Soil Microbes Mediate Productivity Differences Between Natural and Plantation Forests
Abstract
1. Introduction
2. Results
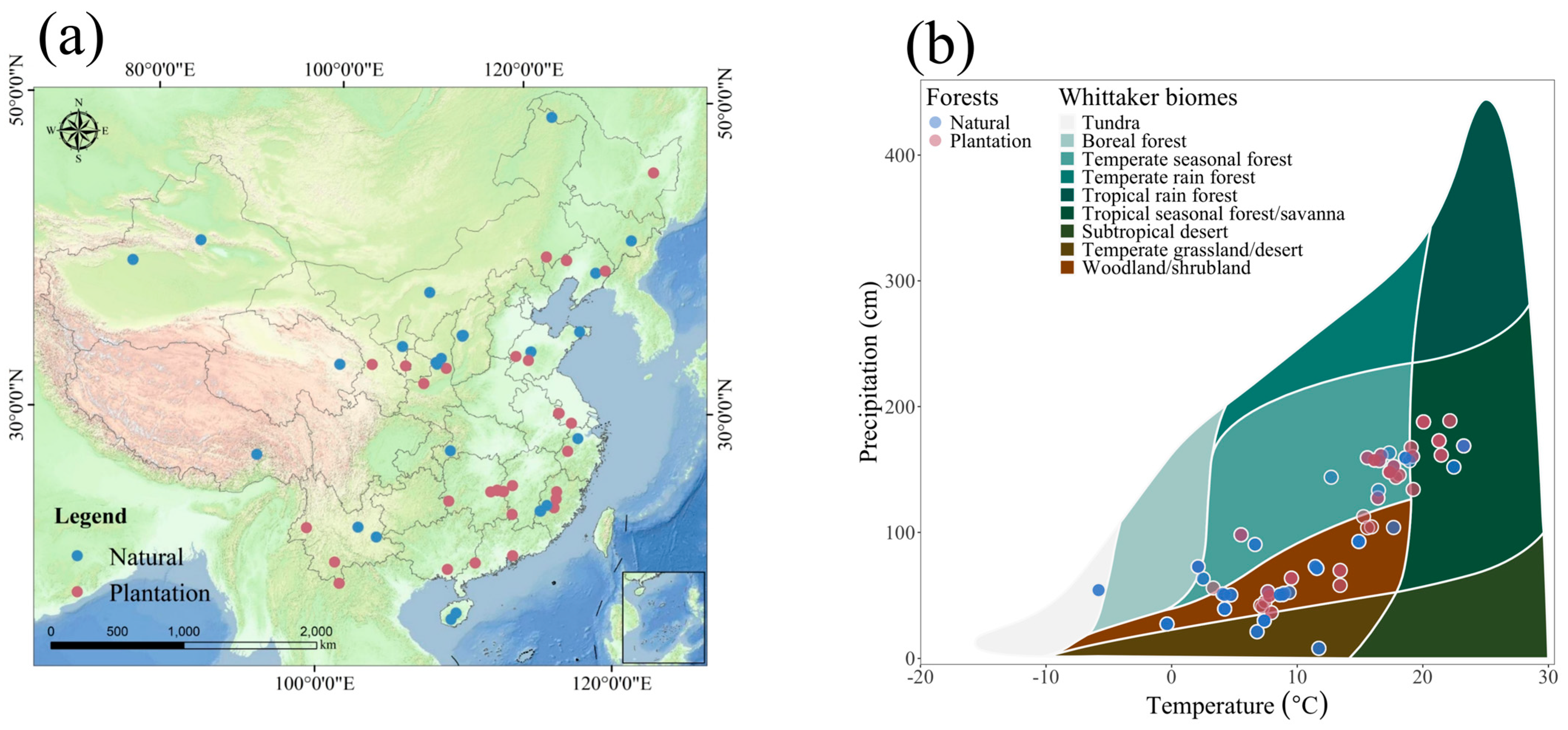
2.1. Spatial Heterogeneity in Forest Distribution and Climatic Conditions
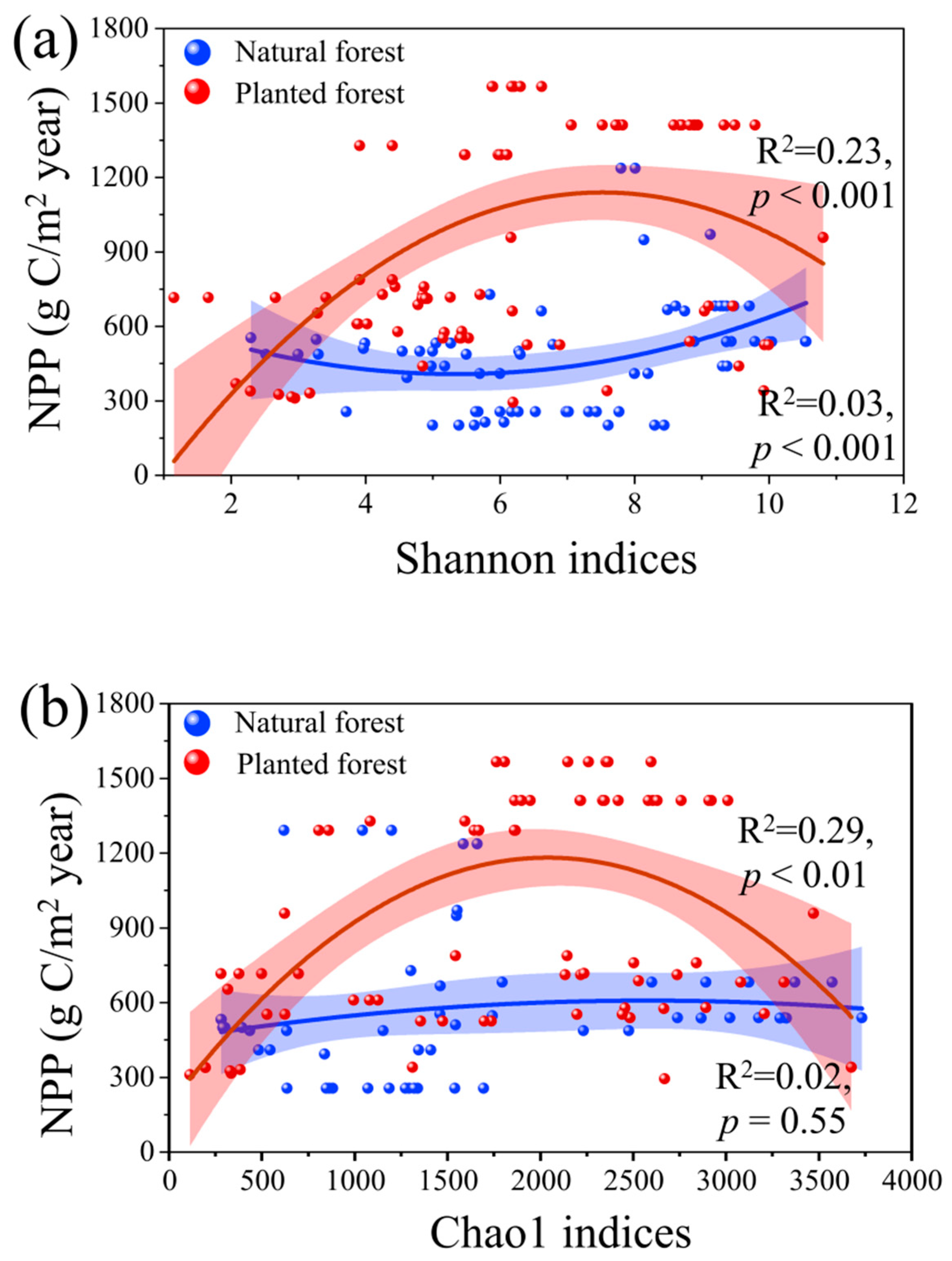
2.2. Asymmetric Relationships Between NPP and Microbial Indices in Natural vs. Plantation Forests
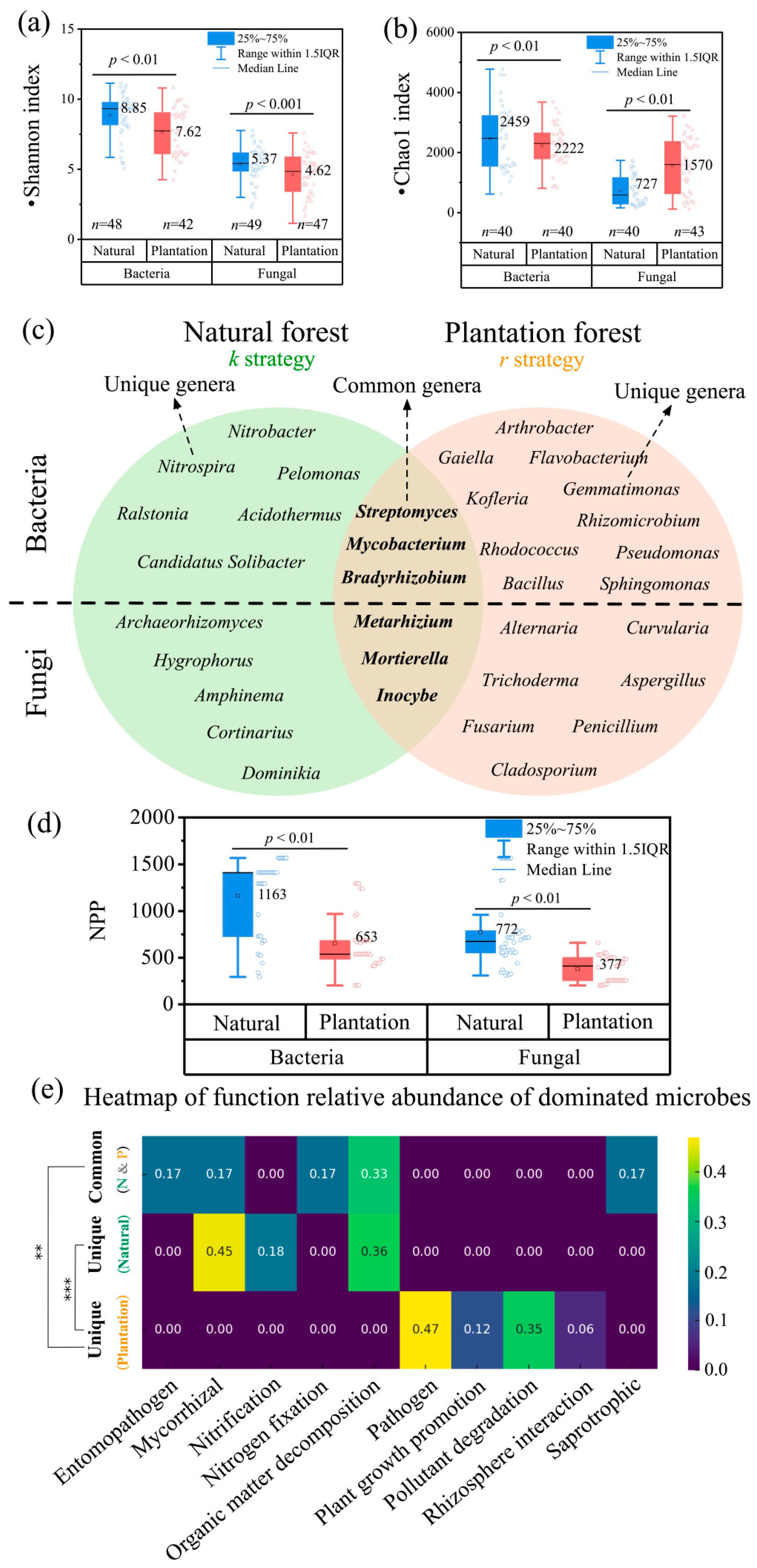
2.3. Contrasting Microbial Community Structures and Functional Profiles
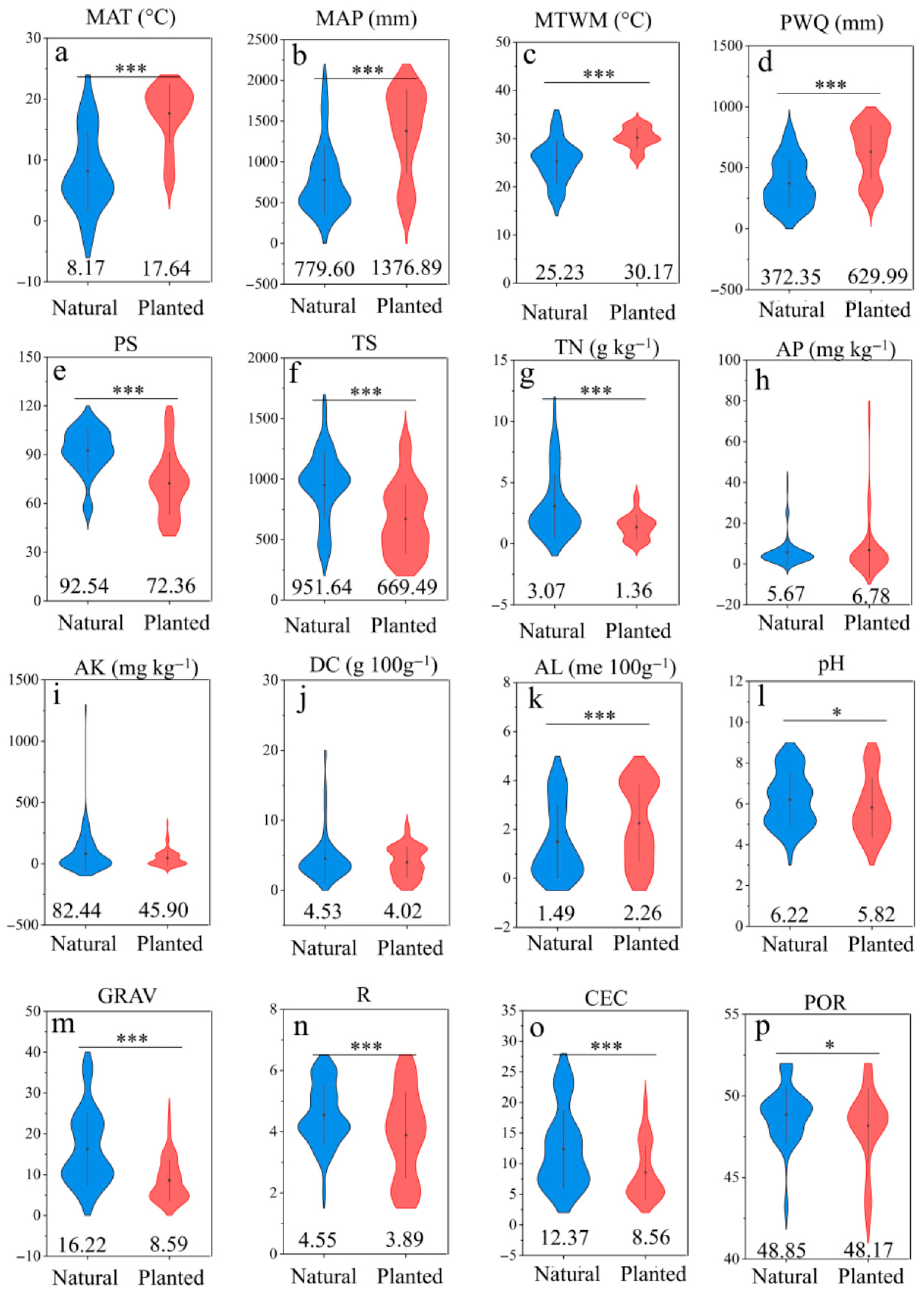
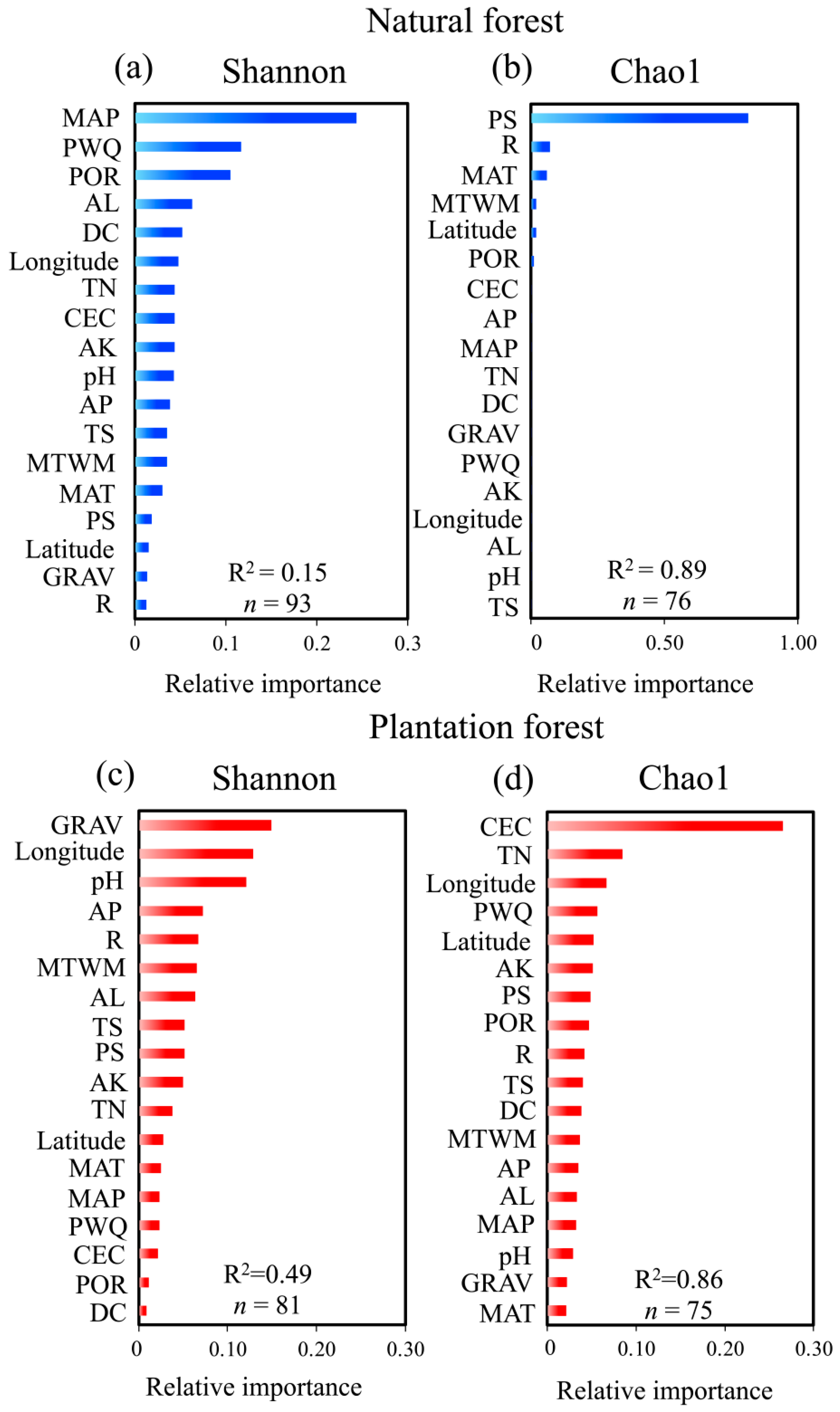
2.4. Drivers of Microbial Diversity: Climatic, Topographic, and Edaphic Controls
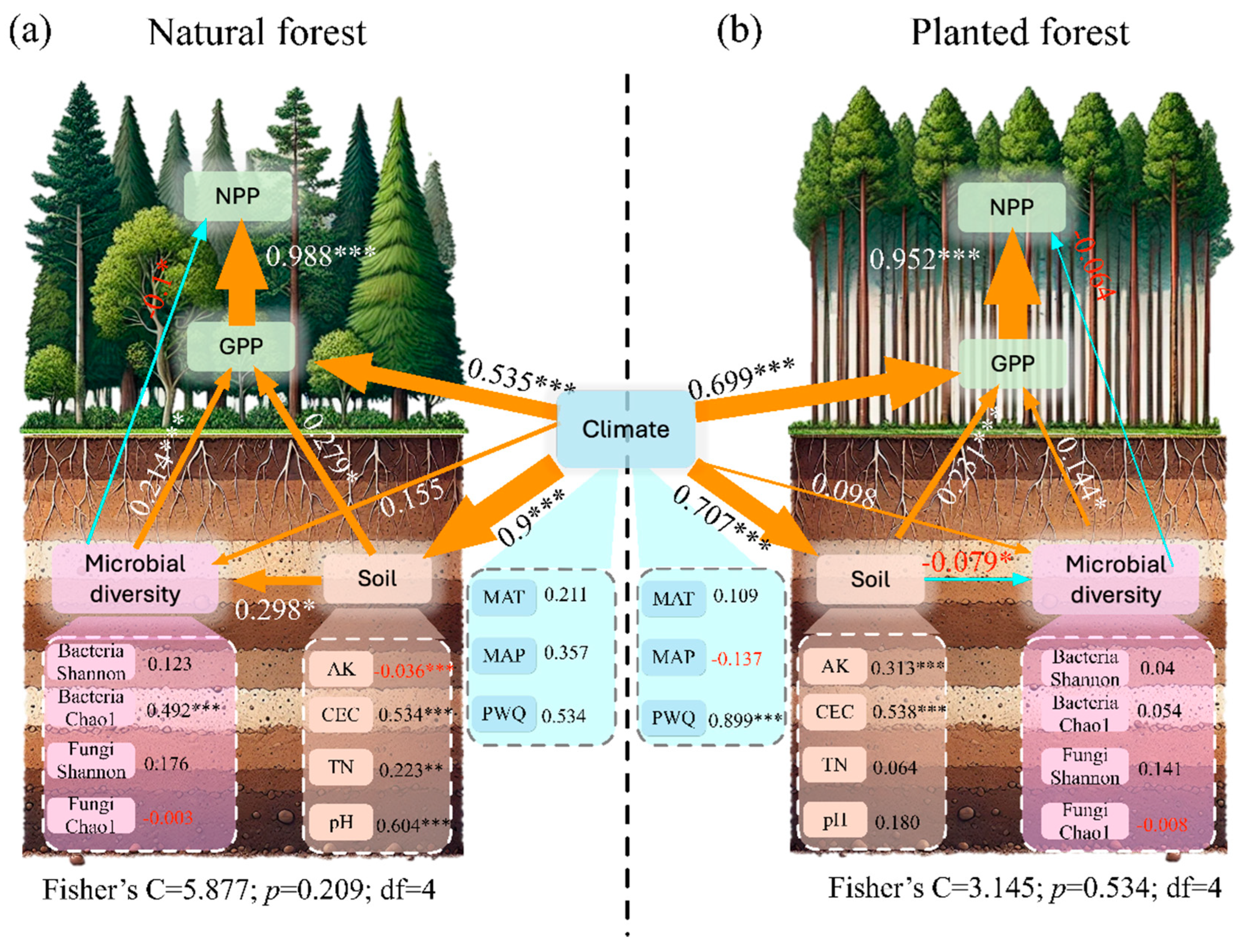
2.5. Structural Equation Model Analysis
3. Discussion
3.1. Spatial Distribution, Climatic Context, and Diversity–Productivity Relationships
3.2. A Comparative Study on the Differences in Microbial Community Diversity and Ecological Functions Between Plantation and Natural Forests
3.3. The Comprehensive Effects of Climate, Soil Factors, and Microbial Diversity on Forest Productivity
3.4. Limitations and Future Directions
4. Method
4.1. Microbial Data Collection
4.2. Environmental Data
4.3. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Montibeller, B.; Marshall, M.; Mander, Ü.; Uuemaa, E. Increased carbon assimilation and efficient water usage may not compensate for carbon loss in European forests. Commun. Earth Environ. 2022, 3, 194. [Google Scholar] [CrossRef]
