, and purification. Phytochemistry 2018, 154, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Sels, J.; Mathys, J.; De Coninck, B.M.; Cammue, B.P.; De Bolle, M.F. Plant pathogenesis-related (PR) proteins: A focus on PR peptides. Plant Physiol. Biochem. 2008, 46, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.M.; Liu, P.Q.; Xu, Y.J.; Xiao, S. Protein trafficking during plant innate immunity. J. Integr. Plant Biol. 2016, 58, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Andreev, Y.A.; Korostyleva, T.V.; Slavokhotova, A.A.; Rogozhin, E.A.; Utkina, L.L.; Vassilevski, A.A.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. Genes encoding hevein-like defense peptides in wheat: Distribution, evolution, and role in stress response. Biochimie 2012, 94, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Rogozhin, E.A.; Musolyamov, A.K.; Andreev, Y.A.; Oparin, P.B.; Berkut, A.A.; Vassilevski, A.A.; Egorov, T.A.; Grishin, E.V.; Odintsova, T.I. Novel antifungal α-hairpinin peptide from Stellaria media seeds: Structure, biosynthesis, gene structure and evolution. Plant Mol. Biol. 2014, 84, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Lobo, D.S.; Pereira, I.B.; Fragel-Madeira, L.; Medeiros, L.N.; Cabral, L.M.; Faria, J.; Bellio, M.; Campos, R.C.; Linden, R.; Kurtenbach, E. Antifungal Pisum sativum defensin 1 interacts with Neurospora crassa cyclin F related to the cell cycle. Biochemistry 2007, 46, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Graham, M.A.; Silverstein, K.A.; Cannon, S.B.; VandenBosch, K.A. Computational identification and characterization of novel genes from legumes. Plant Physiol. 2004, 135, 1179–1197. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, A.K.; Brunstedt, J.; Nielsen, J.E.; Mikkelsen, J.D.; Roepstorff, P.; Nielsen, K.K. Processing, disulfide pattern, and biological activity of a sugar beet defensin, AX2, expressed in Pichia pastoris. Protein Exp. Purif. 1999, 16, 377–387. [Google Scholar] [CrossRef] [PubMed]
- de Zélicourt, A.; Letousey, P.; Thoiron, S.; Campion, C.; Simoneau, P.; Elmorjani, K.; Marion, D.; Simier, P.; Delavault, P. Ha-DEF1, a sunflower defensin, induces cell death in Orobanche parasitic plants. Planta 2007, 226, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Do, H.M.; Lee, S.C.; Jung, H.W.; Sohn, K.H.; Hwang, B.K. Differential expression and in sutu localization of a pepper defensin (CADEF1) gene in response to pathogen infection, abiotic elicitors and environmental stresses in Capsicum anuum. Plant Sci. 2004, 166, 1297–1305. [Google Scholar]
- Fujiumura, M.; Ideguchi, M.; Minami, Y.; Watanabe, K.; Tadera, K. Purification, characterization and sequencing of novel antimicrobial peptides Tu-AMP1 and Tu-AMP2 from bulbs of tulip (Tulipa gesneriana L.). Biosci. Biotechnol. Biochem. 2004, 63, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.E.; Bassett, C.L.; Artlip, T.S.; Webb, R.P.; Janisiewicz, W.J.; Norelli, J.L. Characterization of a defensin from bark and fruit tissues of peach and antimicrobial activity of a recombinant defensin in the yeast, Pichia pastoris. Physiol. Plant. 2003, 119, 563–572. [Google Scholar] [CrossRef]
- Bloch, C.J.; Richardson, M.A. A new family of small (5 kDa) protein inhibitors of insect α-amylases from seeds of sorghum (Sorghum bicolor (L.) Moebch.) have sequence homologies with wheat γ-purothionins. FEBS J. 1991, 279, 101–104. [Google Scholar] [CrossRef]
