The Occurrence of Flavonoids and Related Compounds in Flower Sections of Papaver nudicaule
Abstract
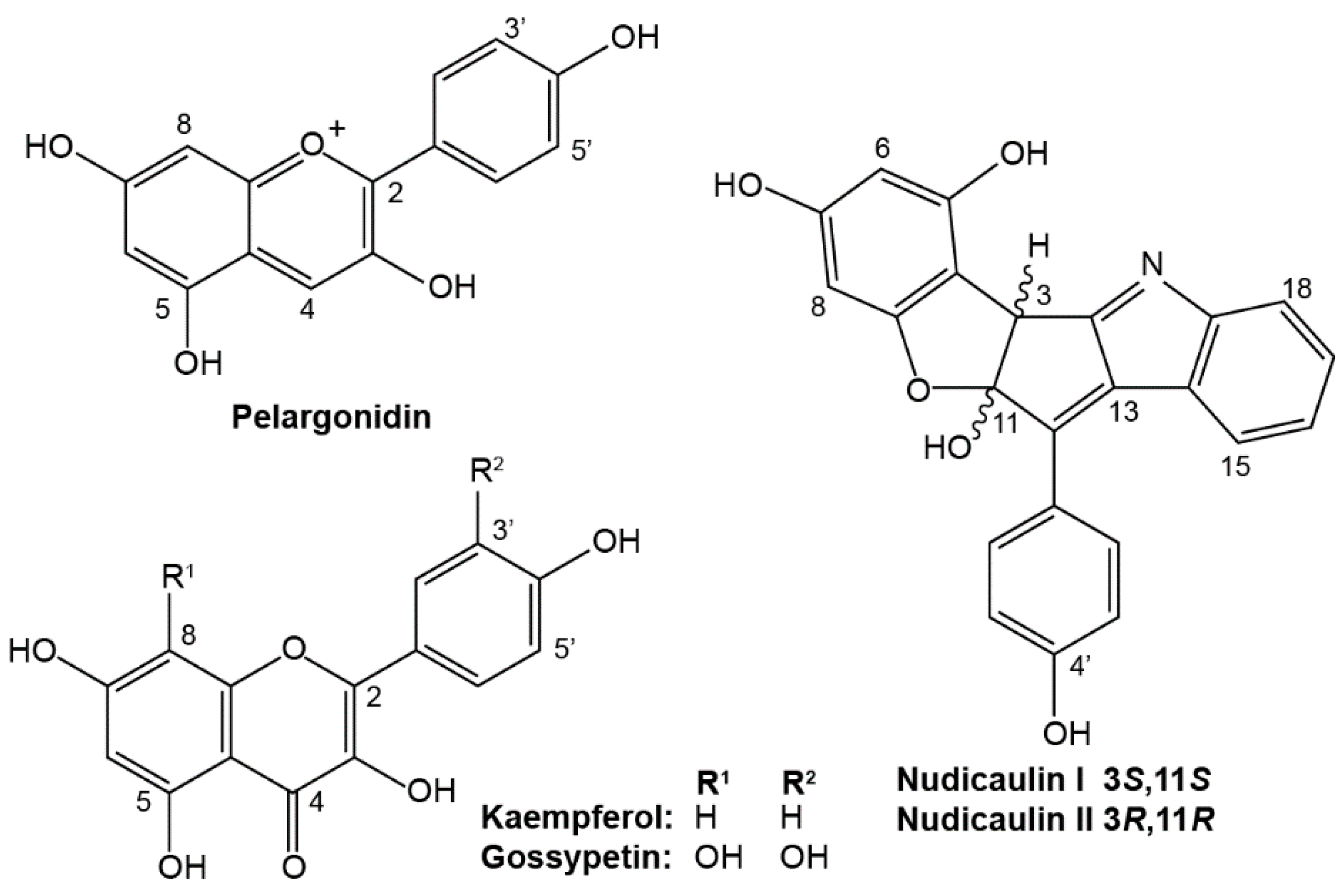
:1. Introduction
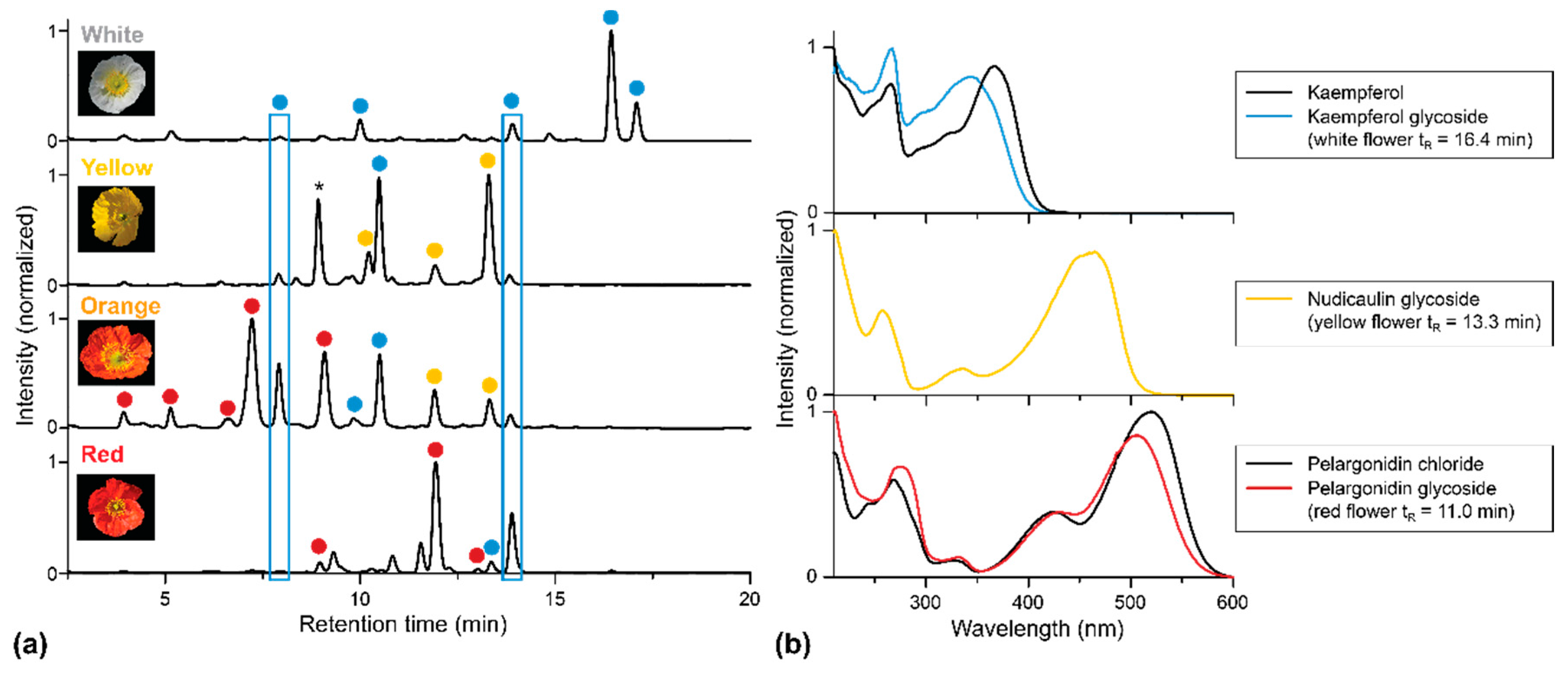
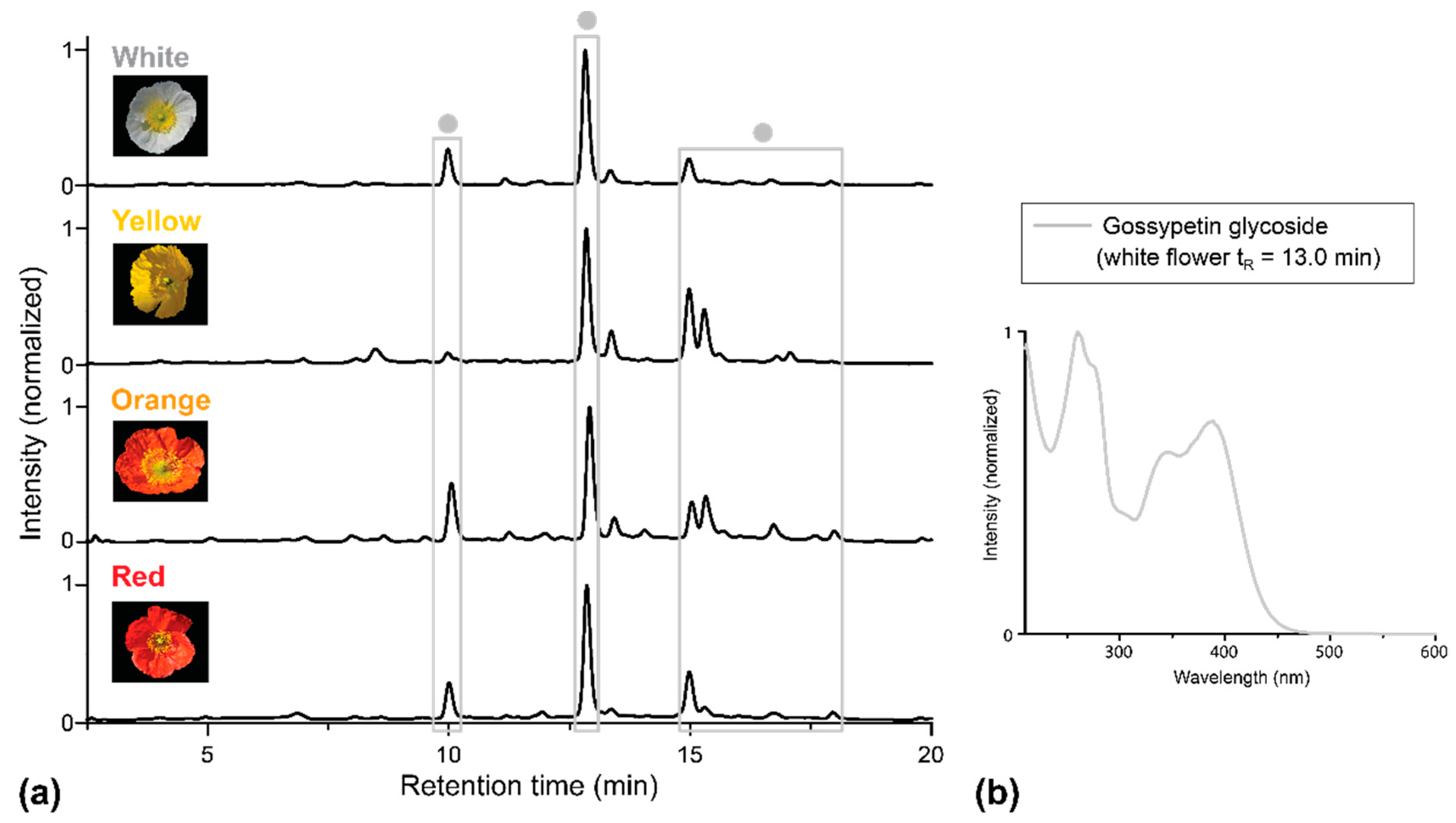
2. Results
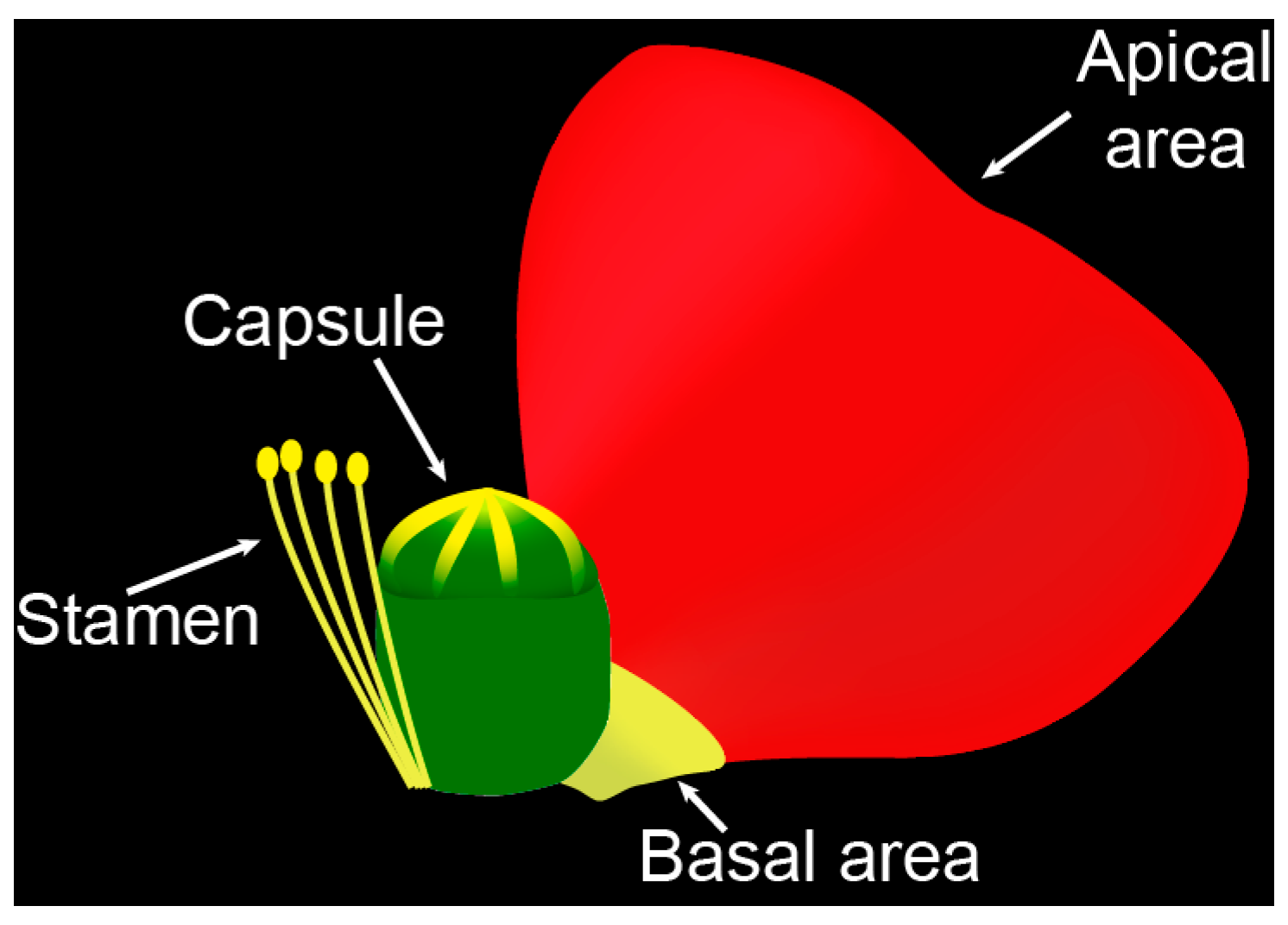
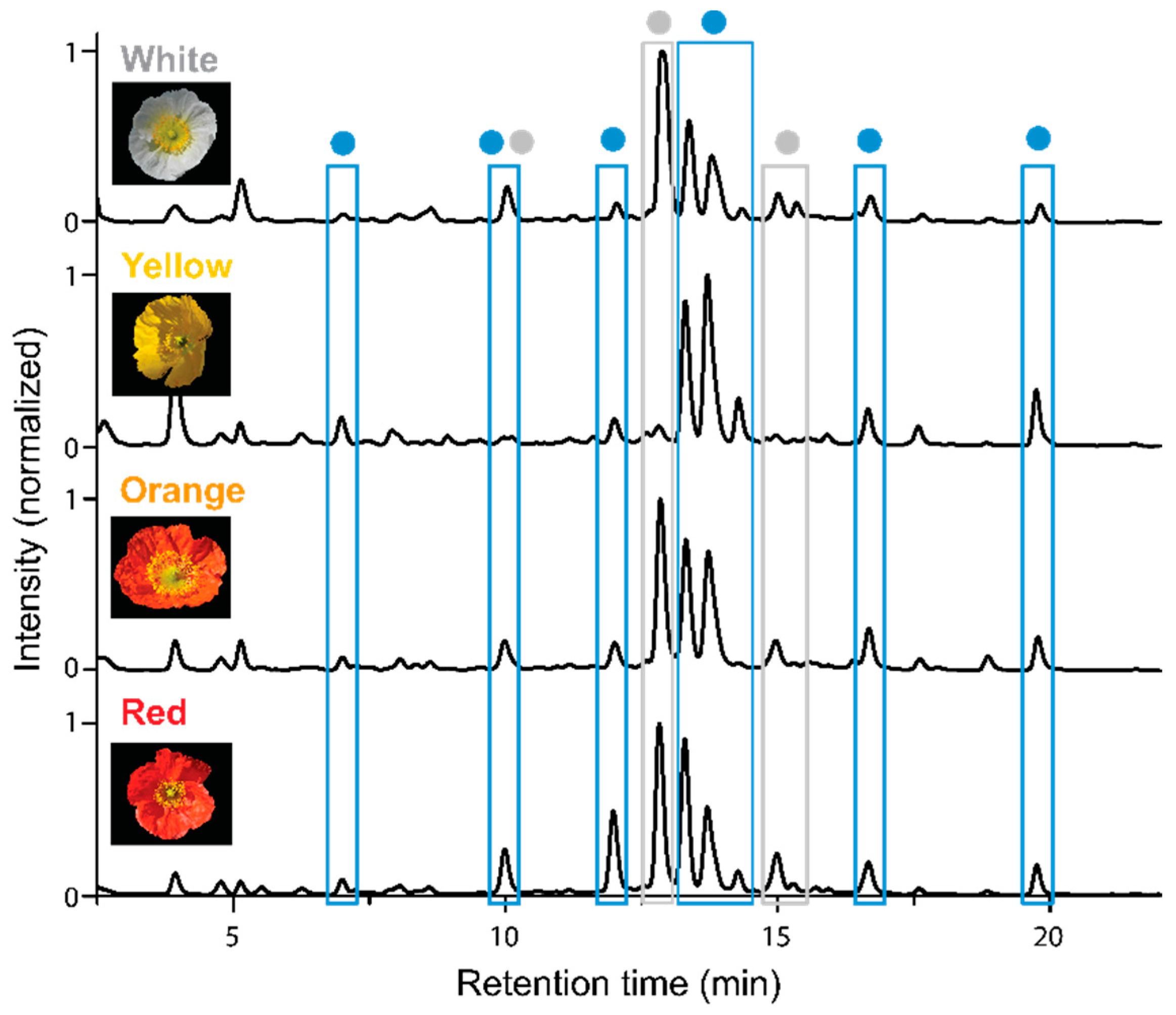
2.1. Apical and Basal Petal Areas
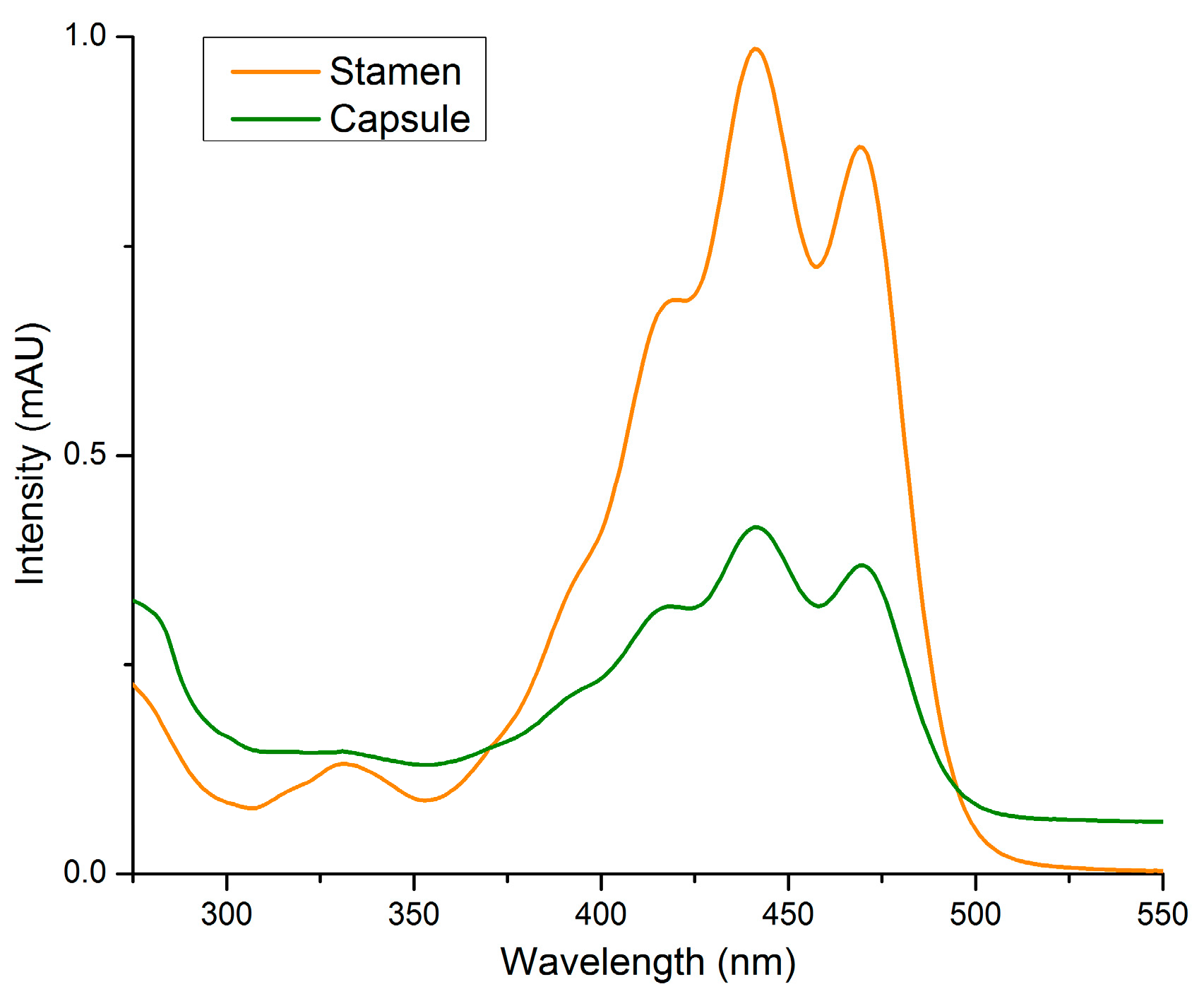
2.2. Stamens and Capsules
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. HPLC-PDA Analysis of Flavonoids and Nudicaulins
4.3. UV/Vis Analysis of Carotenoids
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Parihar, A.; Grotewold, E.; Doseff, A.I. Flavonoid Dietetics: Mechanisms and Emerging Roles of Plant Nutraceuticals. In Pigments in Fruits and Vegetables; Chen, C., Ed.; Springer New York: New York, NY, USA, 2015; pp. 93–126. [Google Scholar]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Hanelt, P. Die