- Gong, H.; Wang, G.; Wang, X.; Kuang, Z.; Cheng, T. Trajectories of Terrestrial Vegetation Productivity and Its Driving Factors in China’s Drylands. Geophys. Res. Lett. 2024, 51, e2024GL111391. [Google Scholar] [CrossRef]
- Bastida, F.; Eldridge, D.J.; García, C.; Png, G.K.; Bardgett, R.D.; Delgado-Baquerizo, M. Soil microbial diversity–biomass relationships are driven by soil carbon content across global biomes. ISME J. 2021, 15, 2081–2091. [Google Scholar] [CrossRef] [PubMed]
- Bastida, F.; García, C.; Fierer, N.; Eldridge, D.J.; Bowker, M.A.; Abades, S.; Alfaro, F.D.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Global ecological predictors of the soil priming effect. Nat. Commun. 2019, 10, 3481. [Google Scholar] [CrossRef]
- Crowther, T.W.; Hoogen, J.v.D.; Wan, J.; Mayes, M.A.; Keiser, A.D.; Mo, L.; Averill, C.; Maynard, D.S. The global soil community and its influence on biogeochemistry. Science 2019, 365, eaav0550. [Google Scholar] [CrossRef]
- Bödeker, I.T.M.; Clemmensen, K.E.; de Boer, W.; Martin, F.; Olson, Å.; Lindahl, B.D. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytol. 2014, 203, 245–256. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Guillaume, T.; Kotowska, M.M.; Hertel, D.; Knohl, A.; Krashevska, V.; Murtilaksono, K.; Scheu, S.; Kuzyakov, Y. Carbon costs and benefits of Indonesian rainforest conversion to plantations. Nat. Commun. 2018, 9, 2388. [Google Scholar] [CrossRef]
- Ding, K.; Zhang, Y.; Yrjälä, K.; Tong, Z.; Zhang, J. The introduction of Phoebe bournei into Cunninghamia lanceolata monoculture plantations increased microbial network complexity and shifted keystone taxa. For. Ecol. Manag. 2022, 509. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, W. Plant–soil microbe feedbacks drive seedling establishment during secondary forest succession: The ‘successional stage hypothesis’. J. Plant Ecol. 2023, 16, rtad021. [Google Scholar] [CrossRef]
- Mao, J.; Wang, J.; Liao, J.; Xu, X.; Tian, D.; Zhang, R.; Peng, J.; Niu, S. Plant nitrogen uptake preference and drivers in natural ecosystems at the global scale. New Phytol. 2025, 246, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Hu, H.; Zohner, C.M.; Huang, W.; Chen, J.; Sun, Y.; Ding, J.; Zhou, J.; Yan, X.; Zhang, J.; et al. Effects of winter soil warming on crop biomass carbon loss from organic matter degradation. Nat. Commun. 2024, 15, 8847. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Zhang, X.; Su, J.; Ji, Y.; Zhao, J.; Gao, J. Nitrogen deposition affects the productivity of planted and natural forests by modulating forest climate and community functional traits. For. Ecol. Manag. 2024, 563, 121970. [Google Scholar] [CrossRef]
