Antifungal Activity of Acorus calamus Essential Oil Against Rice Blast Fungus Magnaporthe oryzae and Its Composition Characterization
Abstract
1. Introduction
2. Results
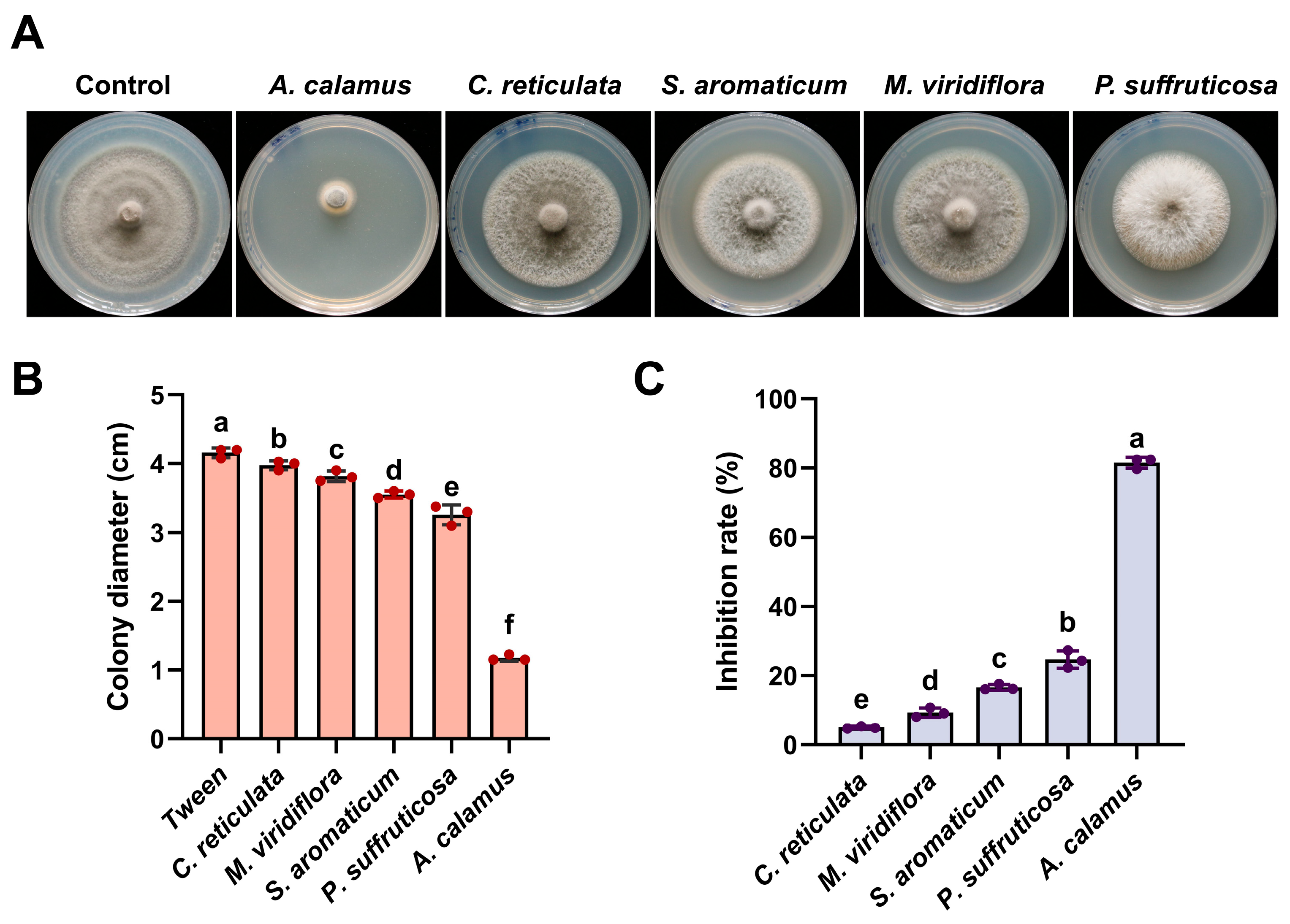
2.1. Primary Screening of the Selected Essential Oil
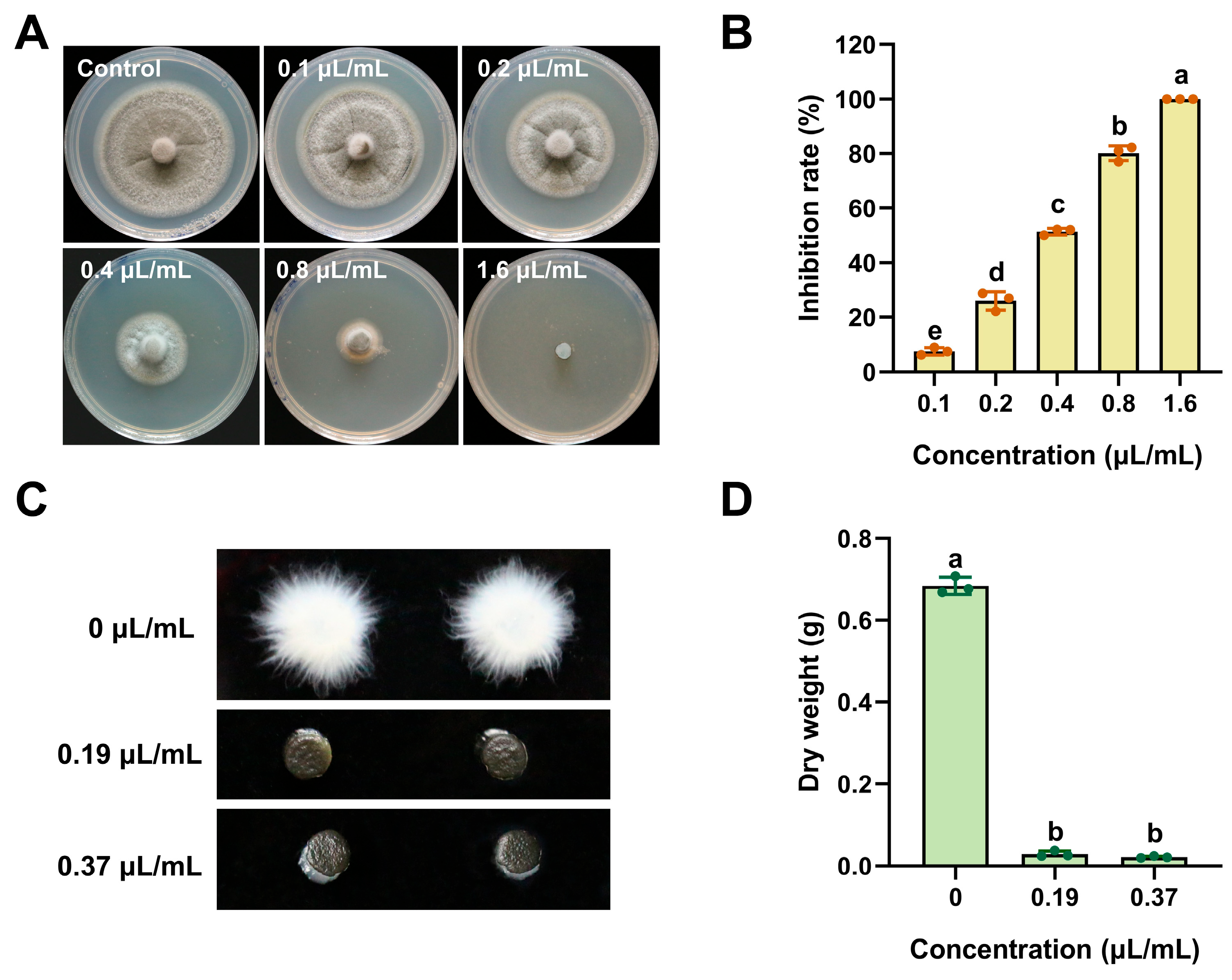
2.2. The Inhibition of ACEO on Mycelial Growth of M. oryzae
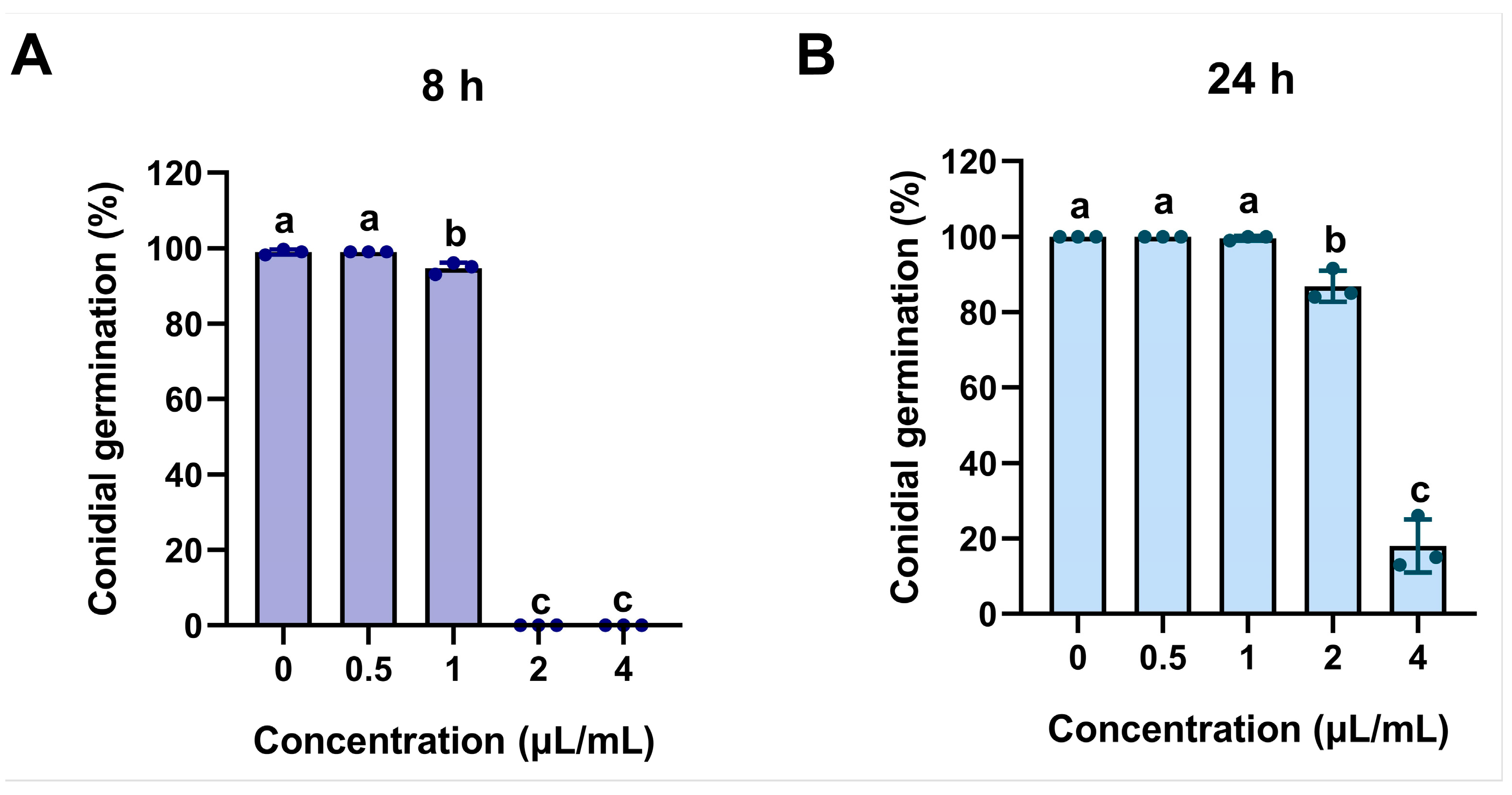
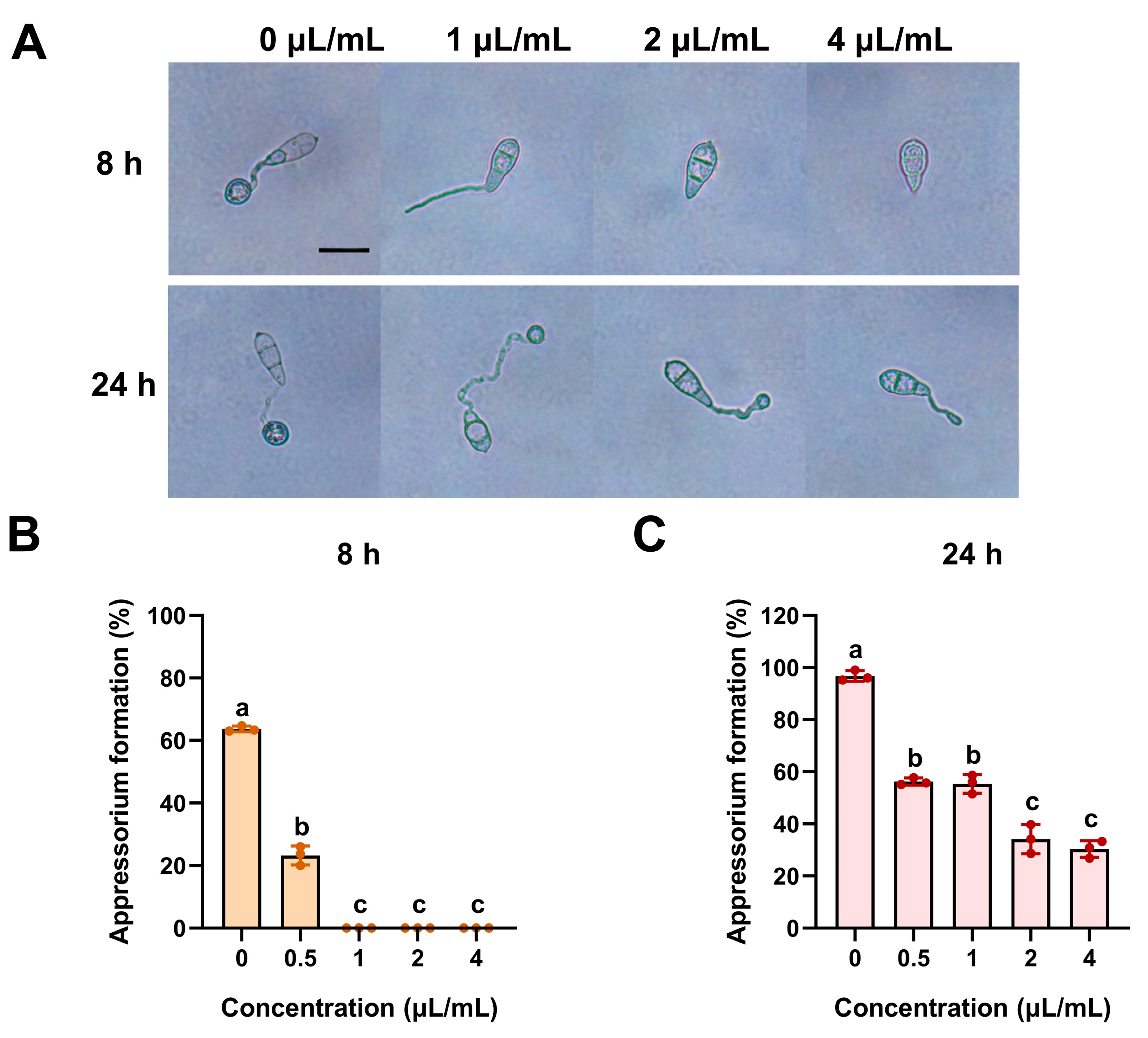
2.3. The Inhibition of ACEO on Conidial Germination and Appressorium Formation of M. oryzae
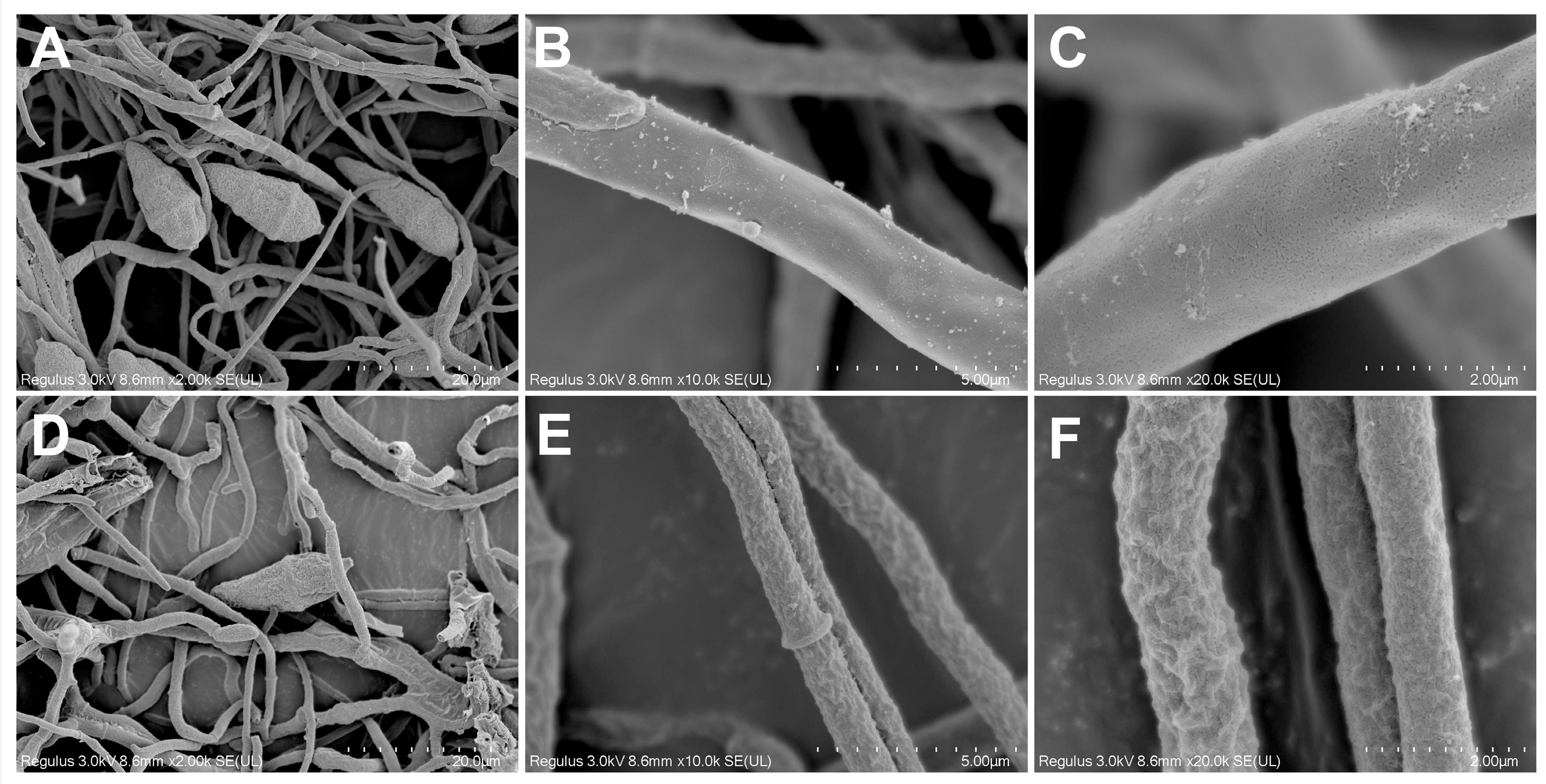
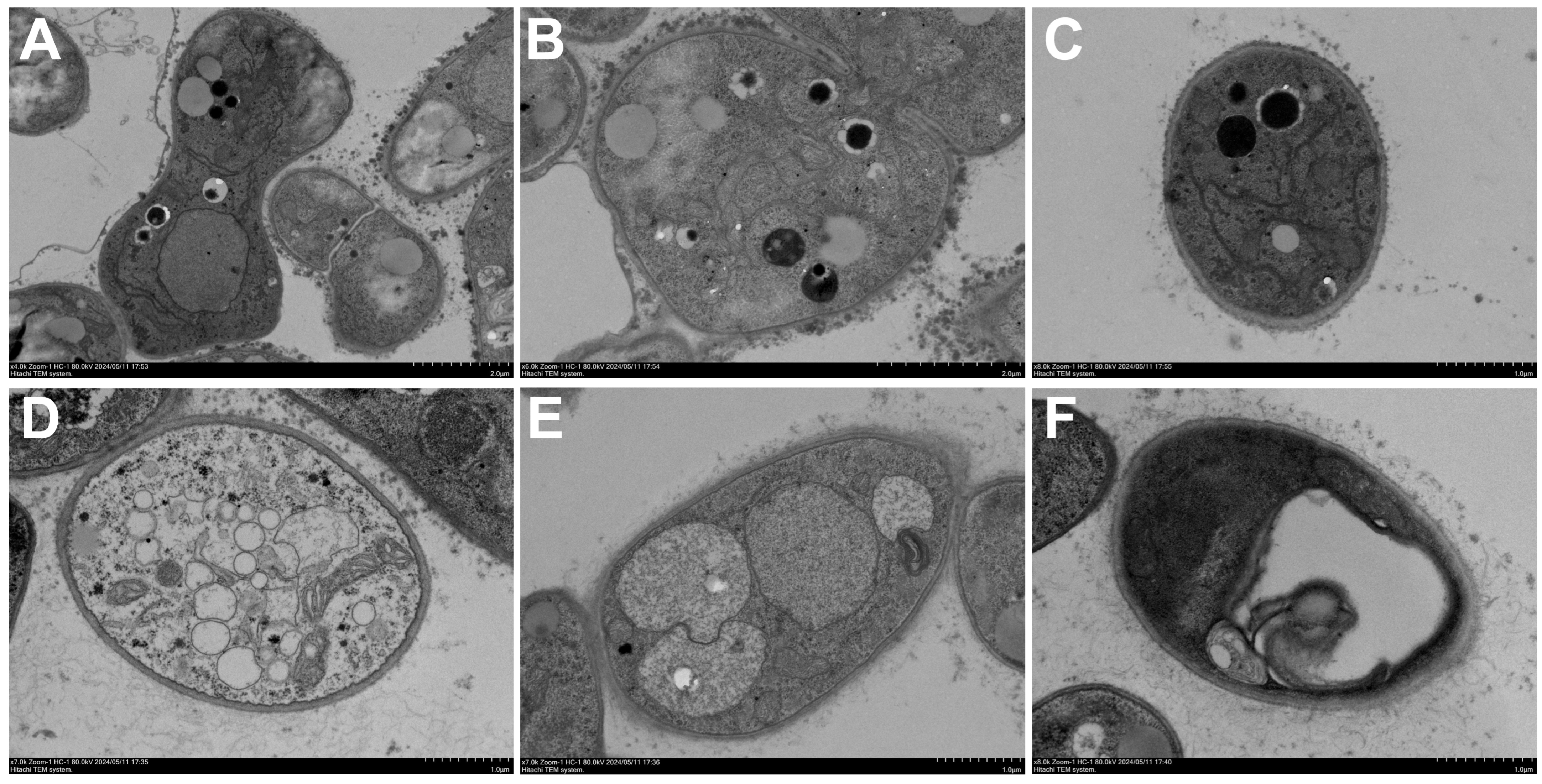
2.4. Effects of ACEO on M. oryzae Morphology
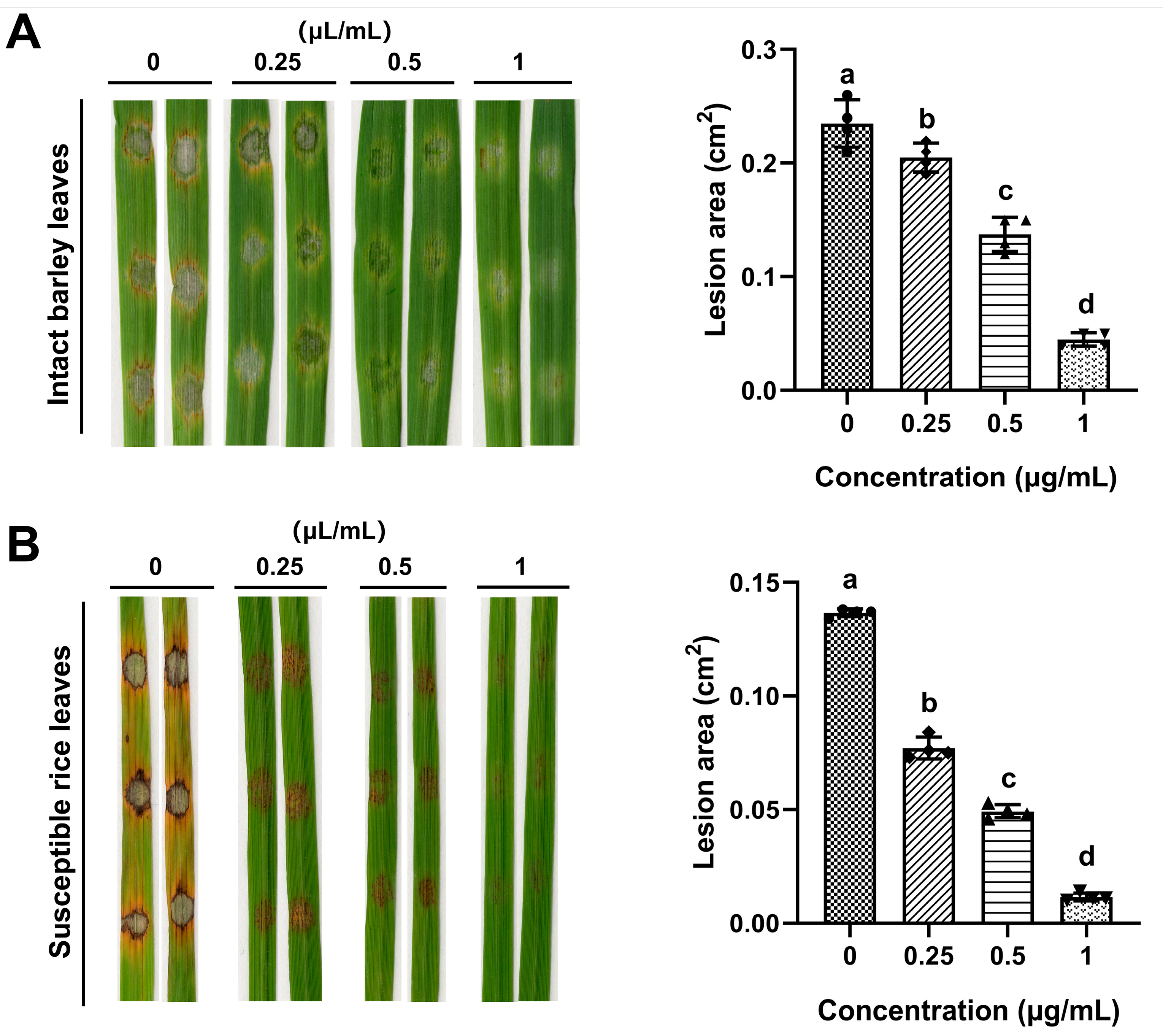
2.5. Effects of ACEO on M. oryzae Pathogenicity
2.6. Chromatography-Mass Spectrometry (GC-MS) Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Mycelial Growth Assay
4.3. Dry Weight Assay
4.4. Conidial Germination and Appressorium Formation Assay
4.5. Scanning Electron Microscopy (SEM) Observations
4.6. Transmission Electron Microscopy (TEM) Observations
4.7. Pathogenicity Tests
4.8. GC-MS Analysis
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Essential oil |
| ACEO | Acorus calamus Essential oil |
| GC-MS | Gas chromatography–mass spectrometry |
| CM | Complete Medium |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| EC50 | Median effective concentration |
| PBS | Phosphate-buffered saline |
References
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Khush, G.S. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol. Biol. 2005, 59, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.H. Pathogen variability and host resistance in rice blast disease. Annu. Rev. Phytopathol. 1981, 18, 167–187. [Google Scholar] [CrossRef]
