Polar Constituents of Salvia willeana (Holmboe) Hedge, Growing Wild in Cyprus
Abstract
:1. Introduction
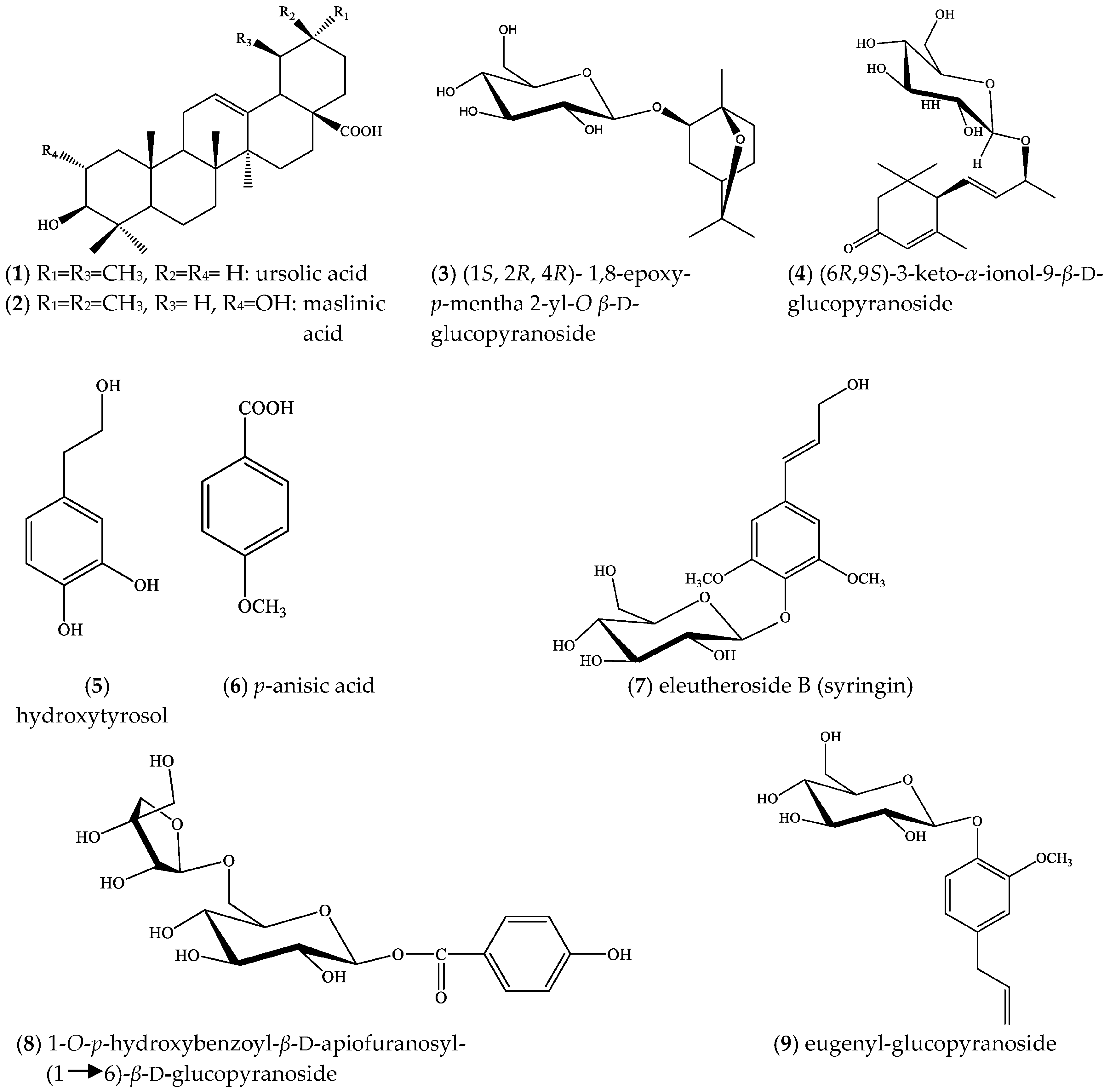
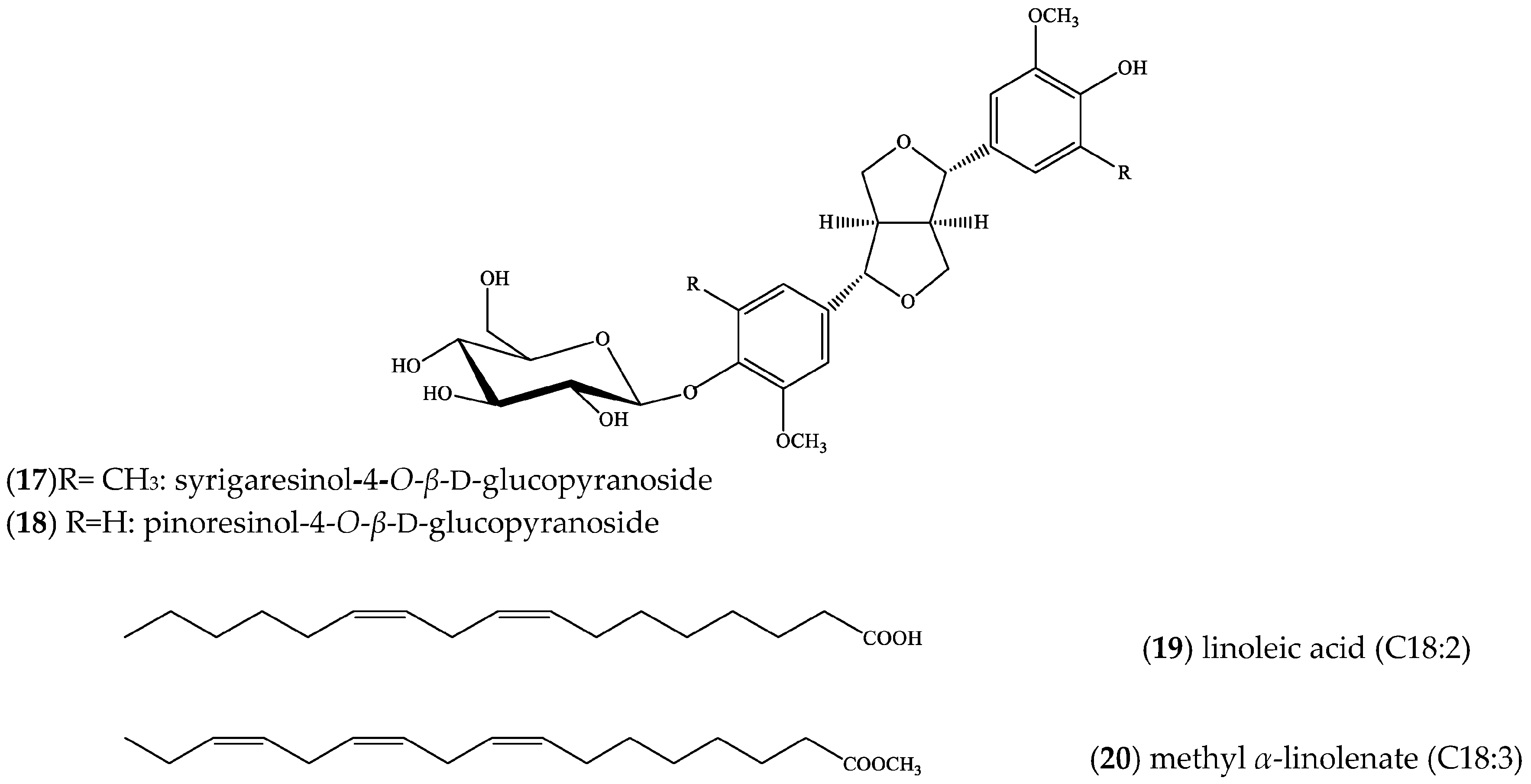
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Equipment and Reagents
4.3. Extraction and Chromatography
4.4. NMR Data of 5, 8, and 13
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lu, Y.; Foo, L.Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Walker, J.B.; Systma, K.J. Staminal Evolution in the genus Salvia (Lamiaceae). Ann. Bot. 2007, 100, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-B.; Ni, Z.-Y.; Shi, Q.-W.; Dong, M.; Kiyota, H.; Gu, Y.-C.; Cong, B. Constituents from Salvia Species and Their Biological Activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Kamatou, G.P.P.; Makunga, N.P.; Ramogola, W.P.N.; Viljoena, A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008, 119, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Grieve, M. A Modern Herbal; Savas Publishing: El Dorado Hills, CA, USA, 1984; ISBN 13 9780486227986. [Google Scholar]
- Dereboylu, A.; Şengonca, N.; Güvensen, A.; Gücel, S. Anatomical and palynological characteristics of Salvia willeana (Holmboe) Hedge and Salvia veneris Hedge endemic to Cyprus. Afr. J. Biotechnol. 2010, 9, 2076–2088. [Google Scholar]
- Bellomaria, B.; Arnold, N.; Valentini, G. Contribution to the study of the essential oils from three species of Salvia growing wild in the eastern Mediterranean region. J. Essent. Oil Res. 1992, 4, 607–614. [Google Scholar] [CrossRef]
- Vonaparti, A.; Karioti, A.; Recio, M.; Máñez, S.; Ríos, J.; Skaltsa, E.; Giner, R. Effects of terpenoids from Salvia willeana in delayed-type hypersensitivity, human lymphocyte proliferation and cytokine production. Nat. Prod. Commun. 2008, 3, 1953–1958. [Google Scholar]