- Zhao, S.; Guo, Y.; Sheng, Q.; Shyr, Y. Advanced Heat Map and Clustering Analysis Using Heatmap3. BioMed Res. Int. 2014, 2014, 986048. [Google Scholar] [CrossRef]
- Duan, S.; He, H.S.; Spetich, M.A.; Wang, W.J.; Fraser, J.S.; Xu, W. Long-term effects of succession, climate change and insect disturbance on oak-pine forest composition in the U.S. Central Hardwood Region. Eur. J. For. Res. 2022, 141, 153–164. [Google Scholar] [CrossRef]
- Fang, K.; Liu, Y.-J.; Zhao, W.-Q.; Liu, J.; Zhang, X.-Y.; He, H.-L.; Kou, Y.-P.; Liu, Q. Stand age alters fungal community composition and functional guilds in subalpine Picea asperata plantations. Appl. Soil Ecol. 2023, 188, 104860. [Google Scholar] [CrossRef]
- Nakayama, M.; Imamura, S.; Taniguchi, T.; Tateno, R. Does conversion from natural forest to plantation affect fungal and bacterial biodiversity, community structure, and co-occurrence networks in the organic horizon and mineral soil? For. Ecol. Manag. 2019, 446, 238–250. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, W.; Li, X.; Wu, J. A global meta-analysis of the impacts of tree plantations on biodiversity. Glob. Ecol. Biogeogr. 2022, 31, 576–587. [Google Scholar] [CrossRef]