- Mendez, E.; Moreno, A.; Colilla, F.; Pelaez, F.; Limas, G.G.; Mendez, R. Primary structure and inhibition of protein synthesis in eukaryotic cell-free system of a novel thionin, γ-hordothionin, from barley endosperm. Eur. J. Biochem. 1990, 194, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Mendez, E.; Rocher, A.; Calero, M.; Girbes, T.; Citores, L.; Soriano, F. Primary structure of ω-hordothionin, a member of a novel family of thionins from barley endosperm, and its inhibition of protein synthesis in eukaryotic and prokaryotic cell-free systems. Eur. J. Biochem. 1996, 239, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Segura, A.; Moreno, M.; Molina, A.; Garcia-Olmedo, F. Novel defensin subfamily from spinach (Spinacea oleracea). FEBS Lett. 1998, 435, 159–162. [Google Scholar] [CrossRef]
- Sharma, P.; Lönneborg, A. Isolation and characterization of a cDNA encoding a plant defensin-like protein from roots of Norway spruce. Plant Mol. Biol. 1996, 31, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-H.; Jian, G.-L.; Zhang, Y.-T.; Ai, T.-M. Bacterial expression of a Trichosanthes lirilowii defensin (TDEF1) and its antifungal activity on Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2007, 28, 62–75. [Google Scholar]
- Colilla, F.J.; Rocher, A.; Mendez, E. Gamma-purothionins: Amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm. FEBS Lett. 1990, 270, 191–194. [Google Scholar] [CrossRef]
- Vriens, K.; Cammue, B.P.; Thevissen, K. Antifungal plant defensins: Mechanisms of action and production. Molecules 2014, 19, 12280–12303. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Terras, F.R.G.; Cammue, B.P.; Osborn, R.W. Plant defensins: Novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995, 108, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, A.F.; Vasconcelos, E.A.; Pelegrini, P.B.; Grossi de Sa, M.F. Antifungal defensins and their role in plant defense. Front. Microbiol. 2014, 5, 116. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Ferket, K.K.; François, I.E.; Cammue, B.P. Interactions of antifungal plant defensins with fungal membrane components. Peptides 2003, 24, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Thevissen, K.; Warnecke, D.C.; François, I.E.; Leipelt, M.; Heinz, E.; Ott, C.; Zähringer, U.; Thomma, B.P.; Ferket, K.K.; Cammue, B.P. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 2004, 279, 3900–3905. [Google Scholar] [CrossRef] [PubMed]
- Egorov, T.A.; Odintsova, T.I.; Pukhalsky, V.A.; Grishin, E.V. Diversity of wheat antimicrobial peptides. Peptides 2005, 26, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Egorov, T.A.; Musolyamov, A.K.; Odintsova, M.S.; Pukhalsky, V.A.; Grishin, E.V. Seed defensins from T. kiharae and related species: Genome localization of defensin-encoding genes. Biochimie 2007, 89, 605–612. [Google Scholar] [PubMed]
- Odintsova, T.I.; Rogozhin, E.A.; Baranov, Y.; Musolyamov, A.K.; Yalpani, N.; Egorov, T.A.; Grishin, E.V. Seed defensins of barnyard grass Echinochloa crusgalli (L.) Beauv. Biochimie 2008, 90, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Osborn, R.W.; De Samblanx, G.W.; Thevissen, K.; Goderis, I.; Torrekens, S.; Van Leuven, F. Isolation and characterization of plant defensins from seeds of Asteraceae, Fabaceae, Hippocastanaceae and Saxifragaceae. FEBS Lett. 1995, 368, 257–262. [Google Scholar] [CrossRef]
