Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction Procedure
2.4. Total Polyphenol Assay by the Folin–Ciocalteau Method
2.5. Flavonoid Assay
2.6. Antioxidant Activity by the DPPH Method
2.7. Total Dry Residue of Extracts
3. Results and Discussion
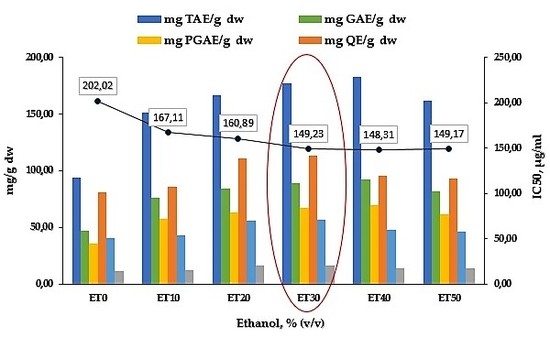
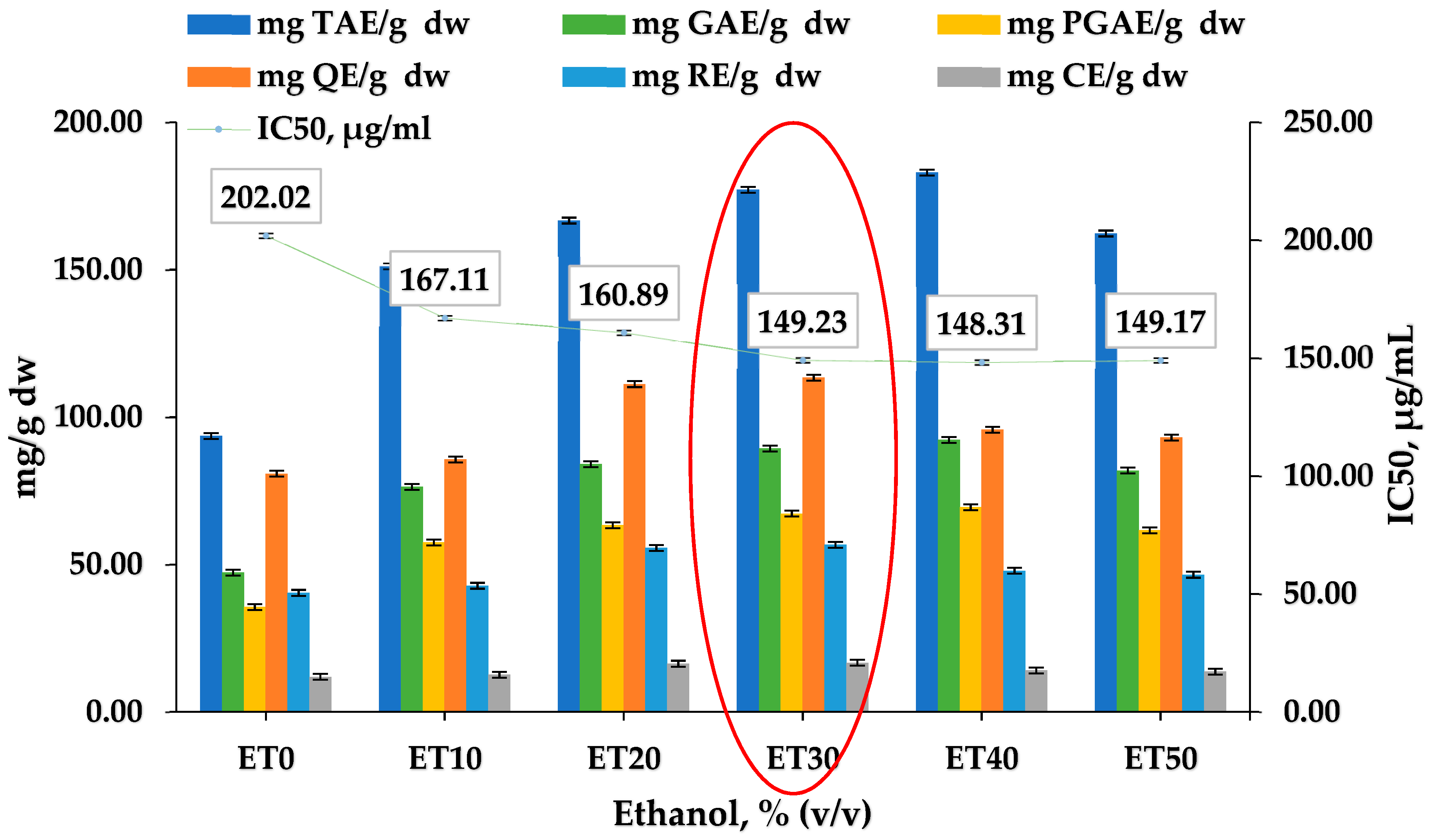
3.1. Effect of the Solvent Used
3.2. Total Polyphenols
3.3. Total Flavonoids
3.4. Antioxidant Capacity
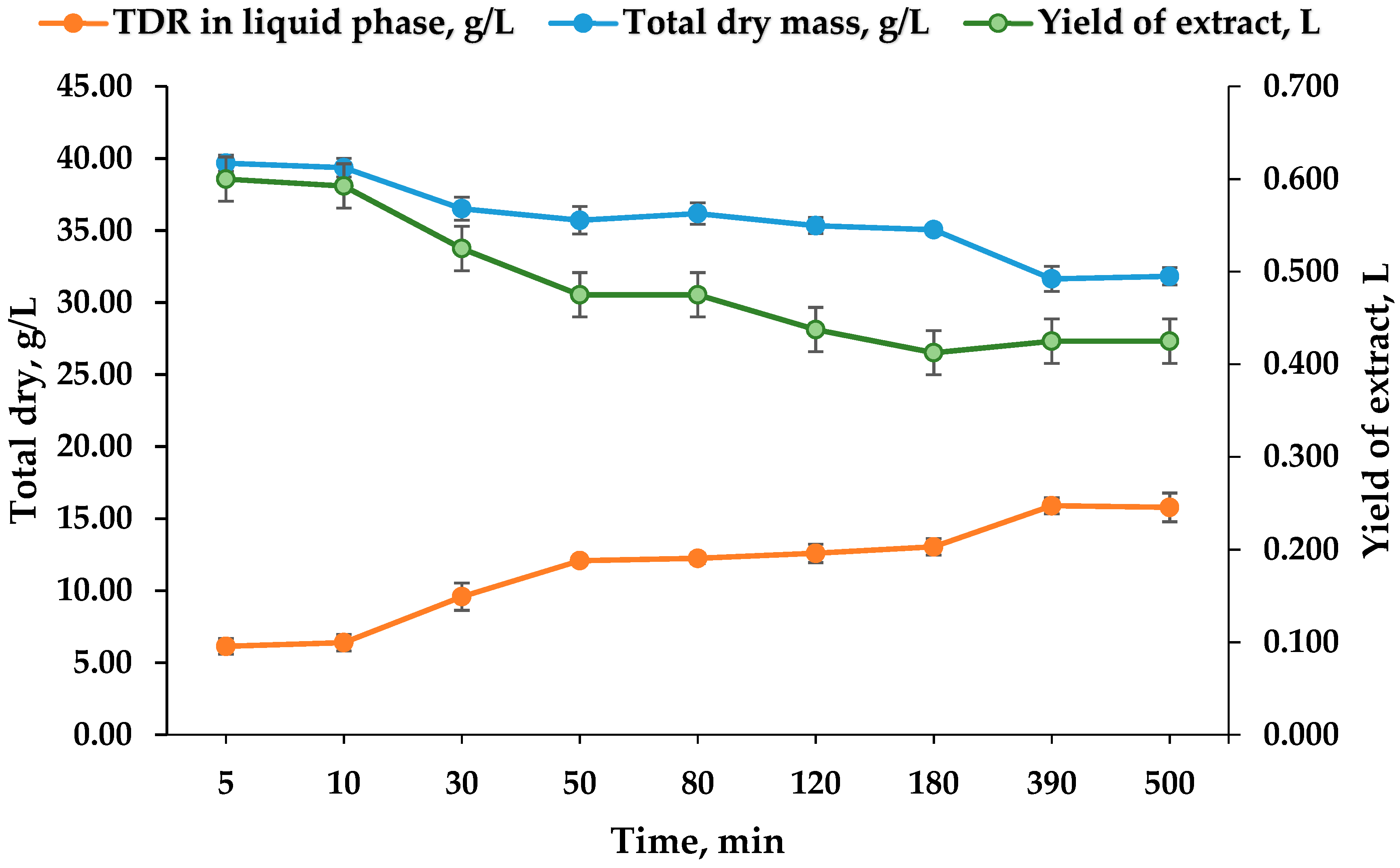
3.5. Total Dry Residue
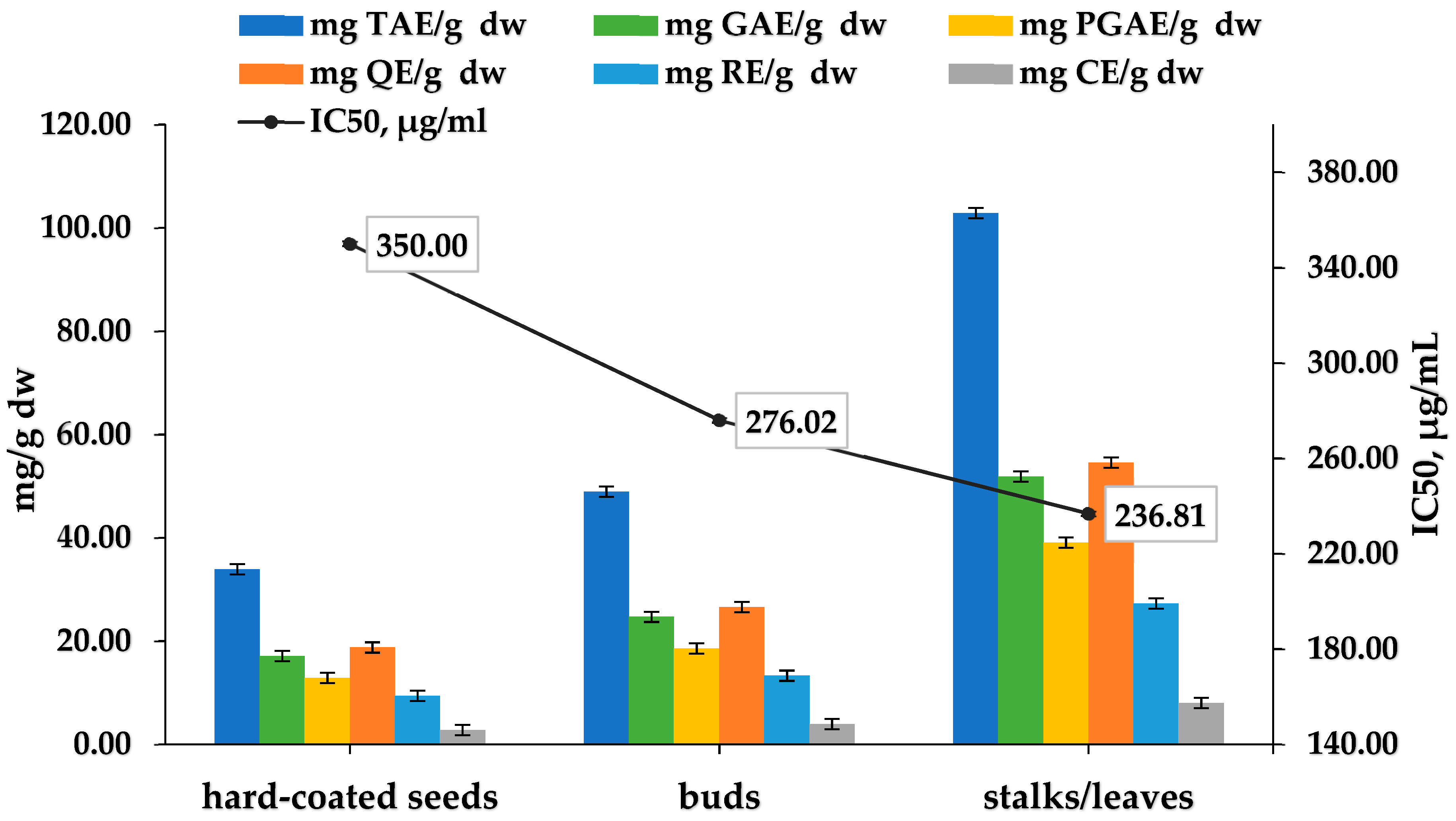
3.6. Evaluation of Cistus incanus Aerial Parts
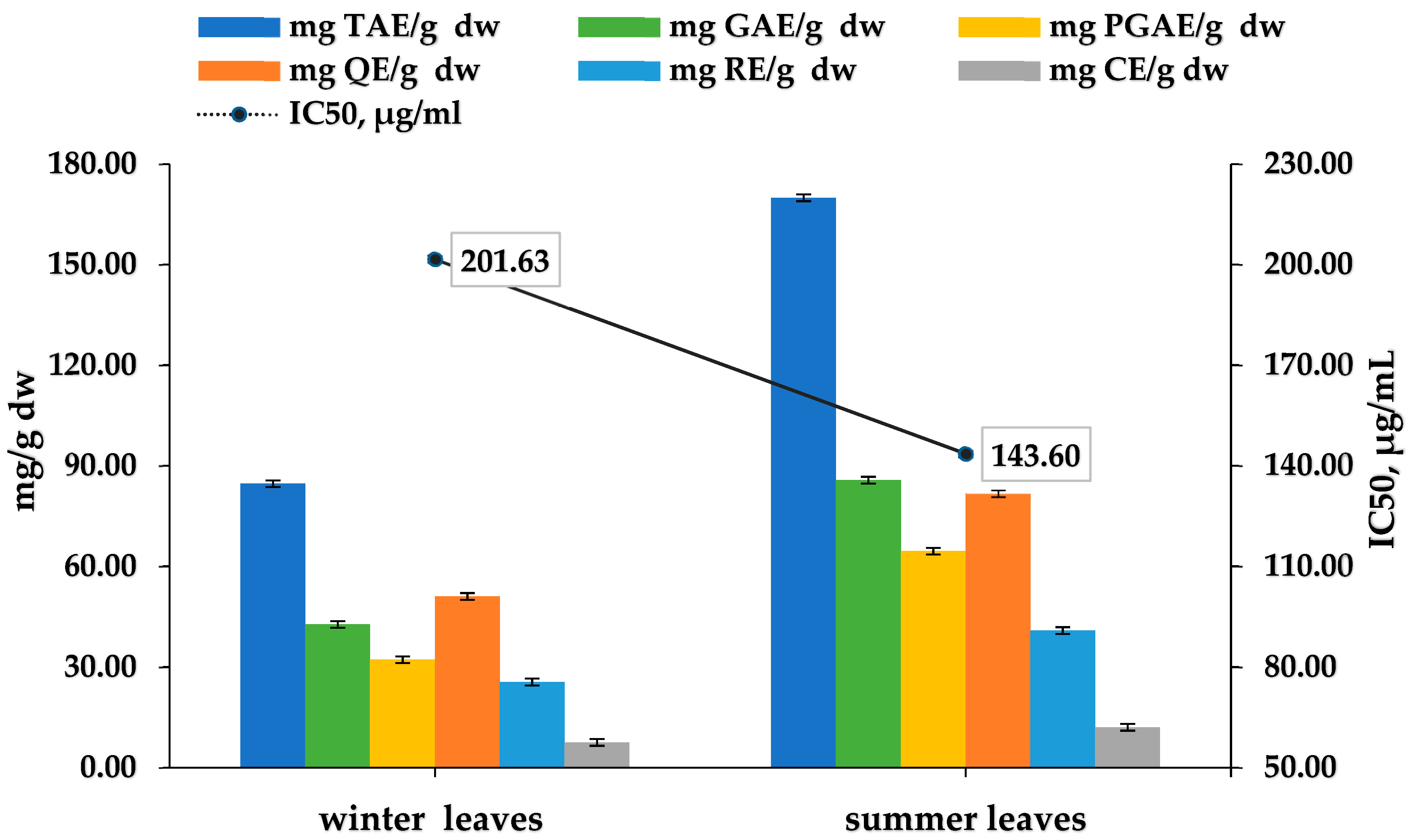
3.7. Evaluation of Cistus incanus Winter and Summer Leaves
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fabricant, D.; Farnsworth, N. The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 2001, 109 (Suppl. 1), 69–75. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Shirzad, H. Toxicity and safety of medicinal plants. J. HerbMed Pharmacol. 2013, 2, 21–22. [Google Scholar]
- Uzunova, S. Plants of Strandja Nature Park; Technoengenring ABC: Burgas, Bulgaria, 2008; ISBN 978-954-92093-6-5. [Google Scholar]
- Gusev, T.; Tzonev, I. Natural Habitats of European Importance in the Protected Area Strandja; Technoengeenering ABC: Malko Tarnovo, Bulgaria, 2014; p. 304. ISBN 978-954-92404-5-0. [Google Scholar]
- Bouamama, H.; Noel, T.; Villard, J.; Benharref, A.; Jana, M. Antimicrobial activities of the leaf extracts of two Moroccan Cistus L. species. J. Ethnopharmacol. 2006, 104, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, I.; Kanellis, K. Isolation and functional analysis of two Cistus creticus cDNAs encoding geranylgeranyl diphosphate synthase. Phytochemistry 2008, 69, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Demetzos, C.; Mitaku, S.; Hotellier, F.; Harvala, A. Heterosides polyphenoliques des feuilles de Cistus creticus L. Ann. Pharm. Fr. 1989, 4, 314–318. [Google Scholar]
- Petereit, F.; Kolodziej, H.; Nahrstedt, A. Proanthocyanidins and Bio Genetically Related Dihydroflavonols from cistus Incanus L. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 729–737. ISBN 978-1-4615-3476-1. [Google Scholar]
- Singleton, L.; Rossi, A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstjc acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Shahidi, F.; Nackz, M. Phenolics in Food and Nutraceuticals; CRC Press: Washington, DC, USA, 2004; ISBN 9781587161384. [Google Scholar]
- Waterhouse, L. Determination of Total Phenolics. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; ISBN 9780471142911. [Google Scholar]
