Blackleg (Leptosphaeria maculans) Severity and Yield Loss in Canola in Alberta, Canada
Abstract
:1. Introduction
2. Results
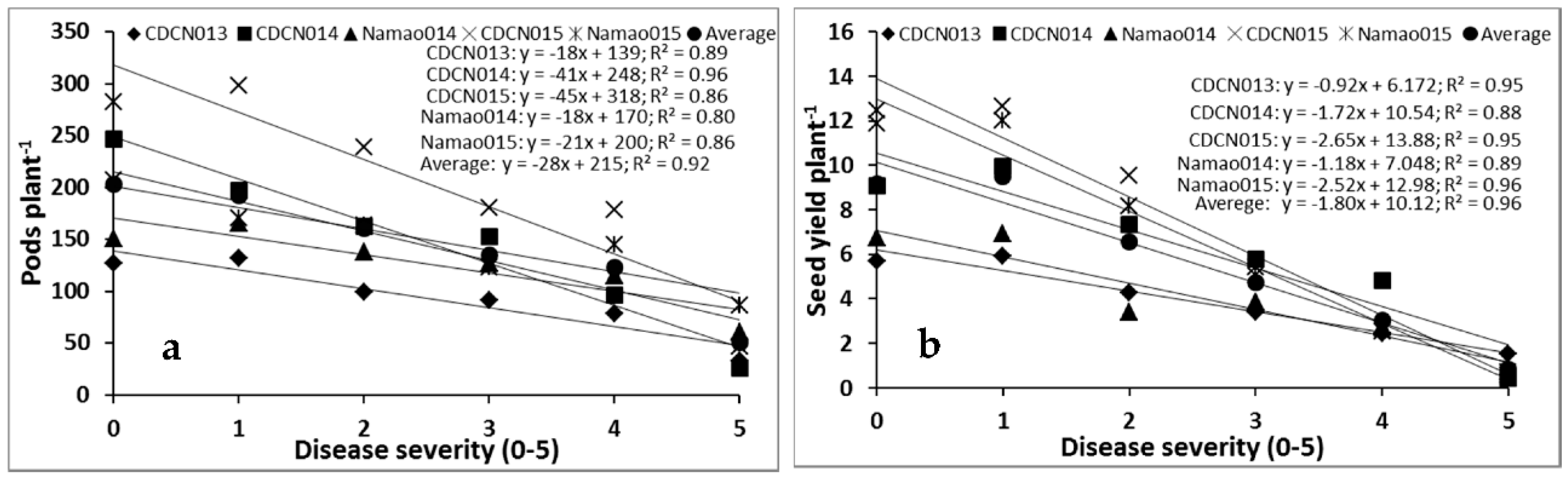
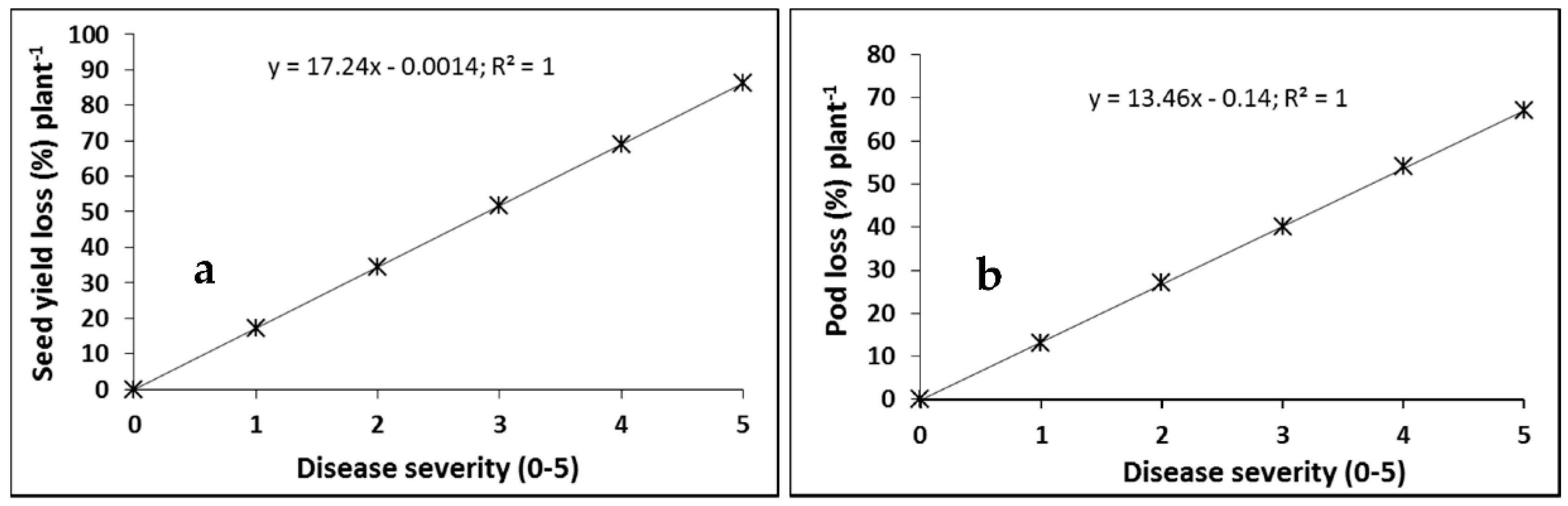
2.1. Cultivar Resistance and Blackleg of Canola
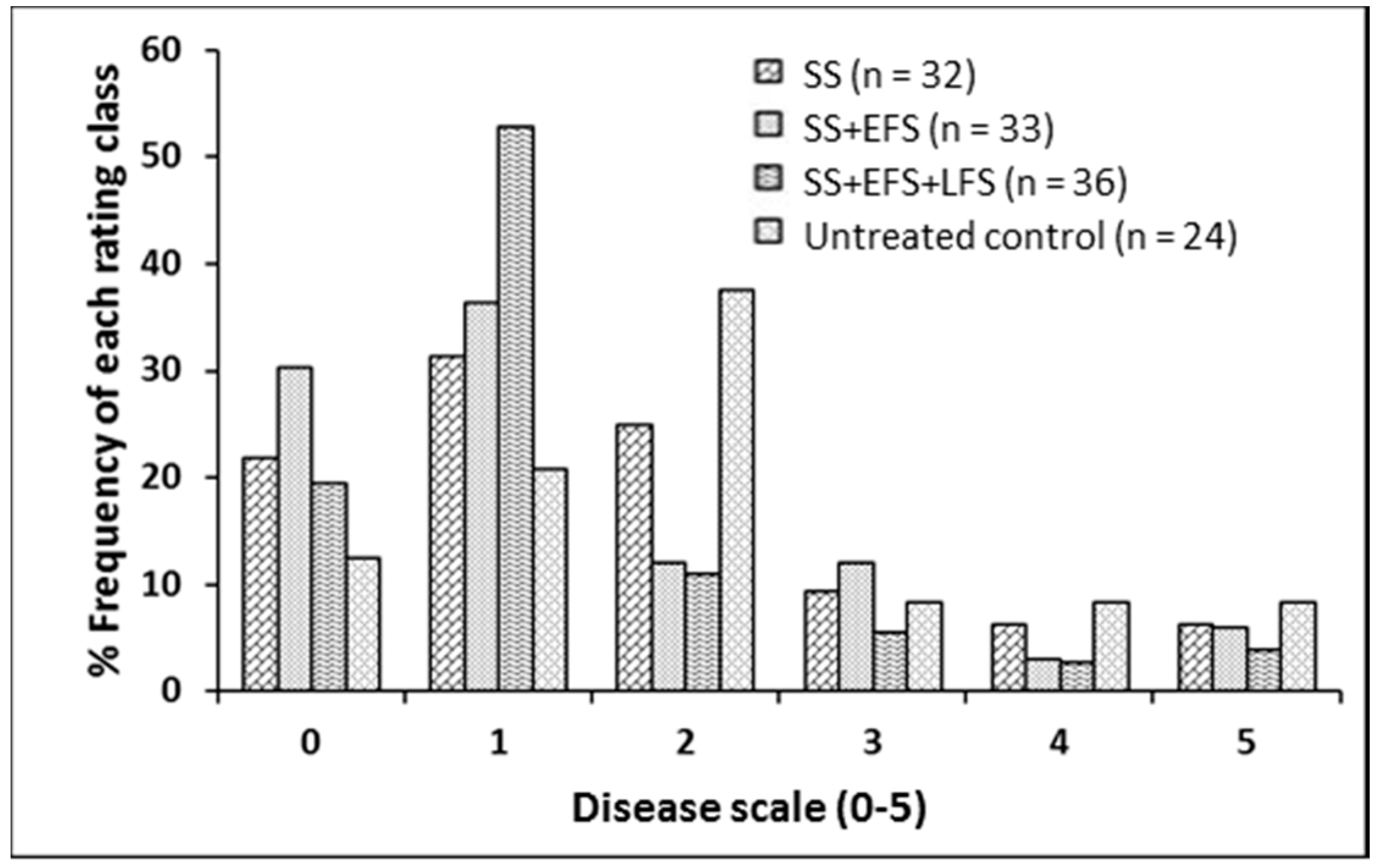
2.2. Foliar Fungicide Application and Blackleg
3. Discussion
4. Materials and Methods
4.1. Cultivar Resistance and Blackleg of Canola
4.2. Foliar Fungicide Application and Blackleg
4.3. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- West, J.S.; Kharbanda, P.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. Worldwide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Dilmaghani, A.; Balesdent, M.H.; Didier, J.P.; Wu, C.; Davey, J. The Leptosphaeria maculans-Leptosphaeria biglobosa species complex in the American continent. Plant Pathol. 2009, 58, 1044–1058. [Google Scholar] [CrossRef]
- Marcroft, S.J.; van de Wouw, A.P.; Salisbury, P.A.; Potter, T.D.; Howlett, B.J. Effect of rotation of canola (Brassica napus) cultivars with different complements of blackleg resistance genes on disease severity. Plant Pathol. 2012, 61, 934–944. [Google Scholar] [CrossRef]
- Bokor, A.; Barbetti, M.J.; Brown, A.G.P.; MacNish, G.C.; Wood, P. Blackleg of rapeseed. J. Agric. West. Aust. 1975, 16, 7–10. [Google Scholar]
- Gugel, R.K.; Petrie, G.A. History, occurrence, impact, and control of blackleg of rapeseed. Can. J. Plant Pathol. 1992, 14, 36–45. [Google Scholar] [CrossRef]
- Hall, R.; Peters, R.D.; Assabgui, R.A. Occurrence and impact of blackleg on oilseed rape in Ontario. Can. J. Plant Pathol. 1993, 15, 305–313. [Google Scholar] [CrossRef]