- Terras, F.R.G.; Schofs, H.M.E.; de Bolle, M.F.C.; Van Leuven, F.; Rees, S.B. In vitro antifungal activity of a radish (Raphanus sativus L.) seed protein homologous to nonspecific lipid lipid transfer proteins. Plant Physiol. 1992, 100, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- De Samblanx, G.W.; Goderis, I.J.; Thevissen, K.; Raemaekers, R.; Fant, F.; Borremans, F.; Acland, D.P.; Osborn, R.W.; Patel, S.; Broekaert, W.F. Mutational analysis of a plant defensin from radish (Raphanus sativus L.) reveals two adjacent sites important for antifungal activity. J. Biol. Chem. 1997, 272, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Jin, X.; Zhao, Y.; Dong, Q.; Jiang, H.; Ma, Q. Evolution of the defensin-like gene family in grass genomes. J. Genet. 2016, 95, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Shelenkov, A.A.; Odintsova, T.I. Prediction of Leymus arenarius (L.) antimicrobial peptides based on de novo transcriptome assembly. Plant Mol. Biol. 2015, 89, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Matić, S.; Bagnaresi, P.; Biselli, C.; Orru’, L.; Amaral Carneiro, G.; Siciliano, I.; Valé, G.; Gullino, M.L.; Spadaro, D. Comparative transcriptome profiling of resistant and susceptible rice genotypes in response to the seedborne pathogen Fusarium fujikuroi. BMC Genomics 2016, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Haddadi, P.; Ma, L.; Wang, H.; Borhan, M.H. Genome-wide transcriptomic analyses provide insights into the lifestyle transition and effector repertoire of Leptosphaeria maculans during the colonization of Brassica napus seedlings. Mol. Plant Pathol. 2016, 17, 1196–1210. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.S.; Rajagopalan, N.; Risseeuw, E.P.; Surpin, M.; Ball, F.J.; Barber, C.J.; Buhrow, L.M.; Clark, S.M.; Page, J.E.; Todd, C.D.; et al. Characterization of Triticum aestivum Abscisic Acid Receptors and a Possible Role for These in Mediating Fusairum Head Blight Susceptibility in Wheat. PLoS ONE 2016, 11, e0164996. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Shelenkov, A.A.; Korostyleva, T.V.; Rogozhin, E.A.; Melnikova, N.V.; Kudryavtseva, A.V.; Odintsova, T.I. Defense peptide repertoire of Stellaria media predicted by high throughput next generation sequencing. Biochimie 2017, 135, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Alamillo, J.M.; Garcia-Olmedo, F. Processing of thionin precursors in barley leaves by a vacuolar proteinase. Eur. J. Biochem. 1997, 243, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Florack, D.E.; Stiekema, W.J. Thionins: Properties, possible biological roles and mechanisms of action. Plant Mol. Biol. 1994, 26, 25–37. [Google Scholar] [CrossRef] [PubMed]
- García-Olmedo, F.; Molina, A.; Alamillo, J.M.; Rodríguez-Palenzuéla, P. Plant defense peptides. Biopolymers 1998, 47, 479–491. [Google Scholar] [CrossRef]