- D’Ávila, L.S.; De Filippi, M.C.C.; Café-Filho, A.C. Sensitivity of Pyricularia oryzae populations to fungicides over a 26-year time frame in Brazil. Plant Dis. 2021, 105, 1771–1780. [Google Scholar] [CrossRef]
- Harata, K.; Daimon, H.; Okuno, T. Trade-off relation between fungicide sensitivity and melanin biosynthesis in plant pathogenic fungi. iScience 2020, 23, 101660. [Google Scholar] [CrossRef]
- Skamnioti, P.; Gurr, S.J. Against the grain: Safeguarding rice from rice blast disease. Trends Biotechnol. 2009, 27, 141–150. [Google Scholar] [CrossRef]
- D’Ávila, L.S.; De Filippi, M.C.C.; Café-Filho, A.C. Fungicide resistance in Pyricularia oryzae populations from southern and northern Brazil and evidence of fitness costs for QoI-resistant isolates. Crop Prot. 2022, 153, 105887–105895. [Google Scholar] [CrossRef]
- Shen, C.; Ding, X.; Rao, W.; Hu, J.; Lin, T.; Zhou, X.Z.; Zheng, Y.; Dong, F.; Fan, G. Comprehensive risk assessment for five SDHI fungicides with AhR agonistic activity to aquatic ecosystems. Pestic. Biochem. Physiol. 2025, 215, 106665. [Google Scholar] [CrossRef] [PubMed]
- Pimentão, A.R.; Cuco, A.P.; Pascoal, C.; Cássio, F.; Castro, B.B. Current trends and mismatches on fungicide use and assessment of the ecological effects in freshwater ecosystems. Environ. Pollut. 2024, 347, 123678. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Cordia, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Rodrigues, T.C.; Gois, I.B.; Fernandes, R.P.M.; Blank, A.F.; Sandes, R.D.D.; Neta, M.T.S.L.; Narain, N.; de Fátima Arrigoni-Blank, M. Chemical characterization and antimicrobial activity of essential oils from Croton grewioides Baill. accessions on the phytopathogen Xanthomonas campestris pv. campestris. Pestic. Biochem. Physiol. 2023, 193, 105454. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Guo, J.; Liang, R.; Wang, X.; Chen, Q.; Wang, J.; Ge, X.; Chen, C.; Sun, J.; Zhao, C.; et al. A review on antifungal activity of plant essential oils. Phytother. Res. 2025, 39, 3736–3761. [Google Scholar] [CrossRef]