- Avani, P.; Amit, P.; Amit, P.; Patel, N. Estimation of flavonoid, polyphenolic content and in vitro antioxidant capacity of leaves of Tephrosia purpurea Linn. (Leguminosae). Int. J. Pharm. Sci. Res. 2010, 1, 66–77. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Yen, G.; Duh, P. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- Sochor, J.; Ryvolova, M.; Krystofova, O.; Salas, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully automated spectrometric protocols for determination of antioxidant activity: Advantages and disadvantages. Molecules 2010, 15, 8618–8640. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe (COE)—European Directorate for the Quality of Medicines (EDQM). Total dry residue. In European Pharmacopea; Council of Europe (COE)—European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2017; p. 276. [Google Scholar]
- Dimcheva, V.; Karsheva, M. Antioxidant activity and polyphenolic content of the Bulgarian wild herb Cistus incanus L. stored under different conditions. J. Chem. Technol. Metall. 2017, 52, 781–790. [Google Scholar]
- Schierstedt, D. Cistus Extracts. U.S. Patent 20100062068 A1, 11 March 2010. [Google Scholar]
- Robbins, R. Phenolic acids in foods: An overview of analytical methodology. In Phenolics in Food and Nutraceuticals; Shahidi, F.N.M., Ed.; CRC Press: Washington, DC, USA, 2004; pp. 483–490. [Google Scholar]
- Fiuza, S.; Gomes, C.; Teixeira, L.; Girão da Cruz, T.; Cordeiro, M.; Milhazes, N.; Borges, F.; Marques, M. Phenolic acid derivatives with potential anticancer properties a structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A. Folin-Ciocalteau Micro Method for Total Phenol in Wine 2005. Available online: http://waterhouse.ucdavis.edu/faqs/folin-ciocalteau-micro-method-for-total-phenol-in-wine (accessed on 15 July 2005).