- Barbetti, M.J.; Khangura, R.K. Managing blackleg in the disease-prone environment of Western Australia. In Proceedings of the 10th International Rapeseed Congress, Canberra, Australia, 26–29 September 1999.
- Zhou, Y.; Fitt, B.D.L.; Welham, S.J.; Gladders, P.; Sansford, C.E.; West, J.S. Effects of severity and timing of stem canker (Leptosphaeria maculans) symptoms on yield of winter oilseed rape (Brassica napus) in the UK. Eur. J. Plant Pathol. 1999, 105, 715–728. [Google Scholar]
- Aubertot, J.N.; Pinochet, X.; Doré, T. The effects of sowing date and nitrogen availability during vegetative stages on Leptosphaeria maculans development on winter oilseed rape. Crop Prot. 2004, 23, 635–645. [Google Scholar] [CrossRef]
- Hall, R. Epidemiology of blackleg of oilseed rape. Can. J. Plant Pathol. 1992, 14, 46–55. [Google Scholar] [CrossRef]
- Mahuku, G.S.; Goodwin, P.H.; Hall, R.; Hsiang, T. Variability in the highly virulent type of Leptosphaeria maculans within and between oilseed rape fields. Can. J. Bot. 1997, 75, 1485–1492. [Google Scholar] [CrossRef]
- Howlett, B.J.; Idnurm, A.; Pedras, M.S.C. Leptosphaeria maculans, the causal agent of blackleg disease of Brassicas. Fungal Genet. Biol. 2001, 33, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rouxel, T.; Balesdent, M.H. The stem canker (blackleg) fungus, Leptosphaeria maculans, enters the genomic era. Mol. Plant Pathol. 2005, 6, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, P.D.; Tewari, J.P. Integrated management of canola diseases using cultural methods. Can. J. Plant Pathol. 1996, 18, 168–175. [Google Scholar] [CrossRef]
- Turkington, T.K.; Clayton, G.W.; Woods, D.L. The impact of soil incorporation of canola residues and stubble application of chemicals on decomposition and inoculum production by Leptosphaeria maculans. Can. J. Plant Pathol. 2000, 22, 155–159. [Google Scholar] [CrossRef]