- Yun, X.; Zhou, Y.; Shi, H.; Li, Z.; Yun, Y.; Xie, M.; Chen, L. Combination of plant essential oils and ice: Extraction, encapsulation and applications in food preservation. Food Chem. 2025, 493, 145793. [Google Scholar] [CrossRef]
- Ayllón-Gutiérrez, R.; Díaz-Rubio, L.; Montaño-Soto, M.; Haro-Vázquez, M.D.P.; Córdova-Guerrero, I. Applications of plant essential oils in pest control and their encapsulation for controlled release: A review. Agriculture 2024, 14, 1766. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Olivero-Verbel, J. Aromatherapy and essential oils: Holistic strategies in complementary and alternative medicine for integral wellbeing. Plants 2025, 14, 400. [Google Scholar] [CrossRef]
- Allagui, M.B.; Moumni, M.; Romanazzi, G. Antifungal activity of thirty essential oils to control pathogenic fungi of postharvest decay. Antibiotics 2023, 13, 28. [Google Scholar] [CrossRef]
- Campos, S.; Espinoza, J.; Fuentes, J.M.; Jofré-Fernández, I.; Tortella, G.; Navarro, D.; Quiroz, A.; Diez, M.C.; Rubilar, O.; Fincheira, P. The impact of essential oils derived from Citrus species to control Botrytis cinerea and their potential physiological actions. Plants 2025, 14, 1859. [Google Scholar] [CrossRef]
- Buonsenso, F.; Schiavon, G.; Spadaro, D. Efficacy and mechanisms of action of essential oils’ vapours against blue mould on apples caused by Penicillium expansum. Int. J. Mol. Sci. 2023, 24, 2900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dai, Y.; Wei, C.; Li, H.; Ma, C.; Zou, Z. Preparation of Cinnamomum camphora essential oil microcapsules using gelatin/gum arabic and evaluation of their antifungal effects on Fusarium spp. Int. J. Biol. Macromol. 2025, 303, 140706. [Google Scholar] [CrossRef]