Typisierung von Papaver nudicaule L. und die Einordnung von P. nudicaule hort. non L. Die Kulturpflanze 1970, 18, 73–88. [Google Scholar] [CrossRef]
- Robinson, G.M.; Robinson, R. A survey of anthocyanins. I. Biochem. J. 1931, 25, 1687–1705. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.M.; Robinson, R. A survey of anthocyanins. II. Biochem. J. 1932, 26, 1647–1664. [Google Scholar] [CrossRef] [PubMed]
- Cornuz, G.; Wyler, H.; Lauterwein, J. Pelargonidin 3-malonylsophoroside from the red Iceland poppy, Papaver nudicaule. Phytochemistry 1981, 20, 1461–1462. [Google Scholar] [CrossRef]
- Tatsis, E.C.; Böhm, H.; Schneider, B. Occurrence of nudicaulin structural variants in flowers of papaveraceous species. Phytochemistry 2013, 92, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Warskulat, A.-C.; Tatsis, E.C.; Dudek, B.; Kai, M.; Lorenz, S.; Schneider, B. Unprecedented utilization of pelargonidin and indole for the biosynthesis of plant indole alkaloids. ChemBioChem 2016, 17, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Schliemann, W.; Schneider, B.; Wray, V.; Schmidt, J.; Nimtz, M.; Porzel, A.; Böhm, H. Flavonols and an indole alkaloid skeleton bearing identical acylated glycosidic groups from yellow petals of Papaver nudicaule. Phytochemistry 2006, 67, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Mo, Y.; Nagel, C.; Taylor, L.P. Biochemical complementation of chalcone synthase mutants defines a role for flavonols in functional pollen. Proc. Natl. Acad. Sci. USA 1992, 89, 7213–7217. [Google Scholar] [CrossRef] [PubMed]
- Acheson, R.M.; Jenkins, C.L.; Harper, J.L.; McNaughton, I.H. Floral pigments in Papaver and their significance in the systematics of the genus. New Phytol. 1962, 61, 256–260. [Google Scholar] [CrossRef]