- Bruix, M.; Jiménez, M.A.; Santoro, J.; González, C.; Colilla, F.J.; Méndez, E.; Rico, M. Solution structure of gamma 1-H and gamma 1-P thionins from barley and wheat endosperm determined by 1H-NMR: A structural motif common to toxic arthropod proteins. Biochemistry 1993, 32, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Stec, B.; Rao, U.; Teeter, M.M. Refinement of purothionins reveals solute particles important for lattice formation and toxicity. Part 2: Structure of beta-purothionin at 1.7 A resolution. Acta Crystallogr. D Biol. Crystallogr. 1995, 51, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Kim, E.; Teeter, M.M.; Suh, S.W.; Stec, B. Crystal structure of alpha-hordothionin at 1.9 Angstrom resolution. FEBS Lett. 2005, 579, 2301–2306. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Debreczeni, J.E.; Sevvana, M.; Gruene, T.; Kahle, B.; Zeeck, A.; Sheldrick, G.M. Structures of viscotoxins A1 and B2 from European mistletoe solved using native data alone. Acta Crystallogr. D Biol. Crystallogr. 2008, 64, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Reimann-Philipp, U.; Schrader, G.; Martinoia, E.; Barkholt, V.; Apel, K. Intracellular thionins of barley. A second group of leaf thionins closely related to but distinct from cell wall-bound thionins. J. Biol. Chem. 1989, 264, 8978–8984. [Google Scholar] [PubMed]
- Stec, B. Plant thionins--the structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Gheysen, G.; Ullah, C.; Verbeek, R.; Shang, C.; Vleesschauwer, D.D.; Höfte, M.; Kyndt, T. The role of thionins in rice defence against root pathogens. Mol. Plant Pathol. 2015, 16, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Zasukhina, G.D.; Odintsova, T.I.; Shulenina, L.V.; Ushenkova, L.N.; Mikhailov, V.F.; Shagirova, Z.M.; Vedernikov, A.N.; Gromov, S.P.; Alfimov, M.V. Antimutagens (β-purothionin and crown compound) as modulators of expression of genes involved in carcinogenesis in human cells. Dokl. Biochem. Biophys. 2012, 446, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Zasukhina, G.D.; Vasilyeva, I.M.; Kadnikov, I.A.; Voronin, M.V.; Odintsova, T.I.; Korostileva, T.V.; Pukhalskii, V.A. Antimutagenic activity of wheat polypeptides in human cells exposed to cadmium chloride. Bull. Exp. Biol. Med. 2013, 155, 370–372. [Google Scholar] [CrossRef] [PubMed]
- Zasukhina, G.D.; Shagirova, J.M.; Babintsev, M.V.; Vasilyeva, I.M.; Rogozhin, E.A.; Odintsova, T.I.; Mikhailov, V.F.; Gromov, S.P.; Vedernikov, A.I.; Alfimov, M.V. Modulation of gene expression by antimutagens in human cells differing in the sensitivity to mutagens. Dokl. Biochem. Biophys. 2013, 453, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Vasil’eva, I.M.; Korostyleva, T.V.; Utkina, L.L.; Slavokhotova, A.A.; Rogozhin, E.A.; Shiian, A.N.; Pukhal’skiĭ, V.A.; Zasukhina, G.D. Antimutagenic activity of wheat beta-purothionin Tk-AMP-BP. Russ. J. Genet. 2011, 47, 1267–1270. [Google Scholar] [CrossRef]
- Pelegrini, P.B.; Franco, O.L. Plant gamma-thionins: Novel insights on the mechanism of action of a multi-functional class of defense proteins. Int. J. Biochem. Cell. Biol. 2005, 37, 2239–2253. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, V.F.; Shishkina, A.A.; Vasilyeva, I.M.; Shulenina, L.V.; Raeva, N.F.; Rogozhin, E.A.; Startsev, M.I.; Zasukhina, G.D.; Gromov, S.P.; Alfimov, M.V. Comparative analysis of natural and synthetic antimutagens as regulators of gene expression in human cells under exposure to ionizing radiation. Russ. J. Genet. 2015, 51, 147–155. [Google Scholar] [CrossRef]