- De la Torre, M.; Bruno, M.; Savona, F.; Rodriquez, B.; Apostolides Arnold, N. Terpenoids from Salvia willeana and S. virgata. Phytochemistry 1990, 29, 668–670. [Google Scholar] [CrossRef]
- Wang, M.; Kikuzaki, H.; Lin, C.; Kahyaoglu, A.; Huang, M.; Nakatani, N.; Ho, C. Acetophenone Glycosides from Thyme (Thymus vulgaris L.). J. Agric. Food Chem. 1999, 47, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Yogiashi, Y.; Matsuda, S.; Suzuki, A.; Kikuchi, M. A new phenolic glycoside syringate from the bark of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. J. Nat. Med. 2009, 63, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Kikuzaki, H.; Zhu, N.; Sang, S.; Nakatani, N.; Ho, C.-T. Isolation and structural elucidation of two new glycosides from sage (Salvia officinalis L.). J. Agric. Food Chem. 2000, 48, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Olennikov, D.; Chekhirova, G.V. 6″-Galloylpicein and other phenolic compounds from Arctostaphylos uva-ursi. Chem. Nat. Compd. 2013, 49, 1–7. [Google Scholar] [CrossRef]
- Özgen, U.; Sevindik, H.; Kazaz, C.; Yigit, D.; Kandemir, A.; Secen, H.; Calis, I. A new sulfated a-Ionone glycoside from Sonchus erzincanicus Matthews. Molecules 2010, 15, 2593–2599. [Google Scholar] [CrossRef] [PubMed]
- Benayache, F.; Boureghda, A.; Ameddah, S.; Marchioni, E.; Benayache, S. Flavonoids from Thymus numidicus Poiret. Pharm. Lett. 2014, 6, 50–54. [Google Scholar]
- Rashed, K.; Ćirić, A.; Glamočlija, J.; Calhelha, R. Antimicrobial and cytotoxic activities of Alnus rugosa L. aerial parts and identification of the bioactive components. Ind. Crops Prod. 2014, 59, 189–196. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 2000, 55, 263–267. [Google Scholar] [CrossRef]