- Kou, Z.X.; Dang, Y.; Liu, L.; Wu, X.H.; Fu, Y. Antifungal activity of Artemisia capillaris essential oil against Alternaria species causing black spot on Yanbian Pingguoli pear in China. Plants 2025, 14, 3146. [Google Scholar] [CrossRef]
- Andrade-Hoyos, P.; Hernández-Arenas, M.; Mendieta-Moctezuma, A.; Barrios-Gómez, E.J.; Romero-Arenas, O.; Ríos-Meléndez, S.; Parraguirre-Lezama, C.; Ibarra-Torres, P. Effect of essential oils on postharvest management of anthracnose associated with Colletotrichum gloeosporioides (Penz.) Penz & Sacc., in mango. Plants 2025, 14, 3249. [Google Scholar] [CrossRef]
- Divya, G.; Gajalakshmi, S.; Mythili, S.; Sathiavelu, A. Pharmacological activities of Acorus calamus: A review. Asian J. Biomed. Pharmaceut. Sci. 2011, 1, 57–64. [Google Scholar]
- Sharma, V.; Singh, I.; Chaudhary, P. Acorus calamus (the healing plant): A review on its medicinal potential, micropropagation and conservation. Nat. Prod. Res. 2014, 28, 1454–1466. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, O.P.; Saxena, B.P. Effect of sweet flag rhizome oil (Acorus calamus) on hemogram and ultrastructure of hemocytes of the tobacco armyworm, Spodoptera litura (lepidoptera: Noctuidae). Micron 2008, 39, 544–551. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, W.; Yang, C.; Xue, D.; Huang, Y. Isolation and characterization of insecticidal activity of (z)-asarone from Acorus calamus L. Insect Sci. 2008, 15, 229–236. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhou, L.G.; Liu, Z.L.; Du, S.S. Identification of insecticidal constituents of the essential oil of Acorus calamus rhizomes against Liposcelis bostrychophila badonnel. Molecules 2013, 18, 5684–5696. [Google Scholar] [CrossRef]
- Al-Mijalli, S.H.; Mrabti, H.N.; Abdallah, E.M.; Assaggaf, H.; Qasem, A.; Alenazy, R.; Bouyahya, A.; Alshabrmi, F.M.; El Hachlafi, N. Acorus calamus as a promising source of new antibacterial agent against Pseudomonas aeruginosa and Staphylococcus aureus: Deciphering volatile compounds and mode of action. Microb. Pathog. 2025, 200, 107357. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Rui, W.; Peng, X.; Huang, Q.; Zhang, W. Organic carbon fractions affected by long-term fertilization in a subtropical paddy soil. Nutr. Cycl. Agroecosystems 2010, 86, 153–160. [Google Scholar] [CrossRef]
- Perumal, A.B.; Li, X.; Su, Z.; He, Y. Preparation and characterization of a novel green tea essential oil nanoemulsion and its antifungal mechanism of action against Magnaporthae oryzae. Ultrason. Sonochemistry 2021, 76, 105649. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, Z.; Yu, G.; Yan, C.; Liu, Y.; Liu, S.; Deng, S. Antifungal activity and action mechanism of thymol against rice blast fungus Magnaporthe oryzae. Pestic. Biochem. Physiol. 2025, 215, 106646. [Google Scholar] [CrossRef]
- Ghahari, S.; Alinezhad, H.; Nematzadeh, G.A.; Tajbakhsh, M.; Baharfar, R. Chemical composition, antioxidant and biological activities of the essential oil and extract of the seeds of Glycine max (Soybean) from North Iran. Curr. Microbiol. 2017, 74, 522–531. [Google Scholar] [CrossRef]
- Joycharat, N.; Thammavong, S.; Voravuthikunchai, S.P.; Plodpai, P.; Mitsuwan, W.; Limsuwan, S.; Subhadhirasakul, S. Chemical constituents and antimicrobial properties of the essential oil and ethanol extract from the stem of Aglaia odorata Lour. Nat. Prod. Res. 2014, 28, 2169–2172. [Google Scholar] [CrossRef]
- Phongpaichit, S.; Pujenjob, N.; Rukachaisirikul, V.; Ongsakul, M. Antimicrobial activities of the crude methanol extract of Acorus calamus Linn. Songklanakarin J. Sci. Technol. 