- Guo, X.W.; Fernando, W.G.D. Seasonal and diurnal patterns of spore dispersal by Leptosphaeria maculans fron canola stubble in relation to environmental conditions. Plant Dis. 2005, 89, 97–104. [Google Scholar] [CrossRef]
- Marcroft, S.J.; Sprague, S.J.; Pymer, S.J.; Salisbury, P.A.; Howlett, B.J. Crop isolation, not extended rotation length, reduces blackleg (Leptosphaeria maculans) severity of canola (Brassica napus) in south-eastern Australia. Aust. J. Exp. Agric. 2004, 44, 601–606. [Google Scholar] [CrossRef]
- Kutcher, H.R.; Brandt, S.A.; Smith, E.G.; Ulrich, D.; Malhi, S.S.; Johnston, A.M. Blackleg disease of canola mitigated by resistant cultivars and four-year crop rotations in western Canada. Can. J. Plant Pathol. 2013, 35, 209–221. [Google Scholar] [CrossRef]
- Kutcher, H.R.; Yu, F.; Brun, H. Improving blackleg disease management of Brassica napus from knowledge of genetic interactions with Leptosphaeria maculans. Can. J. Plant Pathol. 2010, 32, 29–34. [Google Scholar] [CrossRef]
- Chen, Y.; Fernando, W.G.D. Prevalence of pathogenicity groups of Leptosphaeria maculans in western Canada and North Dakota, USA. Can. J. Plant Pathol. 2006, 28, 533–539. [Google Scholar] [CrossRef]
- Kutcher, H.R.; Keri, M.; McLaren, D.L.; Rimmer, S.R. Pathogenic variability of Leptosphaeria maculans in western Canada. Can. J. Plant Pathol. 2007, 29, 388–393. [Google Scholar] [CrossRef]
- Pratt, S. Canola Sector Hopes China’s Blackleg Fears Addressed. Available online: http://www.producer.com/2014/08/canola-sector-hopes-chinas-blackleg-fears-addressed (accessed on 7 May 2015).