- Kul’ko, A.B.; Kisil’, O.V.; Sadykova, V.S.; Mikhailov, V.F.; Vasilyeva, I.M.; Shulenina, L.V.; Zasukhina, G.D.; Rogozhin, E.A. Investigation of thionins from blackseed (Nigella sativa L.) possess cytotoxic, regulatory and antifungal activity. Antibiotiki I khimioterapiya 2016, 61, 8–16. (In Russian) [Google Scholar]
- Vasilchenko, A.S.; Smirnov, A.N.; Zavriev, S.K.; Grishin, E.V.; Vasilchenko, A.V.; Rogozhin, E.A. Novel thionins from black seed (Nigella sativa L.) demonstrate antimicrobial activity. Int. J. Pept. Res. Ther. 2017, 23, 171–180. [Google Scholar] [CrossRef]
- Giudici, A.M.; Regente, M.C.; Villalaín, J.; Pfüller, K.; Pfüller, U.; De La Canal, L. Mistletoe viscotoxins induce membrane permeabilization and spore death in phytopathogenic fungi. Physiol. Plant. 2004, 121, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Rodríguez, J.J.; Ochoa-Zarzosa, A.; López-Gómez, R.; López-Meza, J.E. Plant antimicrobial peptides as potential anticancer agents. Biomed. Res. Int. 2015, 2015, 735087. [Google Scholar] [CrossRef] [PubMed]
- Teeter, M.M.; Ma, X.Q.; Rao, U.; Whitlow, M. Crystal structure of a protein-toxin alpha 1-purothionin at 2.5A and a comparison with predicted models. Proteins 1990, 8, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Loeza-Ángeles, H.; Sagrero-Cisneros, E.; Lara-Zárate, L.; Villagόmes-Gόmez, E.; Lόpez-Meza, J.E.; Ochoa-Zarzosa, A. Thionin Thi2.1 from Arabidopsis thaliana expressed in endothelial cells shows antibacterial, antifungal and cytotoxic activity. Biotechnol. Lett. 2008, 10, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Van Parijs, J.W.F.; Broekaert, J.; Goldstein, I.J.; Peumans, W.J. Hevein: An antifungal protein from rubber-tree (Hevea brasiliensis) latex. Planta 1991, 183, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Raikhel, N.V.; Lee, H.-I. Structure and function of chitin-binding proteins. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1993, 44, 591–615. [Google Scholar] [CrossRef]
- Beintema, J.J. Structural features of plant chitinases and chitin-binding proteins. FEBS Lett. 1994, 350, 159–163. [Google Scholar] [CrossRef]
- De Bolle, M.F.; David, K.M.; Rees, S.B.; Vanderleyden, J.; Cammue, B.P.; Broekaert, W.F. Cloning and characterization of a cDNA encoding an antimicrobial chitin-binding protein from amaranth, Amaranthus caudatus. Plant Mol. Biol. 1993, 22, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Mariën, W.; Terras, F.R.; De Bolle, M.F.; Proost, P.; Van Damme, J.; Dillen, L.; Claeys, M.; Rees, S.B.; Vanderleyden, J.; et al. Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry 1992, 31, 4308–4314. [Google Scholar] [CrossRef] [PubMed]
- Lipkin, A.; Anisimova, V.; Nikonorova, A.; Babakov, A.; Krause, E.; Bienert, M.; Grishin, E.; Egorov, T. An antimicrobial peptide Ar-AMP from amaranth (Amaranthus retroflexus L.) seeds. Phytochemistry 2005, 66, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, E.A.; Slezina, M.P.; Slavokhotova, A.A.; Istomina, E.A.; Korostyleva, T.V.; Smirnov, A.N.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. A novel antifungal peptide from leaves of the weed Stellaria media L. Biochimie 2015, 116, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Komakhin, R.A.; Vysotskii, D.A.; Shukurov, R.R.; Voblikova, V.D.; Komakhina, V.V.; Strelnikova, S.R.; Vetchinkina, E.M.; Babakov, A.V. Novel strong promoter of antimicrobial peptides gene pro-SmAMP2 from chickweed (Stellaria media). BMC Biotechnol. 2016, 16, 43. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.H.; Xiang, Y.; Liu, X.Z.; Zhang, Y.; Hu, Z.; Wang, D.C. Two novel antifungal peptides distinct with a five-disulfide motif from the bark of Eucommia ulmoides Oliv. FEBS Lett. 2002, 521, 87–90. [Google Scholar] [CrossRef]