- Ikan, R. Natural Products. A Laboratory Guide, 2nd ed.; Academic Press Inc.: Cambridge, MA, USA, 1991; pp. 9–11. [Google Scholar]
- Carvalho, M.G.; Costa, P.M.; Santos Abreu, H. Flavones from Vernonia diffusa. J. Braz. Chem. Soc. 1999, 10, 163–166. [Google Scholar] [CrossRef]
- Marder, M.; Viola, H.; Wasowski, C.; Fernández, S.; Medina, J.; Paladini, A. 6-Methylapigenin and hesperidin, new valeriana flavonoids with activity on the CNS. Pharmacol. Biochem. Behav. 2003, 75, 537–545. [Google Scholar] [CrossRef]
- Aghel, N.; Ramezani, Z.; Beiranvard, S. Hesperidin from Citrus sinensis Cultivated in Dezful, Iran. Pak. J. Biol. Sci. 2008, 11, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Nizamutdinova, I.T.; Jeong, J.J.; Xu, G.H.; Lee, S.; Kang, S.S.; Kim, Y.S.; Chang, K.C.; Kim, H.J. Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-α-stimulated human umbilical vein endothelial cells. Int. Immunopharmacol. 2008, 8, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Maltese, F.; Erkelens, C.; Kooy, F.; Choi, Y.; Verpoorte, R. Identification of natural epimeric flavanone glycosides by NMR spectroscopy. Food Chem. 2009, 116, 575–579. [Google Scholar] [CrossRef]
- Han, S.; Mok, S.; Kim, H.; Lee, J.; Lee, D.; Lee, S.Y.; Kim, J.; Kim, S.; Lee, S. Determination of hesperidin in mixed tea by HPLC. J Agric. Sci. 2011, 38, 295–299. [Google Scholar]
- Lee, C.K.; Chang, M.H. The chemical constituents from the heartwood of Eucalyptus citriodora. J Chin. Chem. Soc. 2000, 47, 555–560. [Google Scholar] [CrossRef]