- Council of Europe (COE)—European Directorate for the Quality of Medicines (EDQM). Determination of Tannins in Herbal Drugs. In European Pharmacopoeia; Council of Europe (COE)—European Directorate for the Quality of Medicines (EDQM): Strasbourg, France, 2011; p. 243. [Google Scholar]
- Wu, T.; Chu, C.; Chung, G.; Chen, H.; Hsu, S.; Liu, K.; Chen, C. Effects of tannic acid and its related compounds on food mutagens or hydrogen peroxide-induced DNA strands breaks in human lymphocytes. Mutat. Res. 2004, 556, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.; Lynnette, R. Role of plant polyphenols in genomic stability. Mutat. Res. 2001, 475, 89–111. [Google Scholar] [CrossRef]
- Nepka, C.; Sivridis, E.; Antonoglou, O.; Kortsaris, A.; Georgellis, A.; Taitzoglou, I.; Hytiroglou, P.; Papadimitriou, C.; Zintzaras, I.; Kouretas, D. Chemopreventive activity of very low dose dietary tannic acid administration in hepatoma bearing C3H male mice. Cancer Lett. 1999, 141, 57–62. [Google Scholar] [CrossRef]
- Middleton, E. Effect of plant flavonoids on immune and inflammatory cell function. Adv. Exp. Med. Biol. 1998, 439, 175–182. [Google Scholar] [PubMed]
- Rhodes, M.; Keith, P. Analytical problems in the study of flavonoid compounds in onions. Food Chem. 1996, 57, 113–117. [Google Scholar] [CrossRef]
- Havsteen, B. Flavonoids, a class of natural products of high pharmacological potency. Biochem. Pharmacol. 1983, 32, 1141–1148. [Google Scholar] [CrossRef]
- Salvamani, S.; Gunasekaran, B.; Shaharuddin, N.; Ahmad, S.; Shukor, M. Antiartherosclerotic effects of plant flavonoids. BioMed Res. Int. 2014, 2014, 480258. [Google Scholar] [CrossRef] [PubMed]
- Kreft, S.; Knapp, M.; Kreft, I. Extraction of rutin from buckwheat (Fagopyrum esculentum moench) seeds and determination by capillary electrophoresis. J. Agric. Food Chem. 1999, 47, 4649–4652. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.; O’Brien, N. Protection by the flavonoids myricetin, quercetin, and rutin against hydrogen peroxide-induced DNA damage in caco-2 and hep G2 cells. Nutr. Cancer 1999, 34, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Salah, N.; Miller, N.; Paganga, G.; Tijburg, L.; Bolwell, G.; Riceevans, C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-Breaking antioxidants. Arch. Biochem. Biophys. 