- Odintsova, T.I.; Vassilevski, A.A.; Slavokhotova, A.A.; Musolyamov, A.Kh.; Finkina, E.I.; Khadeeva, N.V.; Rogozhin, E.A.; Korostyleva, T.V.; Pukhalsky, V.A.; Grishin, E.V.; et al. A novel antifungal hevein-type peptide from Triticum kiharae seeds with a unique 10-cysteine motif. FEBS J. 2009, 275, 4266–4275. [Google Scholar] [CrossRef] [PubMed]
- Dubovskii, P.V.; Vassilevski, A.A.; Slavokhotova, A.A.; Odintsova, T.I.; Grishin, E.V.; Egorov, T.A.; Arseniev, A.S. Solution structure of a defense peptide from wheat with a 10-cysteine motif. Biochem. Biophys. Res. Commun. 2011, 411, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Utkina, L.L.; Zhabon, E.O.; Slavokhotova, A.A.; Rogozhin, E.A.; Shiian, A.N.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I.; Pukhal’skiĭ, V.A. Heterologous expression of a synthetic gene encoding a novel hevein-type antimicrobial peptide of Leymus arenarius in Escherichia coli cells. Russ. J. Genet. 2010, 46, 1645–1651. [Google Scholar] [CrossRef]
- Naumann, T.A. Modification of recombinant maize ChitA chitinase by fungal chitinase-modifying proteins. Mol. Plant Pathol. 2011, 12, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Shelenkov, A.A.; Andreev, Y.A.; Odintsova, T.I. Hevein-Like Antimicrobial Peptides of Plants. Biochemistry 2017, 82, 1659–1674. [Google Scholar] [CrossRef] [PubMed]
- Naumann, T.A.; Wicklow, D.T.; Price, N.P. Identification of a chitinase-modifying protein from Fusarium verticillioides: Truncation of a host resistance protein by a fungalysin metalloprotease. J. Biol. Chem. 2011, 286, 35358–35366. [Google Scholar] [CrossRef] [PubMed]
- Slavokhotova, A.A.; Naumann, T.A.; Price, N.P.; Rogozhin, E.A.; Andreev, Y.A.; Vassilevski, A.A.; Odintsova, T.I. Novel mode of action of plant defense peptides—Hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS J. 2014, 281, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Istomina, E.A.; Korostyleva, T.V.; Rozhnova, N.A.; Rogozhin, E.A.; Pukhalski, V.A.; Odintsova, T.I. Genes encoding hevein-like antimicrobial peptides WAMPs: Expression in response to phytohormones and environmental factors. Russ. J. Genet. 2016, 52, 1176–1185. [Google Scholar] [CrossRef]
- Duvick, J.P.; Rood, T.; Rao, A.G.; Marshak, D.R. Purification and characterization of a novel antimicrobial peptide from maize (Zea mays L.) kernels. J. Biol. Chem. 1992, 267, 18814–18820. [Google Scholar] [PubMed]
- Marcus, J.P.; Green, J.L.; Goulter, K.C.; Manners, J.M. A family of antimicrobial peptides is produced by processing of a 7S globulin protein in Macadamia integrifolia kernels. Plant J. 1999, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yang, X.X.; Xia, H.C.; Zeng, R.; Hu, W.G.; Li, Z.; Zhang, Z.C. Purification and characterization of Luffin P1, a ribosome-inactivating peptide from the seeds of Luffa cylindrical. Peptides 2003, 24, 799–805. [Google Scholar] [CrossRef]