- Schoeny, A.; Jeuffroy, M.H.; Lucas, P. Influence of take-all epidemics on winter wheat yield formation and yield loss. Phytopathology 2001, 91, 694–701. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.C.; Emmett, R.W. Blackleg (Leptosphaeria maculans (Desm.) Ces. et de Not.) of rapeseed in Victoria: Crop losses and factors which affect disease severity. Aust. J. Agric. Res. 1977, 28, 47–51. [Google Scholar] [CrossRef]
- Rempel, C.B.; Lisieczko, Z.; Hall, R. Epidemiological aspects of resistance in rapeseed to blackleg. In Proceedings of the 8th International Rapeseed Congress, Saskatoon, SK, Canada, 9–11 July 1991; McGregor, D.I., Ed.; Volume 2, pp. 460–464.
- Peters, R.D. Distribution and Characterization of Leptosphaeria maculans Causing Blackleg of Winter Rapeseed in Ontario. Master’s Thesis, University of Guelph, Guelph, ON, Canada, 1990. [Google Scholar]
- Assabgui, R.; Hall, R. Incidence, severity, and impact of blackleg in winter rapeseed in Ontario during 1988 and 1989. Available online: http://www.tandfonline.com/doi/abs/10.1080/07060669009501009 (accessed on 7 May 2016).
- Church, V.J.; Fitt, B.D.L. Incidence and effects of diseases on seven winter oilseed rape cultivars in 1991/92 and 1993. Int. Org. Biol. Contr. Bull. 1995, 18, 62–68. [Google Scholar]
- Sansford, C.E.; Fitt, B.D.L.; Gladders, P.; Lockley, K.D.; Sutherland, K.G. Oilseed Rape: Disease Development, Forecasting and Yield Loss Relationships; Report OS17; Home-Grown Cereals Authority Project: London, UK, 1996. [Google Scholar]
- Sutherland, K.G.; Wale, S.J.; Sansford, C. Effect of different epidemics of Pyrenopeziza brassicae on yield loss in winter oilseed rape. In Proceedings of the 9th International Rapeseed Congress, Cambridge, UK, 1995; pp. 1004–1006.