1995, 322, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Barrajón, E.; Tomás, L.; Morales, A.; Nuria, M.B.; López, S. Rockrose (Cistus sp.) oils. Essential oils in food prevention. Flavor Saf. 2016, 74, 649–657. [Google Scholar]
- Orhan, N.; Aslan, M.; Şüküroğlu, M.; Orhan, D. In vivo and in vitro antidiabetic effect of Cistus laurifolius L. and detection of major phenolic compounds by UPLCTOF-MS analysis. J. Ethnopharmacol. 2013, 146, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Amensour, M.; Sendra, E.; Perez-Alvarez, J.; Skali-Senhaji, N.; Abrini, J.; Fernandez-Lopez, J. Antioxidant activity and chemical content of methanol and ethanol extracts from leaves of rockrose (Cistus ladaniferus). Plant Foods Hum. Nutr. 2010, 65, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Agnieszka, V.; Agnieszka, K.; Krzysztof, W.; Marek, W. Cistus incanus L. commercial products as a good source of polyphenols in human diet. Ind. Crops Prod. 2017, 107, 297–304. [Google Scholar]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N. Influence of biological, environmental and technical factors on phenolic and antioxidant activities of Tunisian halophytes. C. R. Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Saggu, S.; Sakeran, M.; Zidan, N.; Rehman, H.; Ansari, A. Phytochemical, antioxidant and mineral composition of hydroalcoholic extract of chicory (Cichorium intybus L.) leaves. Saudi J. Biol. Sci. 2015, 22, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Saggu, S.; Sakeran, M.; Zidan, N.; Tousson, E.; Mohan, A.; Rehman, H. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food Chem. Toxicol. 2014, 72, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Razali, N.; Razab, R.; Mat Junit, S.; Abdul Aziz, A. Radical scavenging and reducing properties of extracts of cashew shoots (Anacardium occidentale). Food Chem. 2008, 111, 38–44. [Google Scholar] [CrossRef]