- Park, S.S.; Abe, K.; Kimura, M.; Urisu, A.; Yamasaki, N. Primary structure and allergenic activity of trypsin inhibitors from the seeds of buckwheat (Fagopyrum esculentum Moench). FEBS Lett. 1997, 400, 103–107. [Google Scholar] [CrossRef]
- Yamada, K.; Shimada, T.; Kondo, M.; Nishimura, M.; Hara-Nishimura, I. Multiple functional proteins are produced by cleaving Asn-Gln bonds of a single precursor by vacuolar processing enzyme. J. Biol. Chem. 1999, 274, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Conners, R.; Konarev, A.V.; Forsyth, J.; Lovegrove, A.; Marsh, J.; Joseph-Horne, T.; Shewry, P.; Brady, R.L. An unusual helix-turn-helix protease inhibitory motif in a novel trypsin inhibitor from seeds of Veronica (Veronica hederifolia L.). J. Biol. Chem. 2007, 282, 27760–27768. [Google Scholar] [CrossRef] [PubMed]
- Oparin, P.B.; Mineev, K.S.; Dunaevsky, Y.E.; Arseniev, A.S.; Belozersky, M.A.; Grishin, E.V.; Egorov, T.A.; Vassilevski, A.A. Buckwheat trypsin inhibitor with helical hairpin structure belongs to a new family of plant defence peptides. Biochem. J. 2012, 446, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Nolde, S.B.; Vassilevski, A.A.; Rogozhin, E.A.; Barinov, N.A.; Balashova, T.A.; Samsonova, O.V.; Baranov, Y.V.; Feofanov, A.V.; Egorov, T.A.; Arseniev, A.S.; et al. Disulfide-stabilized helical hairpin structure and activity of a novel antifungal peptide EcAMP1 from seeds of barnyard grass (Echinochloa crus-galli). J. Biol. Chem. 2011, 286, 25145–25153. [Google Scholar] [CrossRef] [PubMed]
- Rogozhin, E.A.; Ryazantsev, D.Y.; Grishin, E.V.; Egorov, T.A.; Zavriev, S.K. Defense peptides from barnyard grass (Echinochloa crusgalli L.) seeds. Peptides 2012, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Ryazantsev, D.Y.; Rogozhin, E.A.; Dimitrieva, T.V.; Drobyazina, P.E.; Khadeeva, N.V.; Egorov, T.A.; Grishin, E.V.; Zavriev, S.K. A novel hairpin-like antimicrobial peptide from barnyard grass (Echinochloa crusgalli L.) seeds: Structure-functional and molecular-genetics characterization. Biochimie 2014, 99, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Utkina, L.L.; Andreev, Y.A.; Rogozhin, E.A.; Korostyleva, T.V.; Slavokhotova, A.A.; Oparin, P.B.; Vassilevski, A.A.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. Genes encoding 4-Cys antimicrobial peptides in wheat Triticum kiharae Dorof. et Migush.: Multimodular structural organization, instraspecific variability, distribution and role in defence. FEBS J. 2013, 280, 3594–3608. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Yuryev, M.; Ryazantsev, D.Y.; Zavriev, S.K.; Feofanov, A.V.; Grishin, E.V.; Rogozhin, E.A. Studying of cellular interaction of hairpin-like peptide EcAMP1 from barnyard grass (Echinochloa crusgalli L.) seeds with plant pathogenic fungus Fusarium solani using microscopy techniques. Scanning 2016, 38, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and mechanisms of action of naturally occurring antifungal peptides. Cell. Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef] [PubMed]
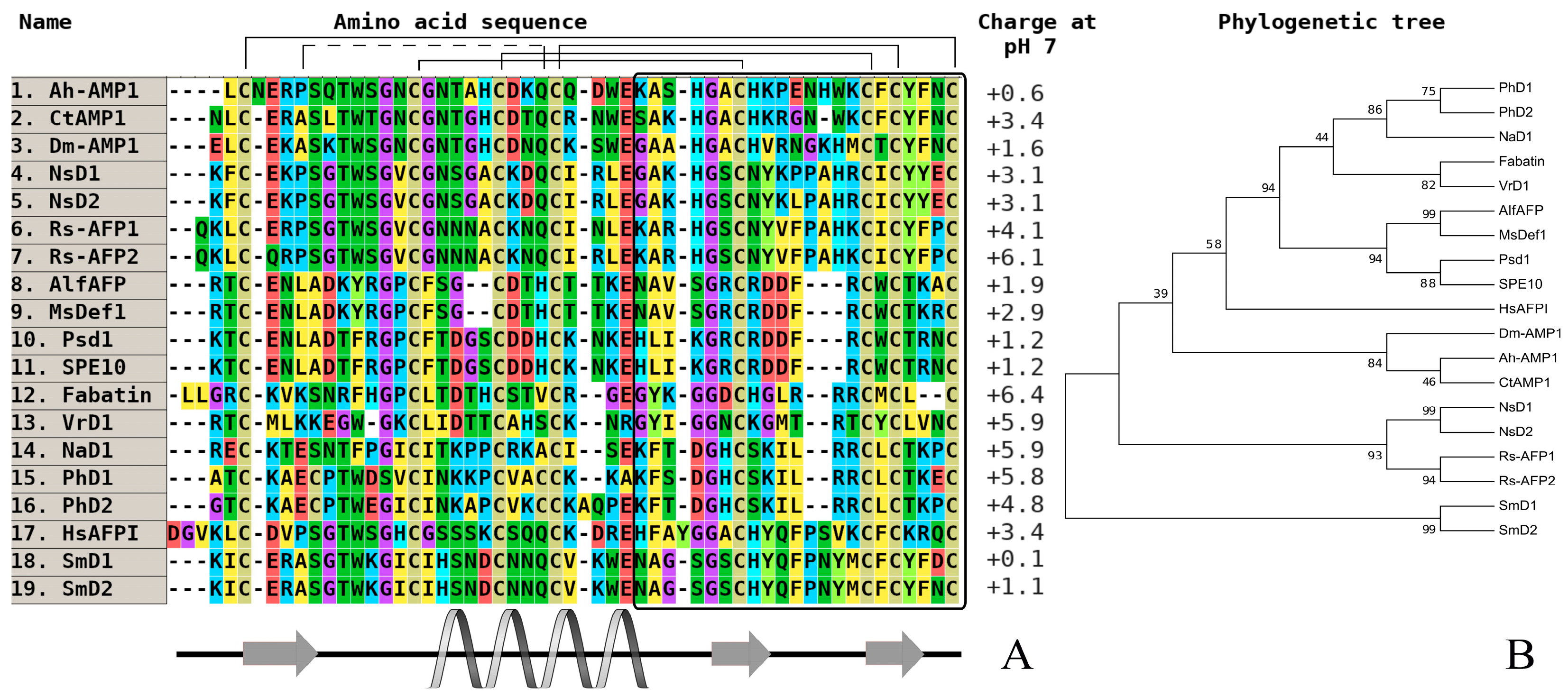
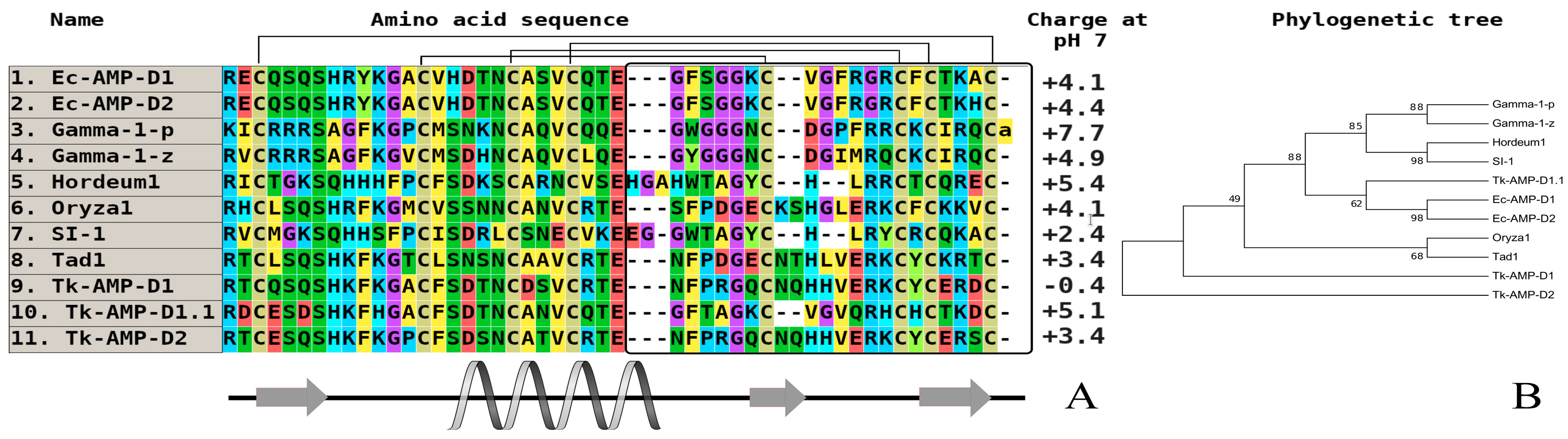
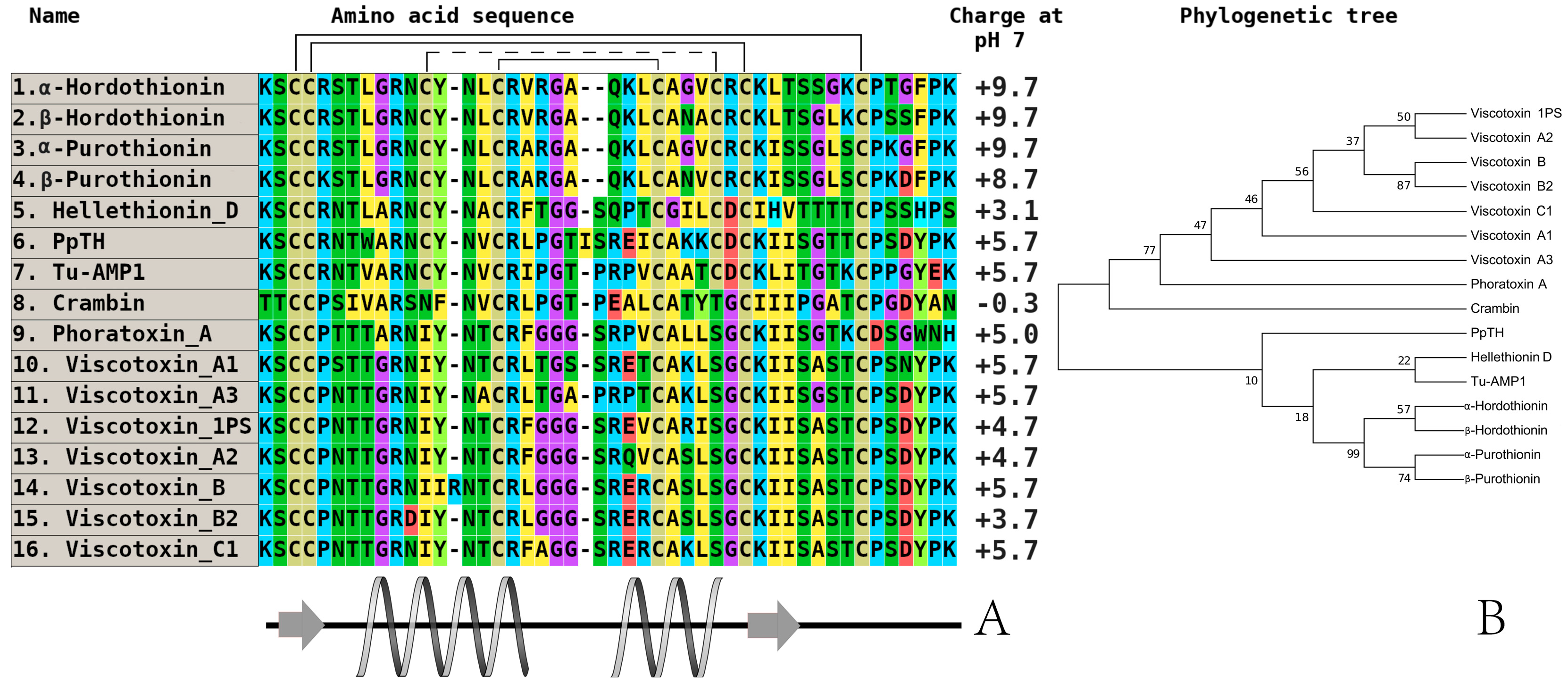
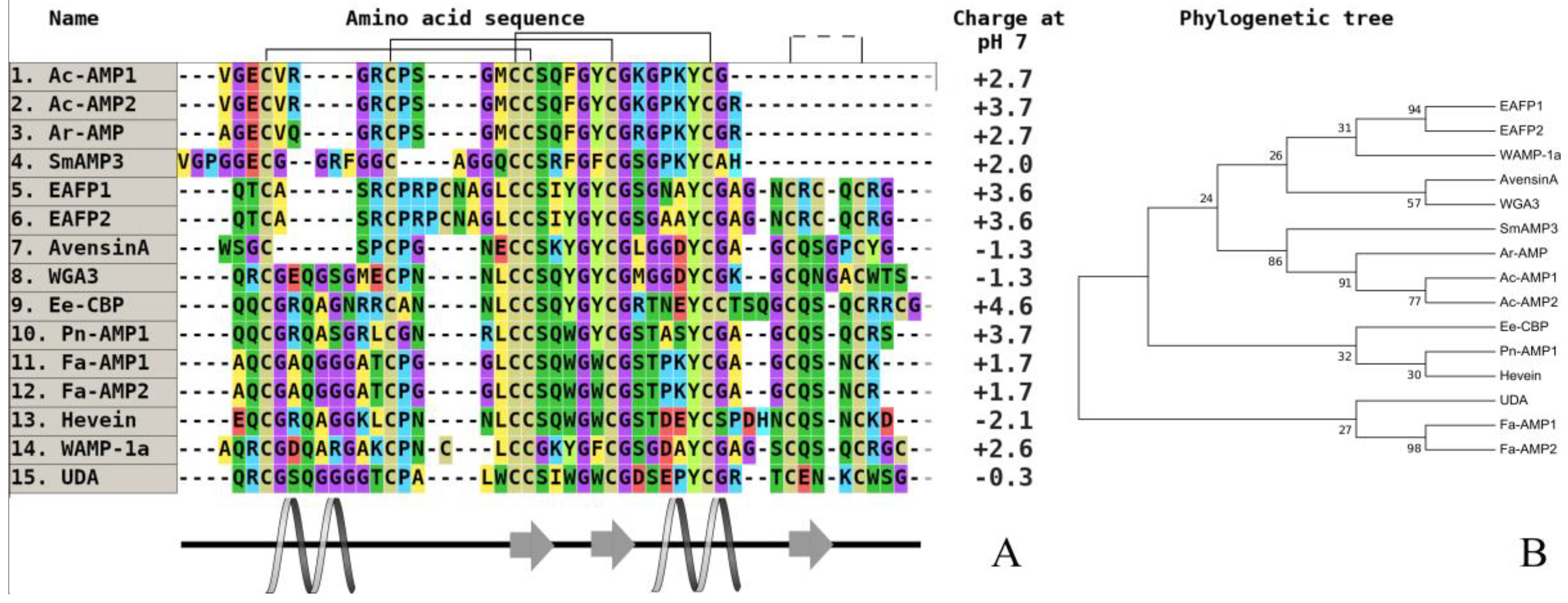