- Fang, K.; Yang, A.-L.; Li, Y.-X.; Zeng, Z.-Y.; Wang, R.-F.; Li, T.; Zhao, Z.-W.; Zhang, H.-B. Native plants change the endophyte assembly and growth of an invasive plant in response to climatic factors. Appl. Environ. Microbiol. 2023, 89, e0109323. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Chen, Y.; Dai, Q.; Hu, J. Dynamic Change in Enzyme Activity and Bacterial Community with long-term rice Cultivation in Mudflats. Curr. Microbiol. 2019, 76, 361–369. [Google Scholar] [CrossRef]
- Qiao, M.; Sun, R.; Wang, Z.; Dumack, K.; Xie, X.; Dai, C.; Wang, E.; Zhou, J.; Sun, B.; Peng, X.; et al. Publisher Correction: Legume rhizodeposition promotes nitrogen fixation by soil microbiota under crop diversification. Nat. Commun. 2024, 15, 3535. [Google Scholar] [CrossRef]
- Florio, A.; Legout, A.; Marechal, M.; Clesse, M.; Delort, A.; Chatelliers, C.C.D.; Gervaix, J.; Shi, Y.; van der Heijden, G.; Zeller, B.; et al. Nitrate leaching from soil under different forest tree species is related to the vertical distribution of Nitrobacter abundance. Sci. Total. Environ. 2025, 967, 178776. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, Y.; Yao, X.; Wang, J.; Zheng, P.; Xi, C.; Hu, B. Dominance of comammox Nitrospira in soil nitrification. Sci. Total. Environ. 2021, 780, 146558. [Google Scholar] [CrossRef]
- Cheng, B.; Zhang, J.; Wang, C.; Li, J.; Chen, F.; Cao, X.; Yue, L.; Wang, Z. Selenium nanomaterials alleviate Brassica chinensis L cadmium stress: Reducing accumulation, regulating microorganisms and activating glutathione metabolism. Chemosphere 2023, 344, 140320. [Google Scholar] [CrossRef]
- Yan, Z.; Jin, H.; Yang, X.; Min, D.; Xu, X.; Hua, C.; Qin, B. Effect of rhizosphere soil microbial communities and environmental factors on growth and the active ingredients of Angelica sinensis in Gansu Province, China. Folia Microbiol. 2024, 70, 673–687. [Google Scholar] [CrossRef]
- Menkis, A.; Urbina, H.; James, T.Y.; Rosling, A. Archaeorhizomyces borealis sp. nov. and a sequence-based classification of related soil fungal species. Fungal Biol. 2014, 118, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Johnson, N.C.; Jansa, J.; Han, J.; Fang, Z.; Zhang, Y.; Jiang, S.; Xi, H.; Mao, L.; Pan, J.; et al. Mycorrhizal effects on crop yield and soil ecosystem functions in a long-term tillage and fertilization experiment. New Phytol. 2024, 242, 1798–1813. [Google Scholar] [CrossRef] [PubMed]
- Baier, R.; Ingenhaag, J.; Blaschke, H.; Göttlein, A.; Agerer, R. Vertical distribution of an ectomycorrhizal community in upper soil horizons of a young Norway spruce (Picea abies [L.] Karst.) stand of the Bavarian Limestone Alps. Mycorrhiza 2006, 16, 197–206. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.-H.; Wang, X.-H.; Wei, T.-Z.; Zhang, M.; He, X.-L. Hygrophorus annulatus, a new edible member of H. olivaceoalbus-complex from southwestern China. Mycoscience 2021, 62, 137–142. [Google Scholar] [CrossRef]
