Detection of Leptosphaeria maculans and Leptosphaeria biglobosa Causing Blackleg Disease in Canola from Canadian Canola Seed Lots and Dockage
Abstract
:1. Introduction
2. Results
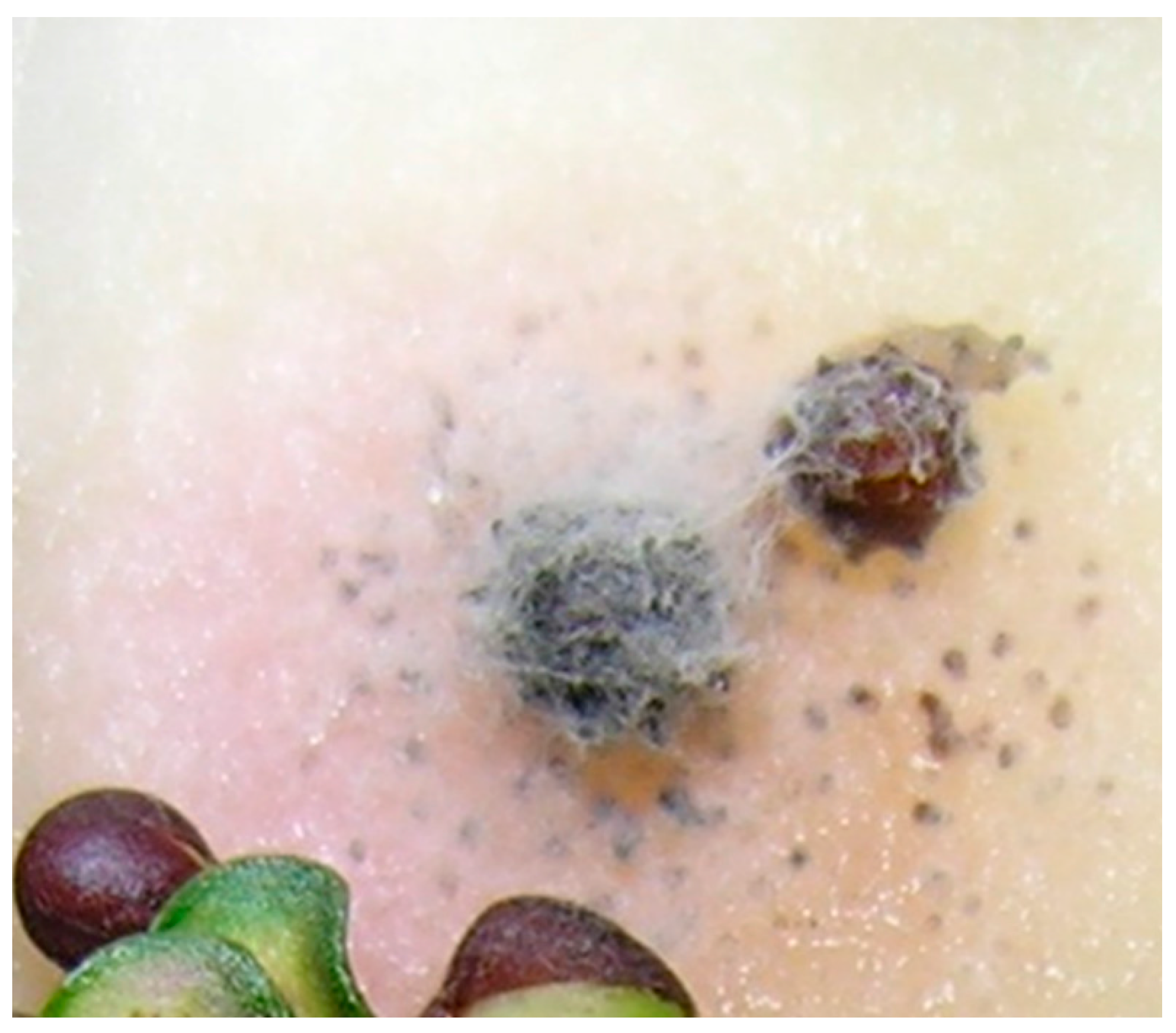
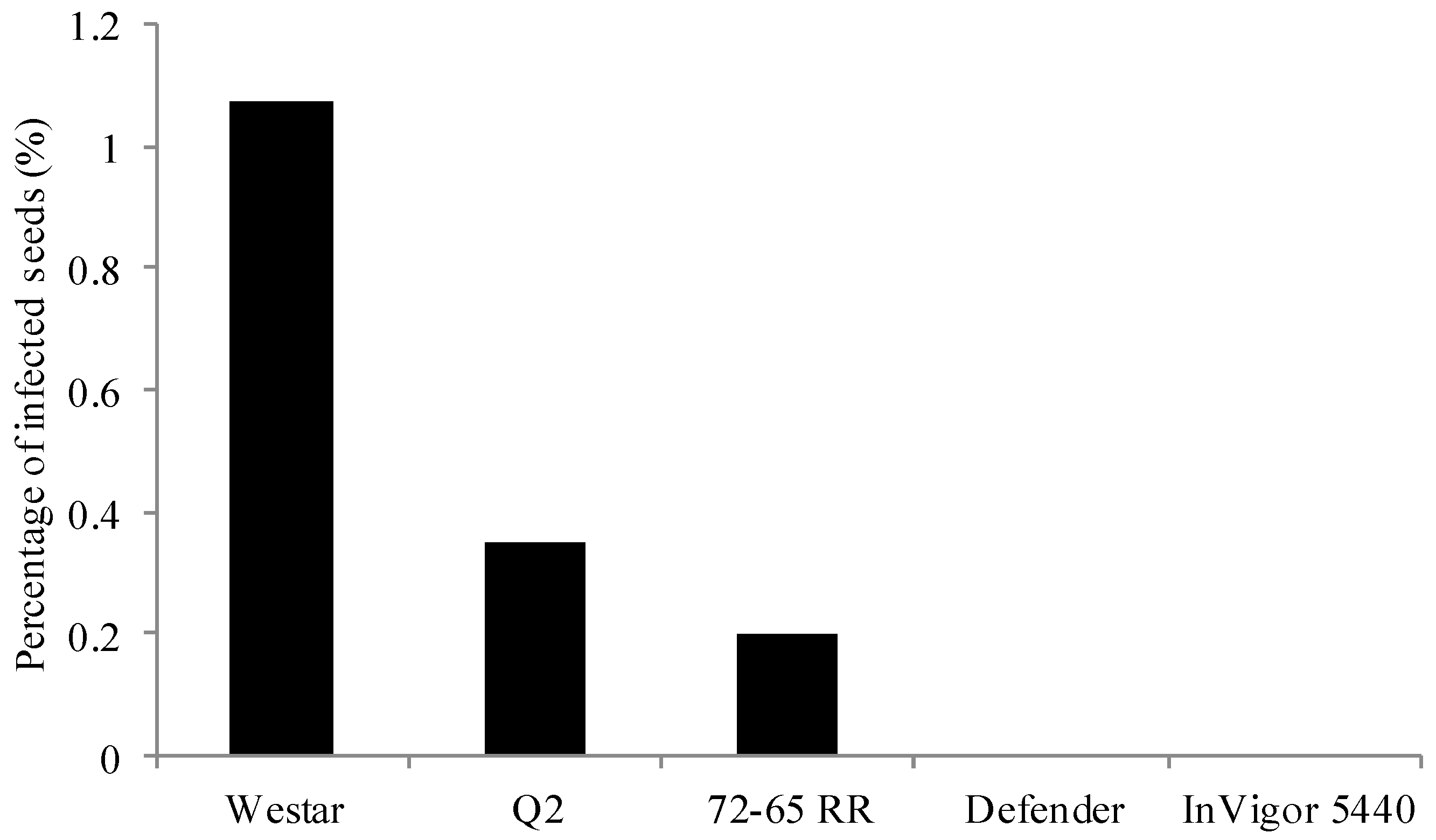
2.1. Frequency and Viability of Leptosphaeria Species in Seed Lots Samples
2.2. Identification of Leptosphaeria Species in Dockage
2.3. Ability of Admixture to Produce Blackleg Inoculum
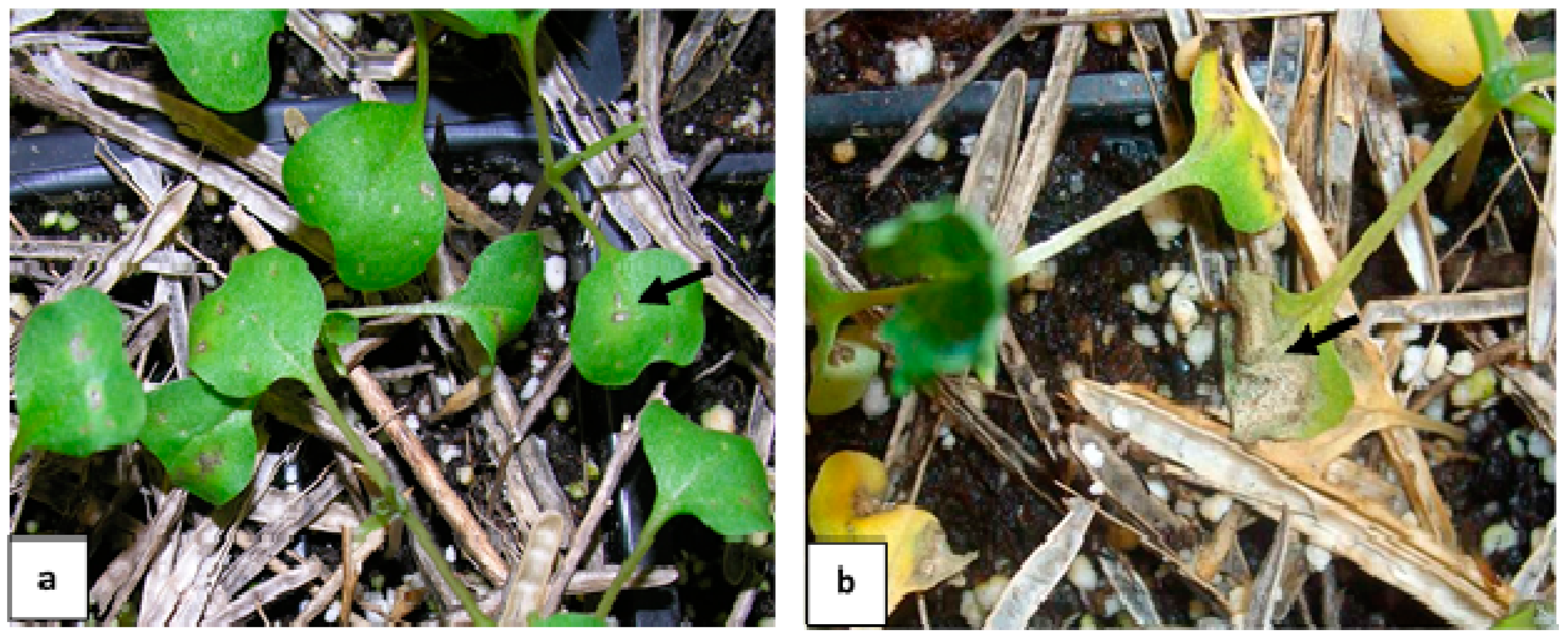
2.4. The Ability of Admixture to Cause Infection
3. Discussion
4. Materials and Methods
4.1. Sample Collection
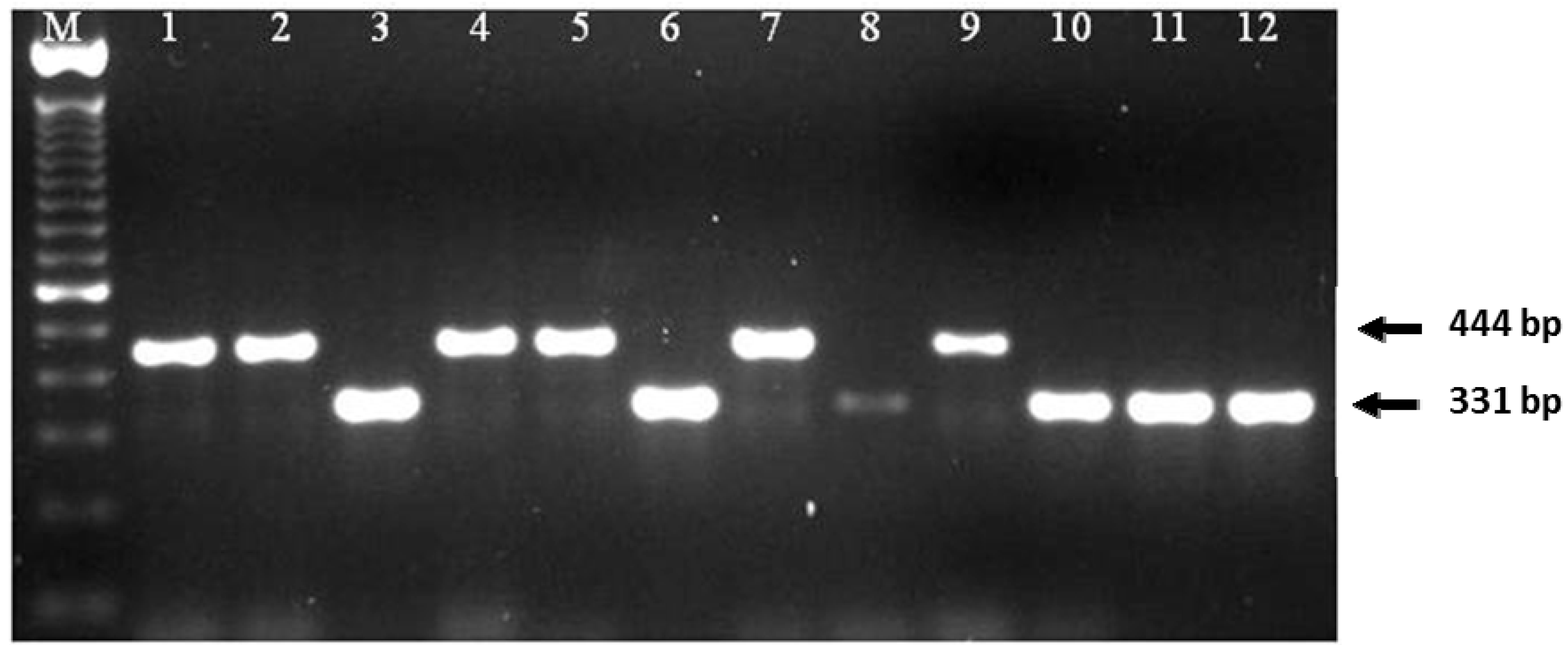
4.2. Identification of Leptosphaeria Species from Seed Samples
4.3. Determination of Leptosphaeria Species in Dockage Samples Collected from Commercial Fields
4.4. The Ability of Admixture to Produce Primary Inoculum
4.5. The Ability of Admixture to Cause Infection
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar]
- Fitt, B.D.L.; Hu, B.C.; Li, Z.Q.; Liu, S.Y.; Lange, R.M.; Kharbanda, P.D.; Butterworth, M.H.; White, R.P. Strategies to prevent spread of Leptosphaeria maculans (phoma stem canker) onto oilseed rape crops in China; costs and benefits. Plant Pathol. 2008, 57, 652–664. [Google Scholar]
- West, J.S.; Biddulph, J.E.; Fitt, B.D.L.; Gladders, P. Epidemiology of Leptosphaeria maculans in relation to forecasting stem canker severity on winter oilseed rape in the UK. Ann. Appl. Biol. 1999, 135, 535–546. [Google Scholar] [CrossRef]
- West, J.S.; Kharbanda, P.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar]