| Peptide/Microbe | α 1 -Purothionin | α -Hordothionin | γ -1-H-Hordothionin | γ -1-P-Purothionin | γ -1-Zeathionin | Tk-AMP-BP1 | Tk-AMP-BP2 |
|---|---|---|---|---|---|---|---|
| Bipolaris sorokiniana | 3.2 | 5.0 | Not tested | Not tested | Not tested | 5.6 | 32.0 |
| Botrytis cinerea | Not tested | 20.0 | Not tested | Not tested | Not tested | Not tested | 32.0 |
| Fusarium oxysporum | 3.9 | 5.0 | 4.0 | 7.6 | 7.0 | 6.0 | Not tested |
| F. solani | Not tested | 5.0 | Not tested | Not tested | Not tested | Not tested | Not tested |
| F. verticillioides | 1.9 | 2.7 | 2.2 | 3.5 | 3.0 | 4.5 | Not tested |
| Neurospora crassa | Not tested | 10.0 | Not tested | Not tested | Not tested | Not tested | Not tested |
| Peptide/Microbe | WAMP1a (+R) | WAMP1b (−R) | WAMP2a (A34K) | WAMP3a (A34E) | WAMP4a (A34N) | LAMP-1a | Ar-AMP |
|---|---|---|---|---|---|---|---|
| Bipolaris sorokiniana | 3.2 | 5.0 | Not tested | Not tested | Not tested | 5.6 | 32.0 |
| Botrytis cinerea | Not tested | 20.0 | Not tested | Not tested | Not tested | Not tested | 32.0 |
| Fusarium oxysporum | 3.9 | 5.0 | 4.0 | 7.6 | 7.0 | 6.0 | Not tested |
| F. solani | Not tested | 5.0 | Not tested | Not tested | Not tested | Not tested | Not tested |
| F. verticillioides | 1.9 | 2.7 | 2.2 | 3.5 | 3.0 | 4.5 | Not tested |
| Neurospora crassa | Not tested | 10.0 | Not tested | Not tested | Not tested | Not tested | Not tested |
| Peptide/Microbe | EcAMP1 | EcAMP2 | EcAMP3 | Tk-AMP-X1 | Tk-AMP-X2 | MBP-1 | Sm-AMP-X |
|---|---|---|---|---|---|---|---|
| Alternaria alternata | 16.0 | >32.0 | 19.8 | Not tested | 28.8 | Not tested | 14.8 |
| Aspergillus niger | >32.0 | >32.0 | 22.4 | Not tested | >32.0 | Not tested | 4.0 |
| B. sorokiniana | 18.2 | >32.0 | 15.0 | Not tested | Not tested | Not tested | >32.0 |
| C. graminicola | >10 | Not tested | Not tested | >30.0 | >30.0 | Not tested | Not tested |
| D. maydis | >10 | Not tested | Not tested | 30.0 | 17.0 | Not tested | Not tested |
| F. graminearum | 4.5 | >32.0 | 5.5 | 7.5 | 7.5 | 4.0 | Not tested |
| F. oxysporum | 8.8 | >32.0 | 9.6 | Not tested | 13.5 | Not tested | 6.8 |
| F. solani | 4.0 | >32.0 | 4.8 | Not tested | 8.5 | Not tested | 8.0 |
| F. verticillioides | 8.1 | >32.0 | 5.2 | 15.0 | 10.0 | Not tested | Not tested |
| P. infestans | 16.3 | >32.0 | 14.0 | Not tested | 25.4 | Not tested | >32.0 |
| P. ultimum | 14.4 | Not tested | Not tested | Not tested | Not tested | Not tested | >32.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogozhin, E.; Ryazantsev, D.; Smirnov, A.; Zavriev, S. Primary Structure Analysis of Antifungal Peptides from Cultivated and Wild Cereals. Plants 2018, 7, 74. https://doi.org/10.3390/plants7030074
Rogozhin E, Ryazantsev D, Smirnov A, Zavriev S. Primary Structure Analysis of Antifungal Peptides from Cultivated and Wild Cereals. Plants. 2018; 7(3):74. https://doi.org/10.3390/plants7030074
Chicago/Turabian StyleRogozhin, Eugene, Dmitry Ryazantsev, Alexey Smirnov, and Sergey Zavriev. 2018. "Primary Structure Analysis of Antifungal Peptides from Cultivated and Wild Cereals" Plants 7, no. 3: 74. https://doi.org/10.3390/plants7030074
APA StyleRogozhin, E., Ryazantsev, D., Smirnov, A., & Zavriev, S. (2018). Primary Structure Analysis of Antifungal Peptides from Cultivated and Wild Cereals. Plants, 7(3), 74. https://doi.org/10.3390/plants7030074