- Hatzakis, E.; Agiomyrgianaki, A.; Kostidis, S.; Dais, P. High-resolution NMR spectroscopy, an alternative fast tool for Qualitative and Quantitative Analysis of Diacylglycerol (DAG) oil. J. Am. Oil Chem. Soc. 2011, 88, 1695–1708. [Google Scholar] [CrossRef]
- Horst, M.; Urbin, S.; Burton, R.; MacMillan, C. Using proton nuclear magnetic resonance as a rapid response research tool for methyl ester characterization in biodiesel. Lipid Technol. 2009, 21, 1–3. [Google Scholar] [CrossRef]
- Güvenalp, Z.; Özbek, H.; Kuruüzüm-Uz, A.; Kazaz, C.; Demirezer, Ö.L. Secondary metabolites from Nepeta heliotropifolia. Turk. J. Chem. 2009, 33, 667–675. [Google Scholar]
- Ragasa, Y.; Ng, V.A.S.; Ebajo, V.D.; Fortin, D.R.; De Los Reyes, M.M.; Shen, C.-C. Triterpenes from Shorea negrosensis. Pharm. Lett. 2014, 6, 453–458. [Google Scholar]
- Raza, R.; Ilyas, Z.; Ali, S.; Muhammad, N.M.; Muhammad, Y.; Khokhar, M.Y.; Iqbal, J. Identification of Highly Potent and Selective α-Glucosidase Inhibitors with Antiglycation Potential, Isolated from Rhododendron arboreum. Rec. Nat. Prod. 2015, 9, 262–266. [Google Scholar]
- Tanaka, J.C.A.; Vidotti, G.J.; da Silva, C.C. A New Tormentic Acid Derivative from Luehea divaricata Mart. (Tiliaceae). J. Braz. Chem. Soc. 2003, 14, 475–478. [Google Scholar] [CrossRef]
- Rungsimakan, S. Phytochemical and Biological Activity Studies on Salvia viridis L. Ph.D. Thesis, Department of Pharmacy and Pharmacology, University of Bath, Bath, UK, 2011. [Google Scholar]
- Woo, W.K.; Han, Y.J.; Un Choi, S.; Ki, H.; Kim, K.H.; Lee, K.R. Triterpenes from Perilla frutescens var. acuta and Their Cytotoxic Activity. Nat. Prod. Sci. 2014, 20, 71–75. [Google Scholar]
- Manns, D. Linalool and cineole type glucosides from Cunila spicata. Phytochemistry 1995, 39, 1115–1118. [Google Scholar] [CrossRef]