- Tétényi, P. Chemodifferentiation of Papavereae from coasts of the Black-Sea to the Atlantic. In Natural Products in the New Millennium: Prospects and Industrial Application; Rauter, A.P., Palma, F.B., Justino, J., Araújo, M.E., Santos, S.P., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2002; pp. 173–181. [Google Scholar]
- Tatsis, E.C.; Schaumlöffel, A.; Warskulat, A.C.; Massiot, G.; Schneider, B.; Bringmann, G. Nudicaulins, yellow flower pigments of Papaver nudicaule: Revised constitution and assignment of absolute configuration. Org. Lett. 2013, 15, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sasaki, R.; Ogata, Y.; Nakamura, Y.; Sakurai, N.; Kitajima, M.; Takayama, H.; Kanaya, S.; Aoki, K.; Shibata, D.; et al. Metabolic profiling of flavonoids in Lotus japonicus using liquid chromatography Fourier transform ion cyclotron resonance mass spectrometry. Phytochemistry 2008, 69, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B.; Kimura, M. HarvestPlus Handbook for Carotenoid Analysi; HarvestPlus Technical Monograph 2; HarvestPlus: Washington, DC, USA; Cali, CO, USA, 2004. [Google Scholar]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants; Ahmad, P., Prasad, M., Eds.; Springer New York: New York, NY, USA, 2012; pp. 159–179. [Google Scholar]
- Cooper-Driver, G.A. Contributions of Jeffrey Harborne and co-workers to the study of anthocyanins. Phytochemistry 2001, 56, 229–236. [Google Scholar] [CrossRef]
- Stich, K.; Halbwirth, H.; Wurst, F.; Forkmann, G. UDP-glucose: Flavonol 7-O-glucosyltransferase activity in flower extracts of Chrysanthemum segetum. Z. Naturforsch. C 1997, 52, 153–158. [Google Scholar] [PubMed]
- Wind, O.; Christensen, S.B.; Mølgaard, P. Colouring agents in yellow and white flowered Papaver radicatum in Northern Greenland. Biochem. Syst. Ecol. 1998, 26, 771–779. [Google Scholar] [CrossRef]