- Lin, W.; Guo, C.; Zhang, H.; Liang, X.; Wei, Y.; Lu, G.; Dang, Z. Electrokinetic-enhanced remediation of phenanthrene-contaminated soil combined with Sphingomonas sp. GY2B and biosurfactant. Appl. Biochem. Biotechnol. 2016, 178, 1325–1338. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, T.; Zhao, H.; Zhang, K.; Cui, J. Temporal and spatial changes in rhizosphere bacterial diversity of mountain Rhododendron mucronulatum. Front. Microbiol. 2023, 14, 1201274. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.M.; Kim, Y.S.; Bang, J.H.; Kim, S.B. Flavobacterium hydrocarbonoxydans sp. nov., isolated from polluted soil. Int. J. Syst. Evol. Microbiol. 2021, 71, 005053. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wu, S.; Pan, H.; Lu, X.; Liu, Y.; Yang, L. Cortinarius and Tomentella fungi become dominant taxa in taiga soil after fire disturbance. J. Fungi 2023, 9, 1113. [Google Scholar] [CrossRef] [PubMed]
- Diggle, S.P.; Whiteley, M. Microbe Profile: Pseudomonas aeruginosa: Opportunistic pathogen and lab rat: This article is part of the Microbe Profiles collection. Microbiology 2020, 166, 30–33. [Google Scholar] [CrossRef]