- West, J.S.; Balesdent, M.H.; Rouxel, T.; Narcy, J.P.; Huang, Y.J.; Roux, J.; Steed, J.M.; Fitt, B.D.L.; Schmit, J. Colonization of winter oilseed rape tissues by ATox+ and B/Tox0 Leptosphaeria maculans (phoma stem canker) in France and England. Plant Pathol. 2002, 51, 311–321. [Google Scholar]
- West, J.S.; Evans, N.; Liu, S.; Hu, B.; Peng, L. Leptosphaeria maculans causing stem canker of oilseed rape in China. Plant Pathol. 2000. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Pereira, E.; Balesdent, M.H.; Brun, H.; Rouxel, T. Molecular phylogeny of the Leptosphaeria maculans––L. biglobosa species complex. Mycol. Res. 2003, 107, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.F.; Song, P.L.; Li, Z.Q.; Li, Q.S.; Hu, B.C. Studies on the biological characteristics of Leptosphaeria biglobosa on Chinese oilseed rape. Chin. J. Oil Crop Sci. 2012, 34, 419–424. [Google Scholar]
- Li, Q.S.; Rong, S.B.; Hu, B.C.; Jiang, Y.F.; Hou, S.M.; Fei, W.X.; Chen, F.X.; Wu, X.J.; Fan, Z.X.; Lei, W.X. Distribution of blackleg disease on oilseed rape in China and its pathogen identification. Chin. J. Oil Crop Sci. 2013, 35, 415–423. [Google Scholar]
- Zhang, X.; White, R.P.; Demir, E.; Jedryczka, M.; Lamge, R.M.; Islam, M.; Li, Z.Q.; Huang, Y.J.; Hall, A.M.; Zhou, G.; et al. Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathol. 2014, 63, 598–612. [Google Scholar]
- Balesdent, M.H.; Jedryczka, M.; Jain, L.; Mendes-Pereira, E.; Bertrandy, J.; Rouxel, T. Conidia as a substrate for internal transcribed spacer-based PCR identification of members of the Leptosphaeria maculans species complex. Phytopathology 1998, 88, 1210–1217. [Google Scholar] [PubMed]
- Ghanbarnia, K.; Fernando, W.G.D.; Crow, G. Comparison of disease severity and incidence at different growth stages of naturally infected canola plants under field conditions by pycnidiospores of Phoma lingam as a main source of inoculum. Can. J. Plant Pathol. 2011, 33, 355–363. [Google Scholar]
- Paul, V.H.; Rawlinson, C.J. Diseases and pests of oilseed rape; Gelsenkirchen-Buer: Verlag Theodore Mann, Germany, 1992. [Google Scholar]
- Wu, J.; Cheng, X.; Xiao, H.; Wang, H.; Yang, L.; Ellis, E.C. Agricultural landscape change in China’s Yangtze Delta, 1942–2002: A case study. Agric. Ecosyst. Environ. 2009, 129, 523–533. [Google Scholar] [CrossRef]
- Peluola, C.; Fernando, W.G.D.; Huvenaars, C.; Kutcher, H.R.; Lahlali, R.; Peng, G. Effect of flooding on the survival of Leptosphaeria spp. in canola stubble. Plant Pathol. 2013, 62, 1350–1356. [Google Scholar]
- Kutcher, H.R.; Balesdent, M.H.; Rimmer, S.R.; Rouxel, T.; Chèvre, A.M.; Delourme, R.; Brun, H. Frequency of avirulence genes in Leptosphaeria maculans in western Canada. Can. J. Plant Pathol. 2010, 32, 77–85. [Google Scholar]
- Zhang, X.; Peng, G.; Kutcher, H.R.; Balesdent, M.-H.; Delourme, R.; Fernando, W.G.D. Breakdown of Rlm3 resistance in the Brassica napus–Leptosphaeria maculans pathosystem in western Canada. Eur. J. Plant Pathol. 2015. [Google Scholar] [CrossRef]
- Liu, S.Y.; Liu, Z.; Fitt, B.D.L.; Evans, N.; Forster, S.J.; Huang, Y.J.; Latunde-Dada, A.O.; Lucas, J.A. Resistance to Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) induced by L. biglobosa and chemical defence activators in field and controlled environments. Plant Pathol. 2006, 55, 401–412. [Google Scholar] [CrossRef]
- Chen, Y.; Fernando, W.G.D. Prevalence of pathogenicity groups of Leptosphaeria maculans in western Canada and North Dakota, USA. Can. J. Plant Pathol. 2006, 1, 533–539. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wu, C.P.; Li, B.; Su, H.; Zhen, S.Z.; An, Y.L. Detection of Leptosphaeria maculans from imported Canola seeds. J. Plant Dis. Protect. 2010, 117, 173–176. [Google Scholar]
- Chigogora, J.L.; Hall, R. Relationship among measures of blackleg in winter oilseed rape and infection of harvested seed by Leptosphaeria maculans. Can. J. Plant Pathol. 1995, 17, 25–30. [Google Scholar] [CrossRef]
- McGee, D.C. Blackleg (Leptosphaeria maculans (Desm.) Ces. et de Not.) of rapeseed in Victoria: sources of infection and relationships between inoculum, environmental factors and disease severity. Aust. J. Agric. Res. 1977, 28, 53–62. [Google Scholar] [CrossRef]
- Hall, R. Epidemiology of blackleg of oilseed rape. Can. J. Plant Pathol. 1992, 14, 46–65. [Google Scholar] [CrossRef]
- Liban, S.H.; Cross, D.J.; Kutcher, H.R.; Peng, G.; Fernando, W.G.D. Race structure and frequency of avirulence genes in the western Canadian Leptosphaeria maculans pathogen population, the causal agent of blackleg in brassica species. Plant Pathol. 2015. [Google Scholar] [CrossRef]
- Barbetti, M.; Khangura, R. Fungal diseases of canola in Western Australia; Bulletin 4406; 2000. Avaliable online: http://www.agric.wa.gov.au/PC_92073.html#flowers (accessed on 12 September 2012).
- Wood, P.M.c.R.; Barbetti, M.J. The role of seed infection in the spread of blackleg of rape in Western Australia. Aust. J. Exp. Agric. Anim. Husb. 1977, 17, 1040–1044. [Google Scholar] [CrossRef]
- Van de Wouw, A.P.; Elliott, V.L.; Ware, A.; Lindbeck, K.; Howlett, B.J.; Marcroft, S.J. Infection of canola pods by Leptosphaeria maculans and subsequent seed contamination. Eur. J. Plant Pathol. 2015. [Google Scholar] [CrossRef]
- Li, C.X.; Wratten, N.; Salisbury, P.A.; Burton, W.A.; Potter, T.D.; Walton, G. Response of Brassica napus and B. juncea germplasm from Australia, China and India to Australian populations of Leptosphaeria maculans. Aust. Plant Pathol. 2008, 37, 162–170. [Google Scholar]
- Ghanbarnia, K.; Fernando, W.G.D.; Crow, G. Developing rainfall- and temperature-based models to describe infection of canola under field conditions caused by pycnidiospores of Leptosphaeria maculans. Phytopathology 2009, 99, 879–886. [Google Scholar] [PubMed]
- Glynn, N.C.; Edwards, S.G. Evaluation of PCR assays for quantifying seed-borne infection by Fusarium and Microdochium seedling blight pathogens. J. Appl. Microbiol. 2010, 108, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, P.D.; Stevens, R.R. Seed Testing for Blackleg of Canola; Alberta Environmental Centre: Vegreville, AB, Canda, 1993; p. 41. [Google Scholar]
- Lee, S.B.; Taylor, J.W. Isolation of DNA from fungal mycelia and single spores. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 282–287. [Google Scholar]
- Demeke, T.; Ratnayaka, I.; Holigroski, M.; Phan, A. Assessment of extraction methods for PCR testing of discontinued or unapproved biotech events in single seeds of canola, flax and soybean. Food Control 2012, 24, 44–49. [Google Scholar]
- Williams, P.H.; Delwiche, P.A. Screening for Resistance to Blackleg of Crucifers in the Seedling Stage. In 1997 Proceedings of Eucarpia Conference; Breeding of Cruciferous Crops: Wageningen, The Netherlands, 1979; pp. 164–170. [Google Scholar]
| Sample | Location | Level of Resistance a | Number of Isolates b | ||
|---|---|---|---|---|---|
| L. maculans | L. biglobosa | Total | |||
| Westar | Vegreville, AB | S | 13 | 39 | 52 |
| Q2 – Field 1 c | Vegreville, AB | MS | 7 | 39 | 46 |
| Q2 – Field 2 c | Vegreville, AB | MS | 0 | 41 | 41 |
| Defender | Vegreville, AB | MR | 2 | 21 | 23 |
| 72-65 RR | Lloydminster, AB | R | 4 | 44 | 48 |
| Defender | Carman, MB | MR | 0 | 3 | 3 |
| Westar | Carman, MB | S | 0 | 0 | 0 |
| Defender | Plum Coulee, MB | MR | 0 | 47 | 47 |
| Westar | Plum Coulee, MB | S | 30 | 132 | 162 |
| Defender | Roland, MB | MR | 0 | 3 | 3 |
| Westar | Roland, MB | S | 0 | 3 | 3 |
| 45H29 | Brandon, MB | R | 0 | 0 | 8 |
| 72-65 RR | Brandon, MB | R | 0 | 0 | 0 |
| CDS10-053 | Brandon, MB | N/A | 0 | 0 | 0 |
| InVigor 5440 | Brandon, MB | R | 0 | 0 | 0 |
| InVigor 5440 | Brandon, MB | R | 0 | 12 | 12 |
| Defender | N. Battleford, SK | MR | 0 | 77 | 77 |
| SC2 | Canora, SK | N/A | 0 | 300 | 300 |
| SC7 | St Brieux, SK | N/A | 1 | 13 | 14 |
| SC9-1 | Pelly, SK | N/A | 0 | 1 | 1 |
| SC9-2 | Pelly, SK | N/A | 2 | 30 | 32 |
| CR47 | Springside, SK | N/A | 0 | 3 | 3 |
| Defender | N. Battleford, SK | MR | 2 | 87 | 89 |
| SC1 | Canora, SK | N/A | 0 | 116 | 116 |
| BHSW2 | Birch Hills, SK | N/A | 0 | 0 | 0 |
| 61 | 1011 | 1072 | |||
| Sample | Location | Level of Resistance a | Seed Infection (%) b | Admixture Infection (%) c | ||
|---|---|---|---|---|---|---|
| L. maculans | L. biglobosa | L. maculans | L. biglobosa | |||
| L150 | North Battleford, SK | R | 0.2 | 0.2 | 10 | 0 |
| 73-45 RR | Lloydminster, SK | R | 0.2 | 0.2 | 10 | 2 |
| 73-45 RR | Speers, SK | R | 0.1 | 0.1 | 6 | 0 |
| 45H29 | Lloydminster, SK | R | 0.1 | 0.1 | 10 | 6 |
| 73-45 RR | Marshall, SK | R | 0.1 | 0.1 | 0 | 2 |
| 45S52 | Speers, SK | MR | 0.2 | 0.2 | 8 | 0 |
| 9553 | Lloydminster, SK | R | 0.4 | 0.2 | 2 | 0 |
| 43E02 | Grand Prairie, AB | MR | 0 | 0 | 0 | 0 |
| 73-45 RR | Rycroft, AB | R | 0 | 0 | 2 | 0 |
| 1012 RR | Girouxville, AB | R | 0 | 0 | 0 | 2 |
| 5440 | Neepawa, MB | R | 0 | 0.1 | 0 | 2 |
| L130 | Griswold, MB | R | 0 | 0.1 | 31 | 0 |
| Nexera | Justice, MB | N/A | 0 | 0 | 0 | 0 |
| 5440 | Neepawa, MB | R | 0 | 0 | 17 | 0 |
| 73-45 | Oak River, MB | N/A | 0.1 | 0 | 0 | 0 |
| 5440 | Dauphine, MB | N/A | 0.1 | 0 | 13 | 0 |
| HEAR | Souris, MB | N/A | 0 | 0 | 8 | 0 |
| INVIGOR | Minnedosa, MB | N/A | 0 | 0 | 2 | 0 |
| D | Duff, SK | N/A | 0 | 0 | 0 | 0 |
| D2 | Duff, SK | N/A | 0 | 0 | 0 | 0 |
| 71-45 | St. Paul, AB | MR | 0 | 0 | 0 | 0 |
| INV5020 | Mallaig, AB | R | 0 | 0.1 | 0 | 0 |
| 6040 RR | Lloydminster, SK | R | 0 | 0 | 0 | 2 |
| 1012 RR | Douglas, MB | R | 0 | 0 | 0 | 2 |
| 1841 RR | Lloydminster, SK | R | 0 | 0 | 0 | 2 |
| INV5440 | Justice, MB | R | 0 | 0 | 0 | 0 |
| INV5020 | Mallaig, AB | R | 0 | 0 | 12 | 0 |
| INV5020 | AB | R | 0 | 0.1 | 0 | 0 |
| INV5440 | AB | R | 0 | 0 | 0 | 0 |
| 6060 RR | Justice, MB | R | 0 | 0.2 | 0 | 0 |
| CI-Nexera | Justice, MB | - | 0 | 0.1 | 0 | 0 |
| 5440 | St. Benedict, SK | R | 0 | 0.1 | 0 | 0 |
| 45H29 | Cudworth, SK | R | 0 | 0 | 0 | 0 |
| 72-65 | St. Benedict, SK | R | 0 | 0 | 0 | 0 |
| K | AB | N/A | 0 | 0 | 0 | 0 |
| L | AB | N/A | 0 | 0 | 0 | 0 |
| M | AB | N/A | 0 | 0 | 0 | 0 |
| Cargill 4 | SK | N/A | 0 | 0 | 10 | 10 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernando, W.G.D.; Zhang, X.; Amarasinghe, C.C. Detection of Leptosphaeria maculans and Leptosphaeria biglobosa Causing Blackleg Disease in Canola from Canadian Canola Seed Lots and Dockage. Plants 2016, 5, 12. https://doi.org/10.3390/plants5010012
Fernando WGD, Zhang X, Amarasinghe CC. Detection of Leptosphaeria maculans and Leptosphaeria biglobosa Causing Blackleg Disease in Canola from Canadian Canola Seed Lots and Dockage. Plants. 2016; 5(1):12. https://doi.org/10.3390/plants5010012
Chicago/Turabian StyleFernando, W. G. Dilantha, Xuehua Zhang, and Chami C. Amarasinghe. 2016. "Detection of Leptosphaeria maculans and Leptosphaeria biglobosa Causing Blackleg Disease in Canola from Canadian Canola Seed Lots and Dockage" Plants 5, no. 1: 12. https://doi.org/10.3390/plants5010012
APA StyleFernando, W. G. D., Zhang, X., & Amarasinghe, C. C. (2016). Detection of Leptosphaeria maculans and Leptosphaeria biglobosa Causing Blackleg Disease in Canola from Canadian Canola Seed Lots and Dockage. Plants, 5(1), 12. https://doi.org/10.3390/plants5010012