- Thompson, W.R.; Meinwald, J.; Aneshansley, D.; Eisner, T. Flavonols: Pigments responsible for ultraviolet absorption in nectar guide of flower. Science 1972, 177, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Pollak, P.E.; Vogt, T.; Mo, Y.; Taylor, L.P. Chalcone synthase and flavonol accumulation in stigmas and anthers of Petunia hybrida. Plant. Physiol. 1993, 102, 925–932. [Google Scholar] [PubMed]
- Barrell, P.J.; Wakelin, A.M.; Gatehouse, M.L.; Lister, C.E.; Conner, A.J. Inheritance and epistasis of loci influencing carotenoid content in petal and pollen color variants of California poppy (Eschscholzia californica Cham.). J. Hered. 2010, 101, 750–756. [Google Scholar] [CrossRef] [PubMed]
| P. nudicaule Cultivar | Apical Area | Basal Area | Capsule | Stamen |
|---|---|---|---|---|
| White | ||||
| Yellow | ||||
| Orange | ||||
| Red |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, B.; Warskulat, A.-C.; Schneider, B. The Occurrence of Flavonoids and Related Compounds in Flower Sections of Papaver nudicaule. Plants 2016, 5, 28. https://doi.org/10.3390/plants5020028
Dudek B, Warskulat A-C, Schneider B. The Occurrence of Flavonoids and Related Compounds in Flower Sections of Papaver nudicaule. Plants. 2016; 5(2):28. https://doi.org/10.3390/plants5020028
Chicago/Turabian StyleDudek, Bettina, Anne-Christin Warskulat, and Bernd Schneider. 2016. "The Occurrence of Flavonoids and Related Compounds in Flower Sections of Papaver nudicaule" Plants 5, no. 2: 28. https://doi.org/10.3390/plants5020028
APA StyleDudek, B., Warskulat, A.-C., & Schneider, B. (2016). The Occurrence of Flavonoids and Related Compounds in Flower Sections of Papaver nudicaule. Plants, 5(2), 28. https://doi.org/10.3390/plants5020028