- Li, H.; Hao, Z.; Wang, X.; Huang, L.; Li, J. Antioxidant activities of extracts and fractions from Lysimachia foenum-graecum Hance. Bioresour. Technol. 2009, 100, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Kitanov, B. Distribution and Collection of Herbs, 1st ed.; Zemizdat: Sofia, Bulgaria, 1987; p. 287. ISBN 9532726431. [Google Scholar]
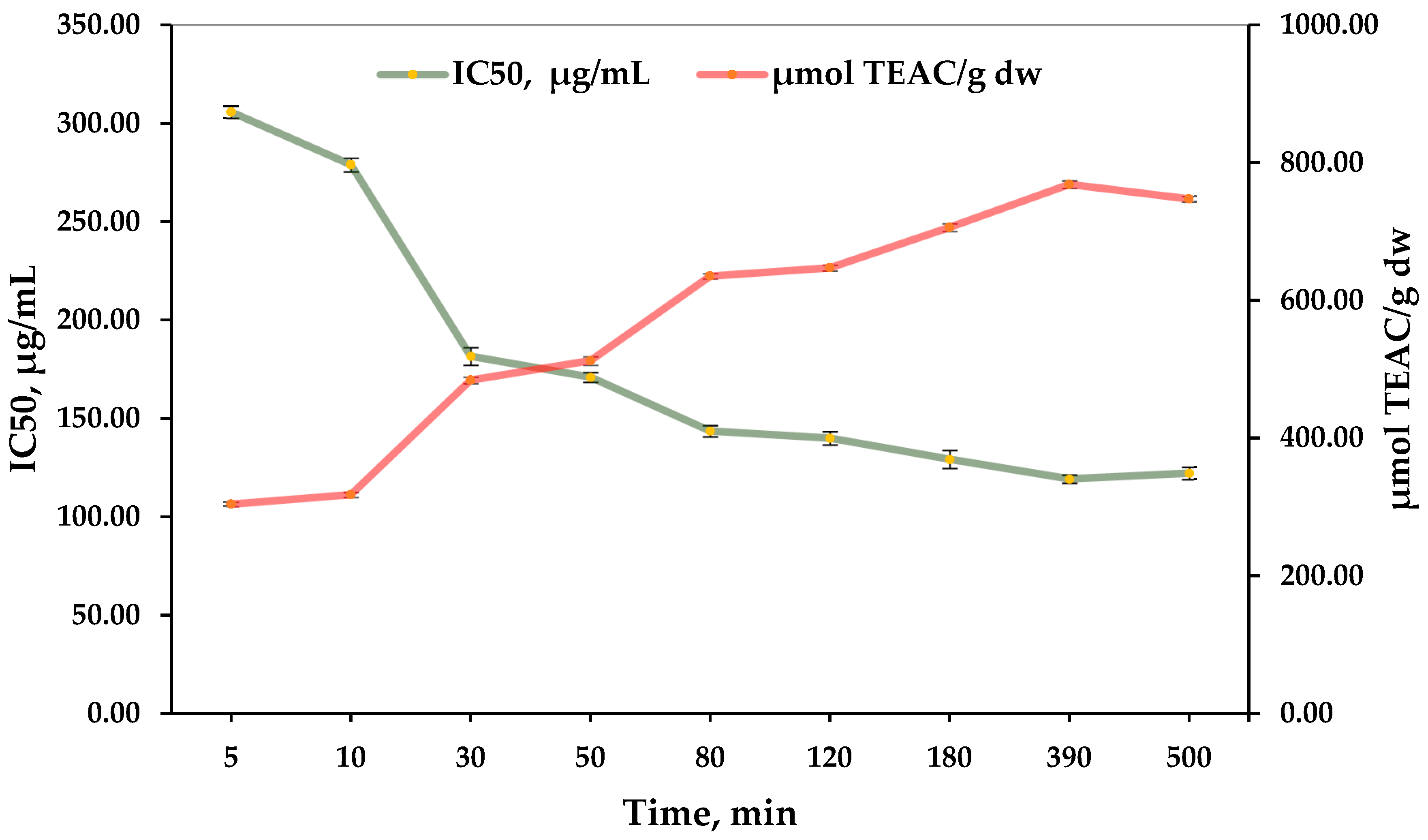
| Extraction Time, min | mg PGAE/g dw ± SD * | mg GAE/g dw ± SD * | mg TAE/g dw ± SD * |
|---|---|---|---|
| 5 | 27.30 ± 1.34 | 36.26 ± 1.76 | 71.88 ± 3.48 |
| 10 | 35.67 ± 1.20 | 47.38 ± 1.62 | 93.91 ± 3.21 |
| 30 | 52.07 ± 2.77 | 69.17 ± 3.65 | 137.09 ± 7.22 |
| 50 | 68.47 ± 2.45 | 90.95 ± 3.23 | 180.27 ± 6.40 |
| 80 | 67.26 ± 1.20 | 89.35 ± 1.62 | 177.08 ± 3.20 |
| 120 | 75.07 ± 2.05 | 99.73 ± 2.71 | 197.66 ± 5.36 |
| 180 | 82.89 ± 0.95 | 110.11 ± 1.25 | 218.24 ± 2.47 |
| 390 | 86.81 ± 2.35 | 115.32 ± 3.10 | 228.56 ± 6.14 |
| 500 | 82.55 ± 1.95 | 109.66 ± 2.57 | 217.34 ± 5.08 |
| Extraction Time, min | mg QE/g dw ± SD * | mg CE/g dw ± SD * | mg RE/g dw ± SD * |
|---|---|---|---|
| 5 | 40.80 ± 4.52 | 6.00 ± 0.75 | 20.40 ± 2.26 |
| 10 | 46.81 ± 5.20 | 6.88 ± 0.76 | 23.40 ± 2.60 |
| 30 | 68.21 ± 4.85 | 10.03 ± 0.71 | 34.10 ± 2.42 |
| 50 | 85.61 ± 3.85 | 12.59 ± 0.57 | 42.80 ± 1.92 |
| 80 | 113.37 ± 2.85 | 16.67 ± 0.42 | 56.68 ± 1.43 |
| 120 | 120.07 ± 3.69 | 17.66 ± 0.54 | 60.04 ± 1.85 |
| 180 | 133.35 ± 4.15 | 19.61 ± 0.61 | 66.67 ± 2.08 |
| 390 | 138.44 ± 6.82 | 20.36 ± 1.00 | 69.22 ± 3.41 |
| 500 | 119.20 ± 5.63 | 17.53 ± 0.83 | 59.60 ± 2.82 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimcheva, V.; Karsheva, M. Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants. Plants 2018, 7, 8. https://doi.org/10.3390/plants7010008
Dimcheva V, Karsheva M. Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants. Plants. 2018; 7(1):8. https://doi.org/10.3390/plants7010008
Chicago/Turabian StyleDimcheva, Vanya, and Maria Karsheva. 2018. "Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants" Plants 7, no. 1: 8. https://doi.org/10.3390/plants7010008
APA StyleDimcheva, V., & Karsheva, M. (2018). Cistus incanus from Strandja Mountain as a Source of Bioactive Antioxidants. Plants, 7(1), 8. https://doi.org/10.3390/plants7010008