- Huang, Y.J.; Hood, J.R.; Eckert, M.R.; Stonard, J.F.; Cools, H.J.; King, G.J.; Rossall, S.; Ashworth, M.; Fitt, B.D.L. Effects of fungicide on growth of Leptosphaeria maculans and L. biglobosa in relation to development of phoma stem canker on oilseed rape (Brassica napus). Plant Pathol. 2011, 60, 607–620. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The strobilurin fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef] [PubMed]
- Grasso, V.; Palermo, S.; Sierotzki, H.; Garibaldi, A.; Gisi, U. Cytochrome b gene structure and consequences for resistance to Qo inhibitor fungicides in plant pathogens. Pest Manag. Sci. 2006, 62, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Chavaillaz, D.; Kaesbohrer, M.; Staub, T.; Felsenstein, F.G. Characterizing resistance risk of Erysiphe graminis f. sp. tritici to strobilurins. Crop Prot. 2001, 20, 87–96. [Google Scholar] [CrossRef]
- Ma, Z.; Luo, Y.; Michailides, T.J. Resistance to Botryosphaeria dothidea from pistachio to iprodione. Plant Dis. 2001, 85, 183–188. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006; p. 813. [Google Scholar]
- Shah, D.A.; and Madden, L.V. Nonparametric analysis of ordinal data in designed factorial experiments. Phytopathology 2004, 94, 33–43. [Google Scholar] [CrossRef] [PubMed]

| Sites/Years | Canola Cultivar/Hybrid | Median Disease Rating | Mean Rank (Ri) | Estimated Relative Effect (pi) | SE | Confidence Interval (95%) for the Estimated Relative Effect | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| CDCN and Namao, 2013 and 2014 | 46S53RR | 1.0 | 25.73 | 0.35041 | 0.028 | 0.30389 | 0.40557 |
| 1950RR | 1.0 | 33.90 | 0.46383 | 0.042 | 0.39920 | 0.53134 | |
| Westar | 2.0 | 49.88 | 0.68576 | 0.049 | 0.60437 | 0.74412 | |
| CDCN and Namao, 2015 | Invigor 5440 | 1.0 | 5.13 | 0.28906 | 0.066 | 0.25906 | 0.39010 |
| Westar | 2.0 | 11.88 | 0.71094 | 0.058 | 0.60990 | 0.74094 | |
| Sites/Years | Canola Cultivar/Hybrid | Resistance | Pods Plant−1 | Yield t·ha−1 |
|---|---|---|---|---|
| 1 CDCN and Namao, 2013 and 2014 | 46S53RR | R | 285 a | 2.66 a |
| 1950RR | MR | 202 b | 2.21 a | |
| Westar | S | 137 c | 1.21 b | |
| SE | 14.67 | 0.13 | ||
| 2 CDCN and Namao, 2015 | Invigor 5440 | MR | 271 a | 1.87 a |
| Westar | S | 150 b | 0.82 b | |
| SE | 7.59 | 0.08 |
| Application Timing | Plants Row−1 | Pods Plant−1 | Yield t·ha−1 |
|---|---|---|---|
| SS | 48 b | 153 bc | 2.64 a |
| SS + EFS | 51 ab | 177 ab | 2.68 a |
| SS + EFS+ LFS | 54 a | 207 a | 2.69 a |
| Untreated control | 37 c | 128 c | 2.03 b |
| SE | 5 | 33 | 0.54 |
| Application Timing | Median Disease Rating | Mean Rank (Ri) | Estimated Relative Effect (pi) | SE | Confidence Interval (95%) for the Estimated Relative Effect | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| SS | 1.5 | 17.00 | 0.51563 | 0.181 | 0.37590 | 0.65114 |
| SS + EFS | 1.0 | 15.13 | 0.45703 | 0.166 | 0.32757 | 0.59778 |
| SS + EFS+ LFS | 1.0 | 10.31 | 0.30664 | 0.134 | 0.21408 | 0.44832 |
| Untreated control | 2.0 | 23.56 | 0.72070 | 0.325 | 0.57514 | 0.80638 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.-F.; Strelkov, S.E.; Peng, G.; Ahmed, H.; Zhou, Q.; Turnbull, G. Blackleg (Leptosphaeria maculans) Severity and Yield Loss in Canola in Alberta, Canada. Plants 2016, 5, 31. https://doi.org/10.3390/plants5030031
Hwang S-F, Strelkov SE, Peng G, Ahmed H, Zhou Q, Turnbull G. Blackleg (Leptosphaeria maculans) Severity and Yield Loss in Canola in Alberta, Canada. Plants. 2016; 5(3):31. https://doi.org/10.3390/plants5030031
Chicago/Turabian StyleHwang, Sheau-Fang, Stephen E. Strelkov, Gary Peng, Hafiz Ahmed, Qixing Zhou, and George Turnbull. 2016. "Blackleg (Leptosphaeria maculans) Severity and Yield Loss in Canola in Alberta, Canada" Plants 5, no. 3: 31. https://doi.org/10.3390/plants5030031
APA StyleHwang, S.-F., Strelkov, S. E., Peng, G., Ahmed, H., Zhou, Q., & Turnbull, G. (2016). Blackleg (Leptosphaeria maculans) Severity and Yield Loss in Canola in Alberta, Canada. Plants, 5(3), 31. https://doi.org/10.3390/plants5030031