- Wang, M.; Shao, Y.; Huang, T.; Wei, G.; Ho, C. Isolation and structural elucidation of aroma constituents bound as glucosides from Sage (Salvia officinalis). J. Agric. Food Chem. 1998, 46, 2509–2511. [Google Scholar] [CrossRef]
- Pabst, A.; Barron, D.; Sémon, E.; Schreier, P. Two diastereomeric 3-oxo-α-ionol β-d-glucosides from Raspberry fruit. Phytochemistry 1992, 31, 1649–1652. [Google Scholar] [CrossRef]
- Takeda, Y.; Zhang, H.; Matsumoto, T.; Otsuka, H.; Oosio, Y.; Honda, G.; Tabata, M.; Fujita, T.; Su, H.; Sezik, E.; et al. Megastigmane glycosides from Salvia nemorosa. Phytochemistry 1997, 44, 117–120. [Google Scholar] [CrossRef]
- Sugiyama, M.; Nagayama, E.; Kikuchi, M. Lignan and Phenylpropanoid glycosides from Osmanthus asiaticus. Phytochemistry 1993, 33, 1215–1219. [Google Scholar] [CrossRef]
- Kim, M.; Moon, H.; Lee, D.; Woo, E. A new lignan glycoside from the Stem bark of Styrax japonica S. et Z. Arch. Pharm. Res. 2007, 30, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yin, C.; Cheng, Z. A new tetrahydrofuran lignan diglycoside from Viola tianshanica Maxim. Molecules 2013, 18, 13636–13644. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, M.; Pae, S.; Oh, K.; Jung, C.; Baek, I.; Lee, S. Analysis of yield of eleutherosides B and E in Acanthopanax divaricatus and A. koreanum Grown with varying cultivation methods. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Lall, N.; Kishore, N.; Binneman, B.; Twilley, D.; Venter, M.; Plessis-Stoman, D.; Boukes, G.; Hussein, A. Cytotoxicity of Syringin and 4-methoxycinnamyl alcohol isolated from Foeniculum vulgare on selected human cell lines. Nat. Prod. Res. 