- Ehling-Schulz, M.; Lereclus, D.; Koehler, T.M. The Bacillus cereus group: Bacillus species with pathogenic potential. Microbiol. Spectr. 2019, 7, GPP3-0032-2018. [Google Scholar] [CrossRef]
- Takai, S.; Mizuno, Y.; Suzuki, Y.; Sasaki, Y.; Kakuda, T.; Kirikae, T. Rhodococcus equi infections in humans: An emerging zoonotic pathogen. Nippon. Saikingaku Zasshi 2024, 79, 15–24. [Google Scholar] [CrossRef]
- Peeters, N.; Guidot, A.; Vailleau, F.; Valls, M. Ralstonia solanacearum, a widespread bacterial plant pathogen in the post–genomic era. Mol. Plant Pathol. 2013, 14, 651–662. [Google Scholar] [CrossRef]
- Haq, I.U.; Mukhtar, Z.; Anwar-Ul-Haq, M.; Liaqat, S. Deciphering host–pathogen interaction during Streptomyces spp. infestation of potato. Arch. Microbiol. 2023, 205, 222. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.-F.; Zhang, H.; Qi, H.; Wang, X.-M.; Wang, J.-D.; Tan, G.-S. A new androstanoid metabolite from a soil fungus Curvularia borreriae strain HS-FG-237. Nat. Prod. Res. 2017, 31, 1080–1084. [Google Scholar] [CrossRef]
- Bai, B.; Liu, C.; Zhang, C.; He, X.; Wang, H.; Peng, W.; Zheng, C. Trichoderma species from plant and soil: An excellent resource for biosynthesis of terpenoids with versatile bioactivities. J. Adv. Res. 2023, 49, 81–102. [Google Scholar] [CrossRef]
- Wang, M.; Xu, Z.; Dong, B.; Zeng, Y.; Chen, S.; