Identification of a Chlorophyll-Deficient Mutant in Maize Associated with Exogenous Vector Insertion
Abstract
1. Introduction
2. Results
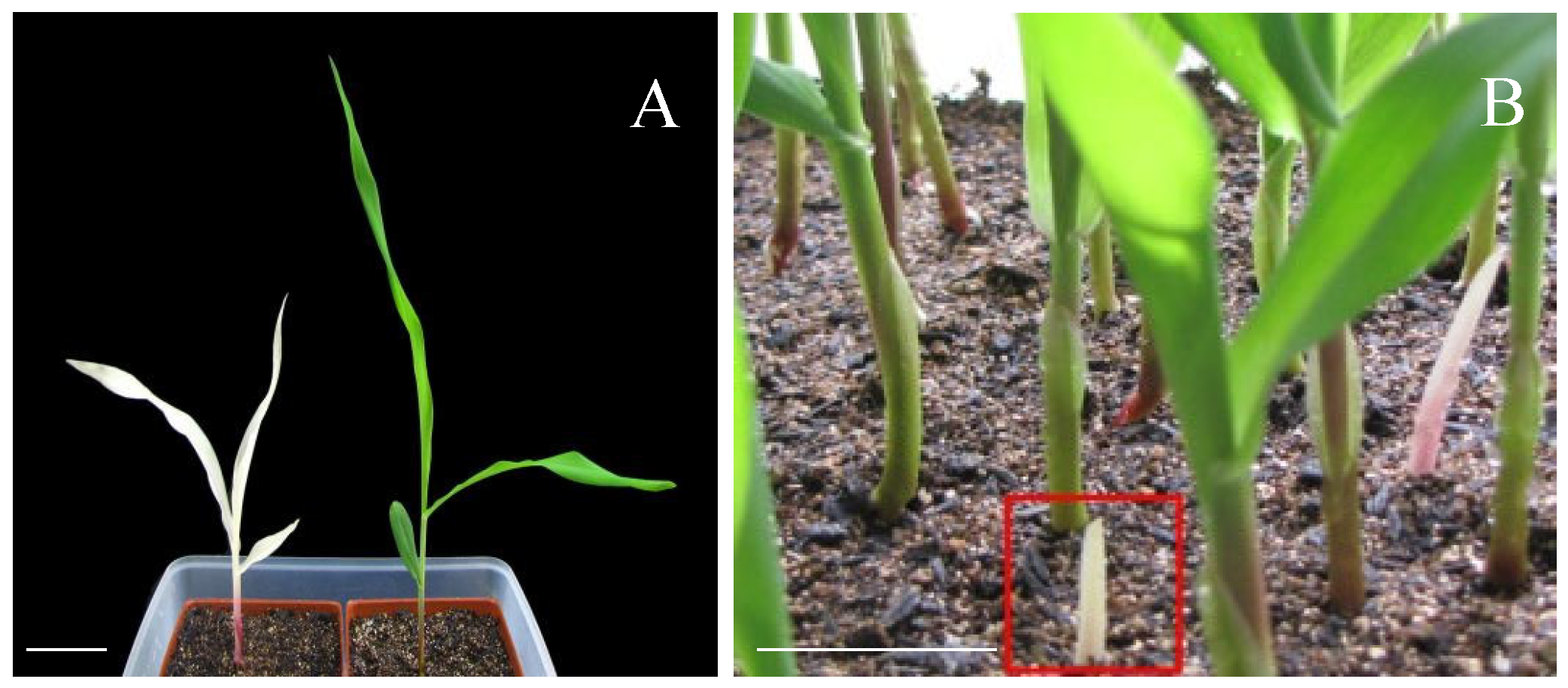
2.1. Phenotypic Characterization of the Chlorophyll-Deficient Mutant (MT)
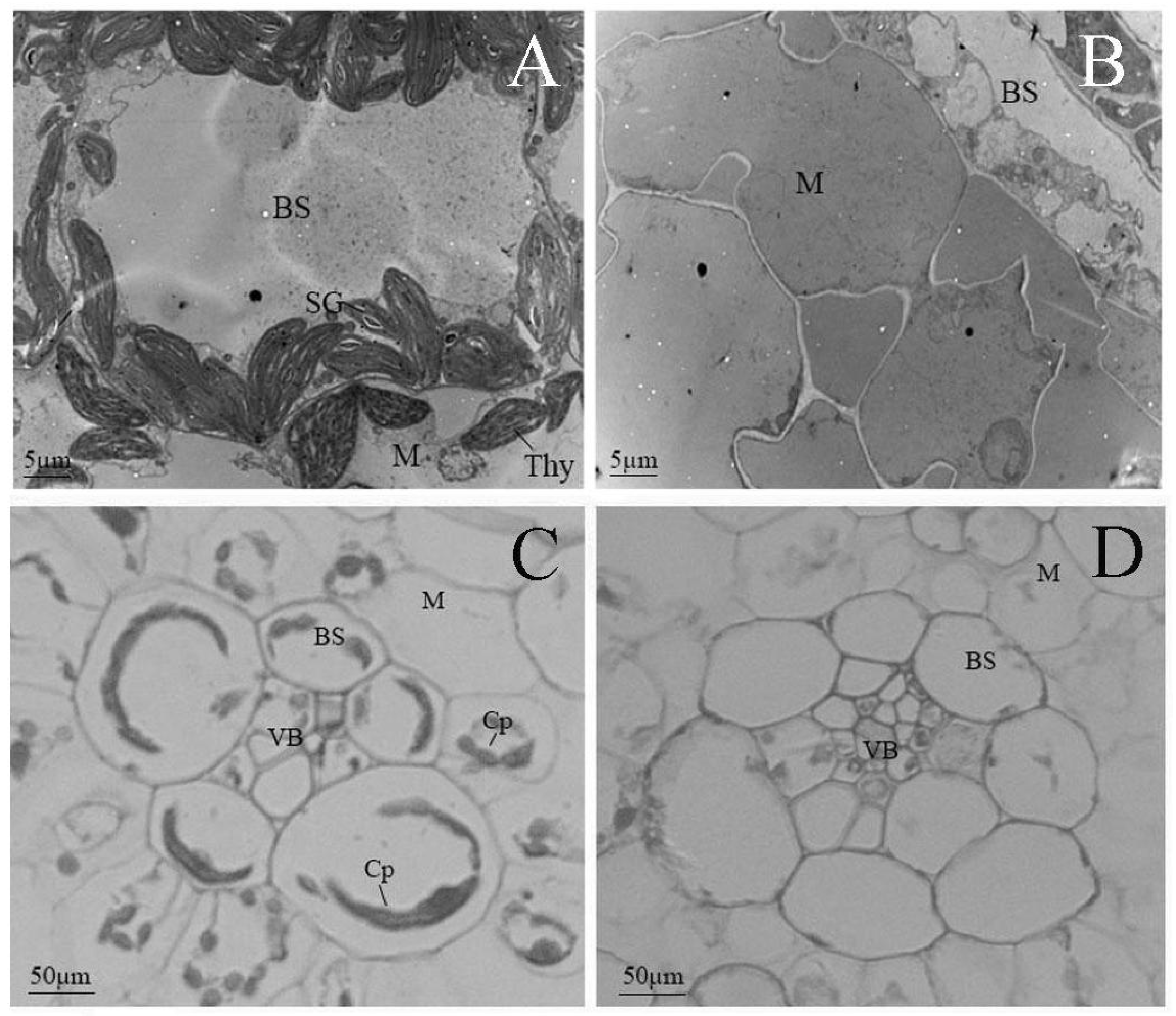
2.2. The Albino Mutant Exhibits Arrested Chloroplast Development in an Early Biogenesis Stage
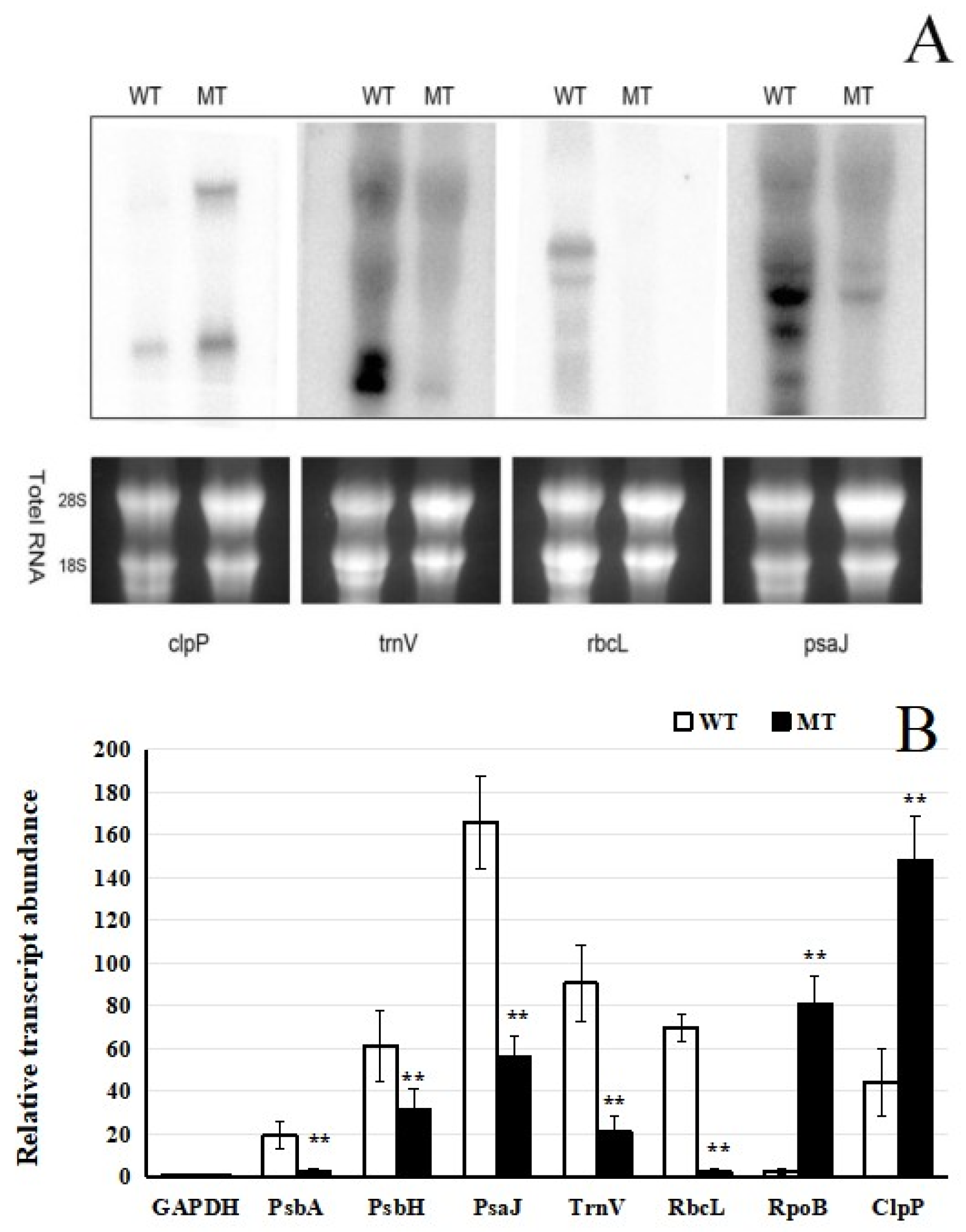
2.3. Expression of Chloroplast Genes in the Mutant
2.4. Association Between Mutant Phenotype and Transgenic Vector
2.5. Chi-Square Analysis Showed That the Chloroplast-Deficient Phenotype Is Caused by a Recessive Nuclear Mutation in a Single Gene
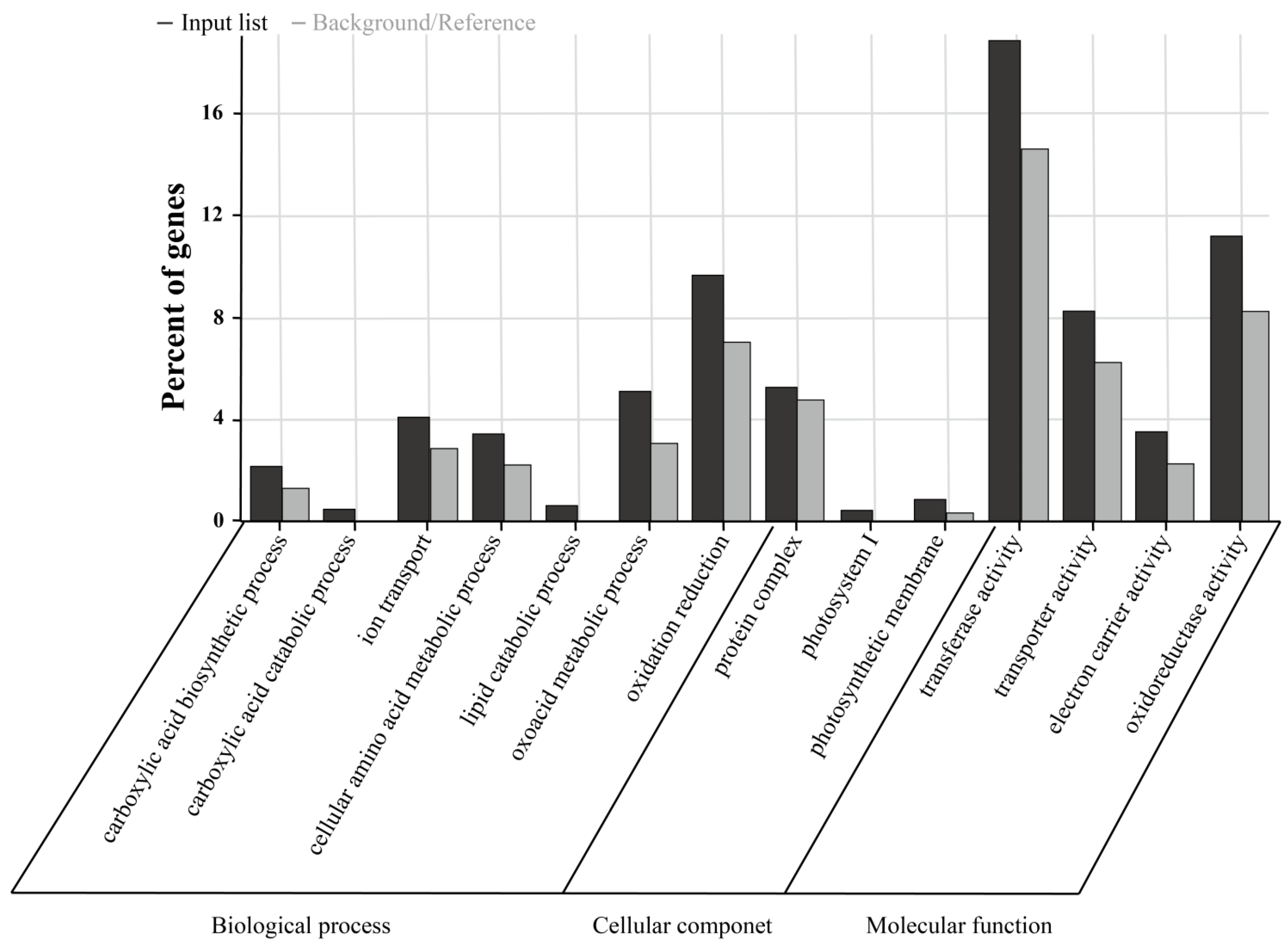
2.6. The BSR-Seq Was Used to Identify Candidate Genes
3. Discussion
3.1. Effects of Chlorophyll-Deficient Mutations on Plastid Transcription
3.2. Mutant Phenotype Linked to the Entire Foreign Vector Sequence
3.3. The BSA (Bulked Segregate Analysis-Sequencing) and BSR Methods Were Used to Identify Candidate Genes
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Microscopy Analysis
4.3. DNA Extraction
4.4. RNA Extraction
4.5. Quantitative Real-Time PCR Analysis (qRT-PCR)
4.6. Statistical Analysis
4.7. RNA Gel-Blot Hybridization Assays
4.8. Co-Segregation Analysis
4.9. BSR Sequencing Data Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, M.Z.; Meng, F.Y.; Mo, F.L.; Chen, X.L.; Zhang, H.; Wang, A.X. Insights into the Molecular Basis of a Yellow Leaf Color Mutant (ym) in Tomato (Solanum lycopersicum). Sci. Hortic. 2022, 293, 110743. [Google Scholar] [CrossRef]
- Li, Y.S.; Wang, X.J.; Lian, X.R.; Liang, G.S.; He, H.J.; Yang, Y.Z.; Zhou, W.Q.; Dong, X.Y.; Zhou, Y.Q.; Liu, Z.X. Fine Mapping of a Yellowing Mutant Gene Zmet9 in Maize. J. Plant Genet. Resour. 2025, 26, 319–330. [Google Scholar]
- Stettler, M.; Eicke, S.; Mettler, T.; Messerli, G.; Hörtensteiner, S.; Zeeman, S.C. Blocking the Metabolism of Starch Breakdown Products in Arabidopsis Leaves Triggers Chloroplast Degradation. Mol. Plant 2009, 2, 1233–1246. [Google Scholar] [CrossRef]
- Asakura, Y.; Hirohashi, T.; Kikuchi, S.; Belcher, S.; Osborne, E.; Yano, S.; Terashima, I.; Barkan, A.; Nakai, M. Maize Mutant Lacking Chloroplast FtsY Exhibits Pleiotropic Defects in the Biogenesis of Thylakoid Membranes. Plant Cell 2004, 16, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Ruppel, N.J.; Logsdon, C.A.; Whippo, C.W.; Inoue, K.; Hangarter, R.P. A Mutation in Arabidopsis Seedling Plastid Development1 Affects Plastid Differentiation in Embryo-Derived Tissues during Seedling Growth. Plant Physiol. 2011, 155, 342–353. [Google Scholar] [CrossRef][Green Version]
- Stern, D.B.; Hanson, M.R.; Barkan, A. Genetics and Genomics of Chloroplast Biogenesis: Maize as a Model System. Trends Plant Sci. 2004, 9, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.K.; Lee, J.Y.; Cho, H.S.; Lee, S.S.; Ha, H.J.; Kim, S.; Choi, D.; Pai, H.S. Inactivation of Organellar Glutamyl- and Seryl-tRNA Synthetases Leads to Developmental Arrest of Chloroplasts and Mitochondria in Higher Plants. J. Biol. Chem. 2005, 280, 37098–37106. [Google Scholar] [CrossRef]
- Oster, U.; Tanaka, R.; Tanaka, A.; Rüdiger, W. Cloning and Functional Expression of the Gene Encoding the Key Enzyme for Chlorophyll b Biosynthesis (CAO) from Arabidopsis thaliana. Plant J. 2000, 21, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, J.H.; Yoo, E.S.; Lee, C.H.; Hirochika, H.; An, G. Differential Regulation of Chlorophyll a Oxygenase Genes in Rice. Plant Mol. Biol. 2005, 57, 805–818. [Google Scholar] [CrossRef]
- Kusaba, M.; Ito, H.; Morita, R.; Iida, S.; Sato, Y.; Fujimoto, M.; Kawasaki, S.; Tanaka, R.; Hirochika, H.; Nishimura, M. Rice NON-YELLOW COLORING 1 is Involved in Light-Harvesting Complex II and Grana Degradation during Leaf Senescence. Plant Cell 2007, 19, 1362–1375. [Google Scholar]
- Yu, Q.B.; Jiang, Y.; Chong, K.; Yang, Z.N. AtECB2, a Pentatricopeptide Repeat Protein, Is Required for Chloroplast Transcript accD RNA Editing and Early Chloroplast Biogenesis in Arabidopsis thaliana. Plant J. 2009, 59, 1011–1023. [Google Scholar]
- Hu, W.; Franklin, K.A.; Sharrock, R.A.; Jones, M.A.; Harmer, S.L.; Lagarias, J.C. Unanticipated Regulatory Roles for Arabidopsis Phytochromes Revealed by Null Mutant Analysis. Proc. Natl. Acad. Sci. USA 2013, 110, 1542–1547. [Google Scholar]
- Xue, Y.J. Mapping and Functional Analysis of Genes Related to Photosynthesis in Maize. Ph.D. Thesis, Jilin University, Changchun, China, 2023. [Google Scholar]
- Kanamaru, K.; Tanaka, K. Roles of Chloroplast RNA Polymerase Sigma Factors in Chloroplast Development and Stress Response in Higher Plants. Biosci. Biotechnol. Biochem. 2004, 68, 2215–2223. [Google Scholar] [CrossRef]
- Sakamoto, W.; Miyagishima, S.Y.; Jarvis, P. Chloroplast Biogenesis: Control of Plastid Development, Protein Import, Division and Inheritance. Arab. Book 2008, 6, e0110. [Google Scholar] [CrossRef]
- Li, Q.; Du, H.W. Advances in the Study of Leaf Color Mutants in Maize. South. Agric. 2019, 13, 14–21. [Google Scholar]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic Gene Transfer: Organelle Genomes Forge Eukaryotic Chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Hedtke, B.; Börner, T.; Weihe, A. Mitochondrial and Chloroplast Phage-Type RNA Polymerases in Arabidopsis. Science 1997, 277, 809–811. [Google Scholar] [CrossRef] [PubMed]
- Hess, W.R.; Börner, T. Organellar RNA Polymerases of Higher Plants. Int. Rev. Cytol. 1999, 190, 1–59. [Google Scholar] [PubMed]
- Liere, K.; Börner, T. Transcription and Transcriptional Regulation in Plastids. In Cell and Molecular Biology of Plastids; Bock, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 121–174. [Google Scholar]
- Wan, P. Evolutionary analysis of chloroplast RNA editing in terrestrial plants. Biotechnol. Bull. 2013, 1, 73–76. [Google Scholar]
- Huang, W.F. Functional Identification and Mechanism Analysis of OsPPR16 during Early Chloroplast Development in Rice. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Wang, X.M.; Qi, Y.F.; Liu, N.; Zhang, Q.X.; Xie, S.; Yu, L.; Li, B.L.; Shao, J.X.; Yu, F.; Liu, X.Y. Interaction of PALE CRESS with PAP2/pTAC2 and PAP3/pTAC10 Affects the Accumulation of Plastid-Encoded RNA Polymerase Complexes in Arabidopsis. New Phytol. 2023, 240, 1433–1448. [Google Scholar] [CrossRef]
- Wu, X.X.; Mu, W.H.; Li, F.; Sun, S.Y.; Cui, C.J.; Kim, C.; Zhou, F.; Zhang, Y. Cryo-EM Structures of the Plant Plastid-encoded RNA Polymerase. Cell 2024, 187, 1127–1144.e21. [Google Scholar] [CrossRef]
- Manoli, A.; Sturaro, A.; Trevisan, S.; Quaggiotti, S.; Nonis, A. Evaluation of Candidate Reference Genes for qPCR in Maize. Plant Physiol. 2012, 169, 807–815. [Google Scholar] [CrossRef]
- Lv, Z.; Zhao, W.; Kong, S.; Li, L.; Lin, S. Overview of Molecular Mechanisms of Plant Leaf Development: A Systematic Review. Front. Plant Sci. 2023, 14, 1293424. [Google Scholar] [CrossRef]
- Li, R.N. Identification of PfkB Type Carbohydrate Kinase Alleles in Maize. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2024. [Google Scholar]
- Cheng, Y.T. Molecular Mechanism of Chloroplast SUFB on SUFBC2D Complex. Ph.D. Thesis, Yangzhou University, Yangzhou, China, 2024. [Google Scholar]
- Li, Z.; Xu, J.Y.; Zhang, T.Q.; Lin, J.F.; Liu, S.C.; Wang, Q.M. Chloroplast Genome Sequencing and Transcriptome Sequencing Provide Insights into the Developmental Mechanism of the Variegated Mutant of Clivia miniata. Plant Cell Rep. 2022, 41, 2093–2106. [Google Scholar]
- Yan, X.Q. Identification and Gene Mapping of a Green-Turning Mutant wsl887 in Rice. Ph.D. Thesis, Guizhou University, Guiyang, China, 2021. [Google Scholar]
- Xing, A.M. Functional Analysis of the Chaperone Protein Gene PDM1 in Arabidopsis thaliana. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2020. [Google Scholar]
- Emanuel, C.; Weihe, A.; Graner, A.; Hess, W.R.; Börner, T. Chloroplast Development Affects Expression of Phage-Type RNA Polymerases in Barley Leaves. Plant J. 2004, 38, 460–472. [Google Scholar] [CrossRef] [PubMed]
- Legen, J.; Kemp, S.; Krause, K.; Profanter, B.; Herrmann, R.G.; Maier, R.M. Comparative Analysis of Plastid Transcription Profiles of Entire Plastid Chromosomes from Tobacco Attributed to Wild-Type and PEP-Deficient Transcription Machineries. Plant J. 2002, 31, 171–188. [Google Scholar] [PubMed]
- Prikryl, J.; Watkins, K.P.; Friso, G.; van Wijk, K.J.; Barkan, A. A Member of the Whirly Family is a Multifunctional RNA- and DNA-Binding Protein That is Essential for Chloroplast Biogenesis. Nucleic Acids Res. 2008, 36, 5152–5165. [Google Scholar] [CrossRef]
- Zhelyazkova, P.; Sharma, C.M.; Förstner, K.U.; Liere, K.; Vogel, J.; Börner, T. The Primary Transcriptome of Barley Chloroplasts: Numerous Noncoding RNAs and the Dominating Role of the Plastid-Encoded RNA Polymerase. Plant Cell 2012, 24, 123–136. [Google Scholar] [CrossRef]
- Lu, P.L.; Luo, X.J. A Natural Variation in PLEIOTROPIC DEVELOPMENTAL DEFECTS Uncovers a Crucial Role for Chloroplast tRNA Modification in Translation and Plant Development. Plant Cell 2020, 32, 1233–1245. [Google Scholar] [CrossRef]
- Jacob, F.; Monod, J. Genetic Regulatory Mechanisms in the Synthesis of Proteins. J. Mol. Biol. 1961, 3, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.Y.; Liu, B.M.; Yang, H.J.; Zhang, Y.P.; Ren, S.H.; Yang, J.Y.; Fu, C. Genetic Analysis and Gene Mapping of Leaf Vein Yellowing Mutant yml in Rice. Acta Agron. Sin. 2022, 48, 3120–3129. [Google Scholar]
- Abe, A.; Kosugi, S.; Yoshida, K.; Natsume, S.; Takagi, H.; Kanzaki, H.; Matsumura, H.; Yoshida, K.; Mitsuoka, C.; Tamiru, M. Genome Sequencing Reveals Agronomically Important Loci in Rice Using MutMap. Nat. Biotechnol. 2012, 30, 174–178. [Google Scholar] [CrossRef]
- Takagi, H.; Tamiru, M.; Abe, A.; Yoshida, K.; Uemura, A.; Yaegashi, H.; Obara, T.; Oikawa, K.; Utsushi, H.; Kanzaki, E. MutMap Accelerates Breeding of a Salt-Tolerant Rice Cultivar. Nat. Biotechnol. 2015, 33, 445–449. [Google Scholar] [PubMed]
- Chi, W.; Ma, J.F.; Zhang, D.Y.; Guo, J.K.; Chen, F.; Lu, C.M.; Zhang, L.X. Rapid Mapping of Quantitative Trait Loci in Rice by Whole Genome Resequencing. Plant J. 2013, 74, 174–183. [Google Scholar] [CrossRef]
- Chi, W.; Ma, J.F.; Zhang, D.Y.; Guo, J.K.; Chen, F.; Lu, C.M.; Zhang, L.X. The Pentratricopeptide Repeat Protein DELAYED GREENING1 Is Involved in the Regulation of Early Chloroplast Development and Chloroplast Gene Expression in Arabidopsis. Plant Physiol. 2008, 147, 573–584. [Google Scholar] [CrossRef][Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Udy, D.B.; Belcher, S.; Williams-Carrier, R.; Gualberto, J.M.; Barkan, A. Effects of Reduced Chloroplast Gene Copy Number on Chloroplast Gene Expression in Maize. Plant Physiol. 2012, 160, 1420–1431. [Google Scholar] [CrossRef]
- Zhou, W.Q.; Yin, J.; Zhou, Y.Q.; Li, Y.S.; He, H.J.; Yang, Y.Z.; Wang, X.J.; Lian, X.R.; Dong, X.Y.; Ma, Z.K.; et al. DSD1/ZmICEb Regulates Stomatal Development and Drought Tolerance in Maize. J. Integr. Plant Biol. 2025, 67, 1487–1500. [Google Scholar]
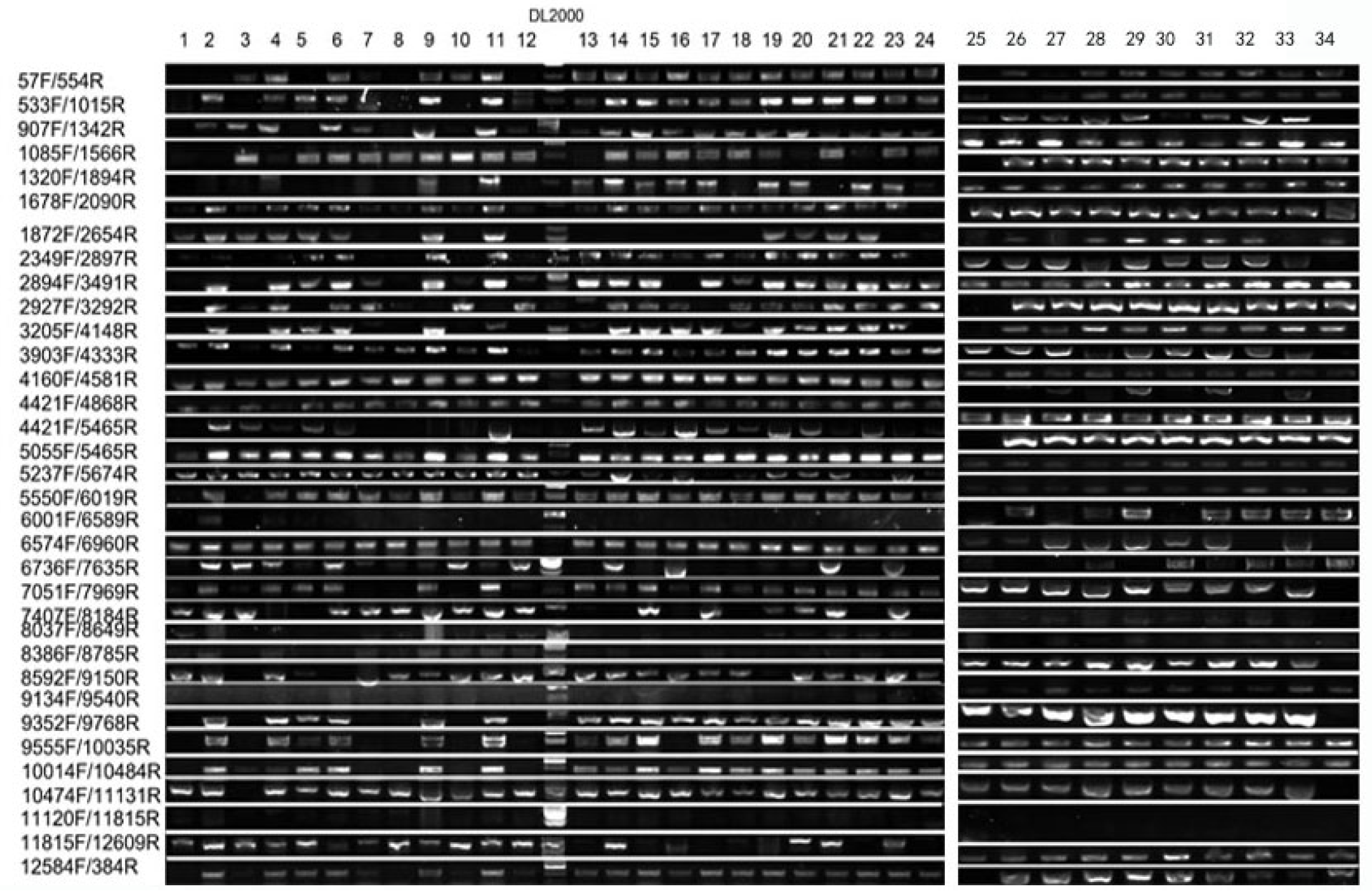

| Forward Primer | Reverse Primer | ||
|---|---|---|---|
| 57F | ATGTGCATGCCAACCACAGGGT | 554R | ATGCGCTCGGCAATGTCCAGTA |
| 533F | TACTGGACATTGCCGAGCGCAT | 1015R | AAAAACGGTTCGTCCTGGCCGT |
| 907F | TTCCGTGAGGACGCATTGACCGA | 1342R | ATGGGTTGCGATGGTCGTCTTGC |
| 1320F | GCAAGACGACCATCGCAACCCAT | 1894R | TTTAATTTCAGCGGCCAGCGCCT |
| 1085F | AACCGTGCGGCTGCATGAAA | 1566R | TTGATCGCGGACACAGCCAAGT |
| 1678F | ACAAGCGGCCTTTGTCGTGT | 2090R | TGGCGTACCGCGTACATCTTCA |
| 1872F | AGGCGCTGGCCGCTGAAATTAAA | 2654R | ACGCTTCGACAGACGGAAAACGG |
| 2349F | AAACCATCCGGCCCGGTACAAA | 2897R | TTTCTGCTTTCCGCCATCGGCT |
| 2894F | GAAAGACGACCTGGTAGAAACCT | 3491R | GTACGTGCTATCCACAGGAAAGA |
| 2927F | AAACACCACGCACGTTGCCA | 3292R | AGCTTGCGCACGGTGAAACA |
| 3205F | CAGAAGCCAGATGGTTGTTCAAG | 4148R | GCGGTATTTTCTCCTTACGCATC |
| 3903F | AAACCTCTGACACATGCAGCTCCC | 4333R | AGCAACGCGGCCTTTTTACGGT |
| 4160F | TTCCGCTTCCTCGCTCACTGACT | 4581R | TGGAGCGAACGACCTACACCGAA |
| 4421F | AAGATACCAGGCGTTTCCCCCT | 4868R | AGCGGTGGTTTGTTTGCCGGAT |
| 4421F | AAGATACCAGGCGTTTCCCCCT | 5465R | TGCGGAGTGCATCAGGCTCTTT |
| 5237F | CAGGTCGCCGTGGGAAAAGACAA | 5674R | ACGGACAGCCGGTATAAAGGGACC |
| 5055F | TCCCAATCAGGCTTGATCCCCAGT | 5465R | TGCGGAGTGCATCAGGCTCTTT |
| 5550F | TTGCTCCAGCCATCATGCCGTT | 6019R | ACAGAGCGTTGCTGCCTGTGAT |
| 6001F | ACAGGCAGCAACGCTCTGTCAT | 6589R | TGGGTTTCTGGCAGCTGGACTT |
| 6574F | AGCTGCCAGAAACCCACGTCAT | 6960R | AGACAAGCACGGTCAACTTCCGT |
| 6736F | TTCAGCAGGTGGGTGTAGAGCGT | 7635R | TGCCGACAGTGGTCCCAAAGATG |
| 7051F | GGGCGTCGTTCTGGGCTCAT | 7969R | CGCTCACTGCCCGCTTTCCA |
| 7407F | TGGGCAATGGAATCCGAGGAGGT | 8184R | CGCAAGACCGGCAACAGGATTCA |
| 8037F | CGTATGTTGTGTGGAATTGTGAG | 8649R | CAGACATCATCGGTATCAGTGAA |
| 8386F | ATCGCGCGCGGTGTCATCTA | 8785R | GCTGCATCTACAGGAGTGGGAGGT |
| 8592F | CCGCTACTGATAGAACCTGCACCG | 9150R | ACAGTGTGCCACCTCGTGAAGGA |
| 9134F | ACGAGGTGGCACACTGTTGTCC | 9540R | TCAACGTTATGGAGAGCAGCGCA |
| 9352F | GCTCCTGGTTAGCCTCTCTTCCGT | 9768R | ACCGTGGACTCAACAACCTTCCT |
| 9555F | GCAACAGACTGAAGCTGTGGGT | 10035R | ACCCAGCAACCAGGACCAGAGT |
| 10014F | ACTCTGGTCCTGGTTGCTGGGT | 10484R | CGATGCTCACCCTGTTGTTTGGTGT |
| 10474F | GGTGAGCATCGACAAAAGAAACA | 11131R | TTTGTCGGGTCATCTTTTCATGC |
| 11120F | TGACCCGACAAACAAGTGCACGG | 11815R | TCGACGCAGTCTAACGGACACCA |
| 11815F | ACGGCGTTTAACAGGCTGGCATT | 12609R | TAGCATTCGCCATTCAGGCTGCG |
| 12584F | TTGCGCAGCCTGAATGGCGAAT | 384R | GTGCAGTTCGGCCCGTTGGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhou, W.; Wang, H.; Liang, C.; He, H.; Li, Y.; Lian, X.; Wang, X.; Dong, X.; Ma, Z.; Liu, Z.; et al. Identification of a Chlorophyll-Deficient Mutant in Maize Associated with Exogenous Vector Insertion. Plants 2026, 15, 266. https://doi.org/10.3390/plants15020266
Zhou W, Wang H, Liang C, He H, Li Y, Lian X, Wang X, Dong X, Ma Z, Liu Z, et al. Identification of a Chlorophyll-Deficient Mutant in Maize Associated with Exogenous Vector Insertion. Plants. 2026; 15(2):266. https://doi.org/10.3390/plants15020266
Chicago/Turabian StyleZhou, Wenqi, Haoyue Wang, Chunxia Liang, Haijun He, Yongsheng Li, Xiaorong Lian, Xiaojuan Wang, Xiaoyun Dong, Zengke Ma, Zhongxiang Liu, and et al. 2026. "Identification of a Chlorophyll-Deficient Mutant in Maize Associated with Exogenous Vector Insertion" Plants 15, no. 2: 266. https://doi.org/10.3390/plants15020266
APA StyleZhou, W., Wang, H., Liang, C., He, H., Li, Y., Lian, X., Wang, X., Dong, X., Ma, Z., Liu, Z., & Zhou, Y. (2026). Identification of a Chlorophyll-Deficient Mutant in Maize Associated with Exogenous Vector Insertion. Plants, 15(2), 266. https://doi.org/10.3390/plants15020266