2015, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Mulkens, A.; Kapetanidis, I. Eugenylglucoside, a new natural phenylpropanoid heteroside from Melissa officinalis. J. Nat. Prod. 1988, 51, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Ushiyama, M.; Furuya, T. Glycosylation of phenolic compounds by root culture of Panax ginseng. Phytochemistry 1989, 28, 3009–3013. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Rosmarinic acid derivatives from Salvia officinalis. Phytochemistry 1999, 51, 91–94. [Google Scholar] [CrossRef]
- Woo, E.; Piao, M. Antioxidative Constituents from Lycopus lucidus. Arch. Pharm. Res. 2004, 27, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Özgen, U.; Mavi, A.; Terzi, Z.; Kazaz, C.; Aşçi, A.; Kaya, Y.; Seçen, H. Relationship between chemical structure and antioxidant activity of luteolin and its glyxosides isolated from Thymus sipyleus subsp. sipyleus var. sipyleus. Rec. Nat. Prod. 2011, 5, 12–21. [Google Scholar]
- Dapkevicius, A.; Beek, T.; Lelyveld, G.; Veldhuizen, A.; Groot, A.; Linssen, J.; Venskutonis, R. Isolation and structure Elucidation of radican scavengers from Thymus vulgaris Leaves. J. Nat. Prod. 2002, 65, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.A.; Abdel-Azim, N.S.; Pieters, L.; Vlietinck, A.J. Isolation and NMR spectra of syringaresinol-β-d-glucoside from Cressa cretica. Fitoterapia 2004, 75, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Park, H.B.; Lee, K.H.; Kim, K.H.; Lee, I.K.; Noh, H.J.; Choi, S.U.; Lee, K.R. Lignans from the roots of Berberis amurensis. Nat. Prod. Sci. 2009, 15, 17–21. [Google Scholar]
- Klimek, B.; Tokar, M. Biologically active compounds from the flowers of Forsythia suspensa Vahl. Acta Pol. Pharm. 1998, 55, 499–504. [Google Scholar]
- Ouyang, M.; Shung, Y.; Zhang, Z.K.; Kuo, Y.H. Inhibitory Activity against Tobacco Mosaic Virus (TMV) Replication of Pinoresinol and Syringaresinol Lignans and Their Glycosides from the Root of Rhus javanica var. roxburghiana. J. Agric. Food Chem. 2007, 55, 6460–6465. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.; Shen, C.; Lin, L. Anti-inflammatory principles from Balanopora laxiflora. J. Food Drug Anal. 2011, 19, 502–508. [Google Scholar]
- Kim, A.R.; Ko, H.J.; Chowdhury, M.A.; Chang, Y.; Woo, E. Chemical constituents of the aerial parts of Artemisia selengensis and their IL-6 inhibitory activity. Arch. Pharm. Res. 2015, 38, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Mastelić, J.; Marijanović, Z. A variety of volatile compounds as Markers in Unifloral Honey from Dalmatian Sage (Salvia officinalis L.). Chem. Biodivers. 2006, 3, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chan, Y. Five new iridoids from roots of Salvia digitaloides. Molecules 2014, 19, 15521–15534. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.G.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer: New York, NY, USA, 1970. [Google Scholar]
- Coll, J.C.; Bowden, B.F. The application of Vacuum Liquid Chromatography to the separation of terpene mixtures. J. Nat. Prod. 1986, 49, 934–936. [Google Scholar] [CrossRef]
- Stahl, E. Thin-Layer Chromatography, a Laboratory Handbook, 2nd ed.; Springer: Berlin/Heideiberg, Germany, 1969. [Google Scholar]
- Neu, R. Chelate von Diarylborsäuren mit aliphatischen Oxyalkylaminen als Reagenzien für den Nachweis von Oxyphenyl-benzo-γ-pyronen. Naturwissenschaften 1957, 44, 181–183. [Google Scholar] [CrossRef]
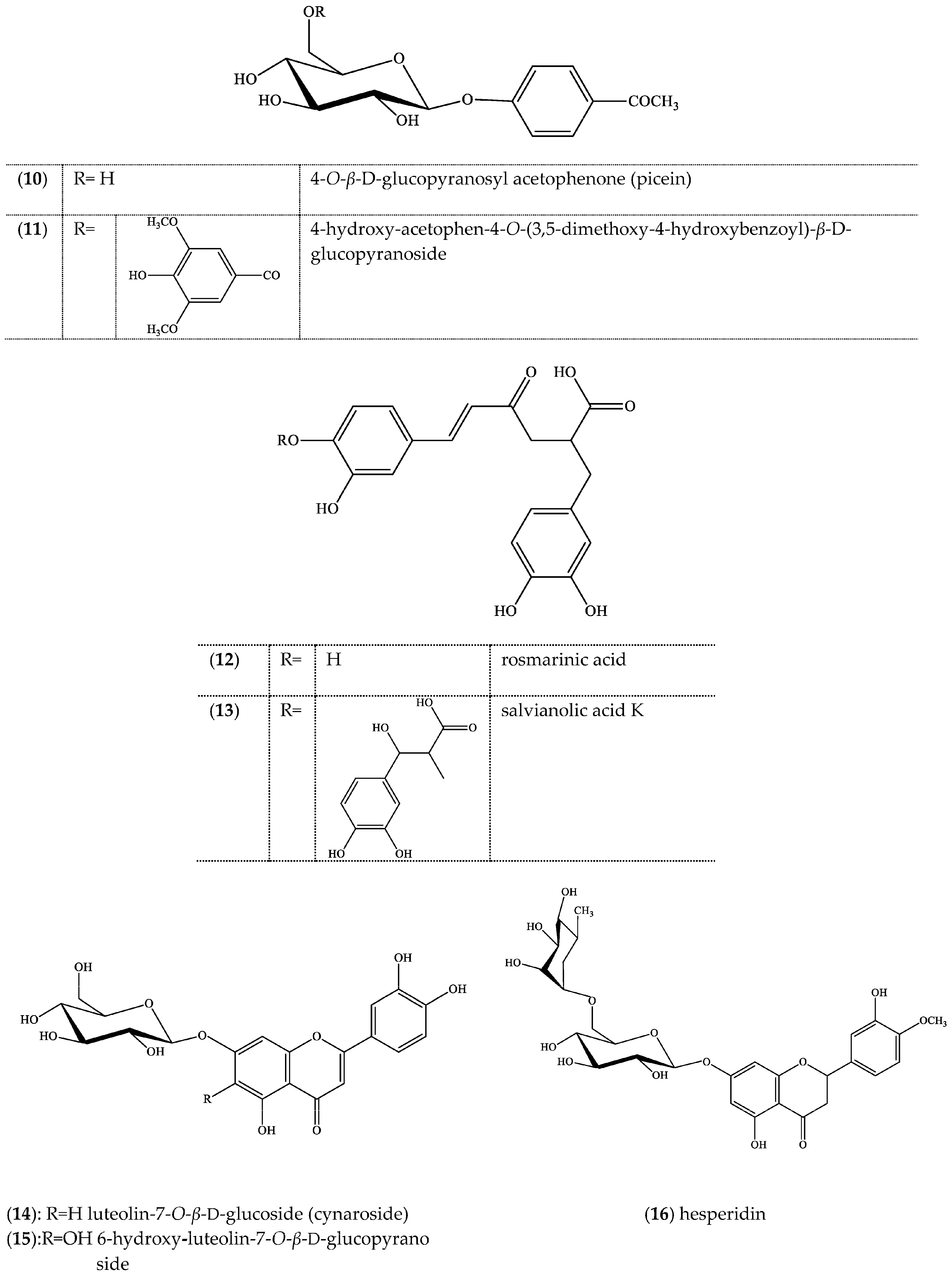
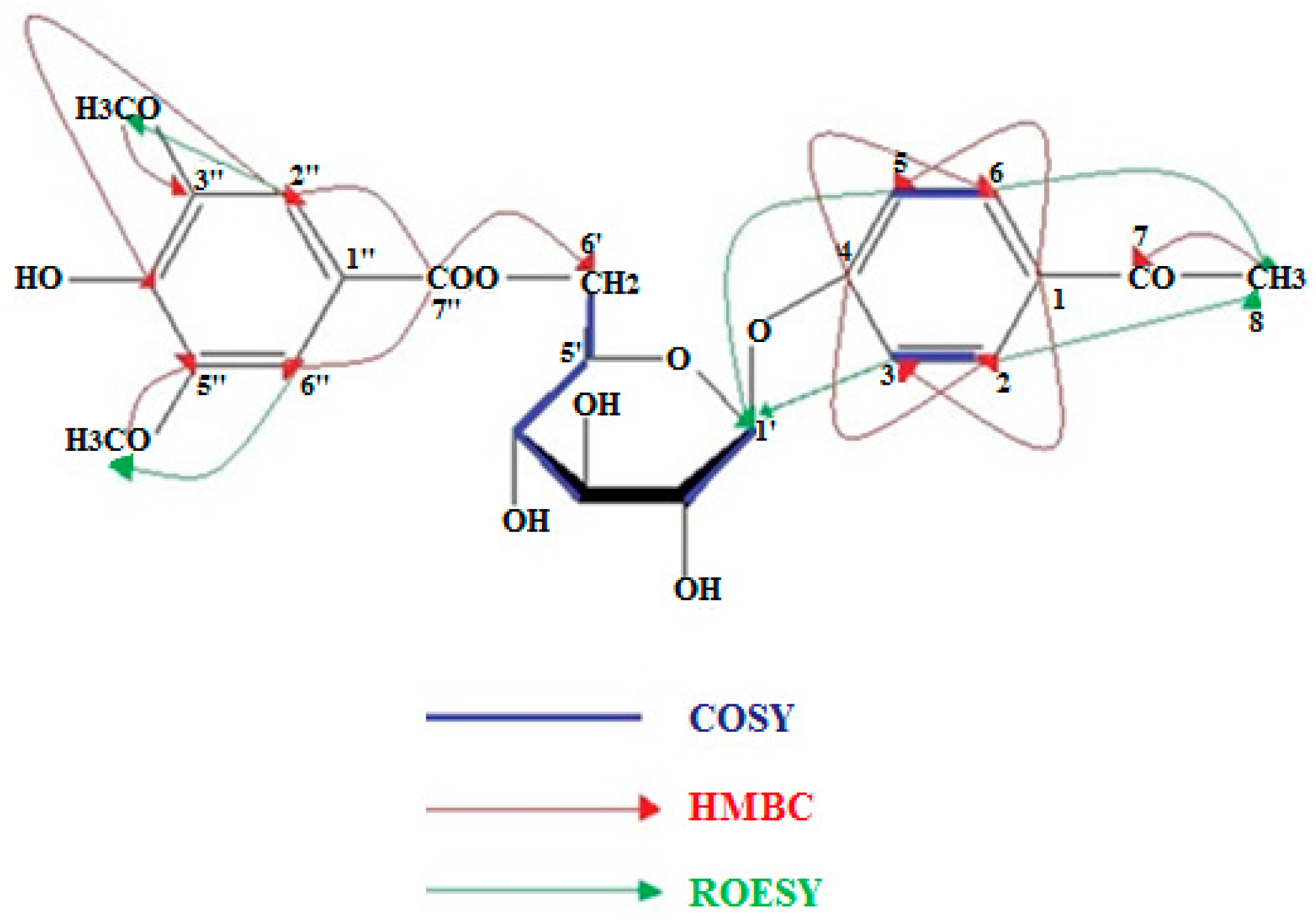
| δC | C | δH | H | J (Hz) | |
|---|---|---|---|---|---|
| 1 | 131.5 | C | - | - | - |
| 2 | 130.1 | CH | 7.69 | 1 | d (J = 9.0) |
| 3 | 116.2 | CH | 7.05 | 1 | d (J = 9.0) |
| 4 | 162.5 | C | - | - | - |
| 5 | 116.2 | CH | 7.05 | 1 | d (J = 9.0) |
| 6 | 130.1 | CH | 7.69 | 1 | d (J = 9.0) |
| 7 | 199.1 | C | - | - | - |
| 8 | 26.5 | CH3 | 2.47 | 3 | s |
| 1′ | 101.7 | CH | 5.04 | 1 | d (J = 7.8) |
| 2′ | 74.5 | CH | 3.53 | 2 | m |
| 3′ | 77.5 | CH | |||
| 4′ | 71.3 | CH | 3.43 | 1 | m |
| 5′ | 75.5 | CH | 3.89 | 1 | dd (J = 8.0, 2.3) |
| 6a′ | 64.8 | CH2 | 4.71 | 2 | dd (J = 11.7, 2.3) |
| 6b′ | 4.46 | dd (J = 11.7, 8.0) | |||
| 1″ | - | C | - | - | - |
| 2″ | 108.3 | CH | 7.33 | 1 | s |
| 3″ | 149.0 | C | - | - | - |
| 4″ | 142.3 | C | - | - | - |
| 5″ | 149.0 | C | - | - | - |
| 6″ | 108.3 | CH | 7.33 | 1 | s |
| 7″ | 167.3 | C | - | - | - |
| 3″, 5″-OCH3 | 56.0 | CH3 | 3.83 | 6 | s |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mailis, T.; Skaltsa, H. Polar Constituents of Salvia willeana (Holmboe) Hedge, Growing Wild in Cyprus. Plants 2018, 7, 18. https://doi.org/10.3390/plants7010018
Mailis T, Skaltsa H. Polar Constituents of Salvia willeana (Holmboe) Hedge, Growing Wild in Cyprus. Plants. 2018; 7(1):18. https://doi.org/10.3390/plants7010018
Chicago/Turabian StyleMailis, Theofilos, and Helen Skaltsa. 2018. "Polar Constituents of Salvia willeana (Holmboe) Hedge, Growing Wild in Cyprus" Plants 7, no. 1: 18. https://doi.org/10.3390/plants7010018
APA StyleMailis, T., & Skaltsa, H. (2018). Polar Constituents of Salvia willeana (Holmboe) Hedge, Growing Wild in Cyprus. Plants, 7(1), 18. https://doi.org/10.3390/plants7010018