2005, 27, 517–523. [Google Scholar]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Ortiz, S.C.; Huang, M.; Hull, C.M. Discovery of fungus-specific targets and inhibitors using chemical phenotyping of pathogenic spore germination. mBio 2021, 12, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, H.; Chen, K.; Feng, J.; Zhang, Y. Antifungal activities and mode of action of Cymbopogon citratus, Thymus vulgraris, and Origanum heracleoticum essential oil vapors against Botrytis cinerea and their potential application to control postharvest strawberry gray mold. Foods 2021, 10, 2451. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, Y.; Chen, Y.; Wang, Y.; Gu, Q.; Song, D. Antifungal activity and mechanism of Litsea cubeba (Lour.) Persoon essential oil against the waxberry spoilage fungi Penicillium oxalicum and its potential application. Int. J. Food Microbiol. 2024, 411, 110512. [Google Scholar] [CrossRef]
- Szczeblewski, P.; Wróblewski, M.; Borzyszkowska-Bukowska, J.; Bairamova, T.; Górska, J.; Laskowski, T.; Samulewicz, A.; Kosno, M.; Sobiech, K.; Teresa Polit, J.; et al. The role of centrifugal partition chromatography in the removal of β-asarone from Acorus calamus essential oil. Sci. Rep. 2022, 12, 22217. [Google Scholar] [CrossRef]
- Loying, R.; Gogoi, R.; Sarma, N.; Borah, A.; Munda, S.; Pandey, S.K.; Lal, M. Chemical compositions, in-vitro antioxidant, anti-microbial, anti-inflammatory and cytotoxic activities of essential oil of Acorus calamus L. rhizome from North-East India. J. Essent. Oil Bear. Plants 2019, 22, 1299–1312. [Google Scholar] [CrossRef]
- Sanli, I.; Ozkan, G.; Şahin-Yeşilçubuk, N. Green extractions of bioactive compounds from citrus peels and their applications in the food industry. Food Res. Int. 2025, 212, 116352. [Google Scholar] [CrossRef]
- Mo, F.; Hu, X.; Ding, Y.; Li, R.; Long, Y.; Wu, X.; Li, M. Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Physiol. 2021, 178, 104942. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Wu, H.B.; Kuang, M.S.; Lan, H.P.; Wen, Y.X.; Liu, T.T. Novel bithiophene dimers from Echinops latifolius as potential antifungal and nematicidal agents. J. Agric. Food Chem. 2020, 68, 11939–11945. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhang, Y.; Wang, C.; Liu, S.; Liao, X. Effects and inhibition mechanism of phenazine-1-carboxamide on the mycelial morphology and ultrastructure of Rhizoctonia solani. Pestic. Biochem. Physiol. 2018, 147, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; ElHachlafi, N.; Al-Mijalli, S.H.; Jeddi, M.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G.; et al. Phytochemical profile, assessment of antimicrobial and antioxidant properties of essential oils of Artemisia herba-alba Asso., and Artemisia dracunculus L.: Experimental and computational approaches. J. Mol. Struct. 2023, 1294, 136479. [Google Scholar] [CrossRef]
| No. | Compound | Area (%) |
|---|---|---|
| 1 | m-Xylene | 0.45 |
| 2 | Camphene | 0.54 |
| 3 | E,E-6,11-Tridecadien-1-ol acetate | 0.21 |
| 4 | trans-β-Ocimene | 0.31 |
| 5 | Camphor | 0.71 |
| 6 | 3-Methyl-2,4,10-trioxatricyclo[3.3.1.13,7]decane | 0.29 |
| 7 | Copaene | 0.25 |
| 8 | β-Elemene | 1.06 |
| 9 | Caryophyllene | 0.81 |
| 10 | 1,5-dimethyl-8-(1-methylethylidene)-1,5-cyclodecadiene | 0.27 |
| 11 | Calarene | 0.64 |
| 12 | 4-trans-propenylveratrole | 2.06 |
| 13 | cis-Isoeugenol methyl ether | 1.23 |
| 14 | Methyl isoeugenol | 0.47 |
| 15 | γ-cadinene | 0.49 |
| 16 | Zizanene | 0.26 |
| 17 | Germacrene D | 0.28 |
| 18 | 6-Epishyobunone | 4.62 |
| 19 | Ledene | 2.78 |
| 20 | Shyobunone | 0.28 |
| 21 | α-Gurjunene | 0.92 |
| 22 | Epishyobunone | 8.07 |
| 23 | δ-Cadinene | 4.52 |
| 24 | Isoshyobunone | 14.92 |
| 25 | (Z)-Methylisoeugenol | 0.75 |
| 26 | α-Calacorene | 4.81 |
| 27 | β-Calacorene | 0.78 |
| 28 | Eremophila ketone | 0.30 |
| 29 | Spathulenol | 0.24 |
| 30 | β-Asarone | 19.83 |
| 31 | α-Asarone | 4.03 |
| 32 | Dehydroxy-isocalamendiol | 2.98 |
| 33 | τ-Cadinol | 0.90 |
| 34 | α-Cadinol | 0.76 |
| 35 | 3−Chloro−2−fluoro−N−(2−phenylethyl)−N−heptylbenzamide | 0.38 |
| 36 | cis-Calamenene | 0.85 |
| 37 | 1,2,3,4-Tetrahydro-3-isopropyl-5-methyl-1-oxonaphthalene | 1.26 |
| 38 | Zierone | 8.30 |
| 39 | Nootkatone | 0.39 |
| 40 | Isocalamenediol | 2.99 |
| 41 | Dibutyl phthalate | 0.25 |
| 42 | Hexamethylcyclotrisiloxane | 0.22 |
| 43 | 2-(Acetoxymethyl)-3-(methoxycarbonyl)biphenylene | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Deng, S.; Wang, Z.; Li, Y.; Liu, Y.; Kong, Z.; Meng, G.; Jin, S.; Zeng, A.; Liu, H.; Liu, S. Antifungal Activity of Acorus calamus Essential Oil Against Rice Blast Fungus Magnaporthe oryzae and Its Composition Characterization. Plants 2026, 15, 332. https://doi.org/10.3390/plants15020332
Deng S, Wang Z, Li Y, Liu Y, Kong Z, Meng G, Jin S, Zeng A, Liu H, Liu S. Antifungal Activity of Acorus calamus Essential Oil Against Rice Blast Fungus Magnaporthe oryzae and Its Composition Characterization. Plants. 2026; 15(2):332. https://doi.org/10.3390/plants15020332
Chicago/Turabian StyleDeng, Shuzhen, Ziyi Wang, Yusi Li, Yiming Liu, Zhiyi Kong, Ge Meng, Saige Jin, Anqi Zeng, Huan Liu, and Shengming Liu. 2026. "Antifungal Activity of Acorus calamus Essential Oil Against Rice Blast Fungus Magnaporthe oryzae and Its Composition Characterization" Plants 15, no. 2: 332. https://doi.org/10.3390/plants15020332
APA StyleDeng, S., Wang, Z., Li, Y., Liu, Y., Kong, Z., Meng, G., Jin, S., Zeng, A., Liu, H., & Liu, S. (2026). Antifungal Activity of Acorus calamus Essential Oil Against Rice Blast Fungus Magnaporthe oryzae and Its Composition Characterization. Plants, 15(2), 332. https://doi.org/10.3390/plants15020332






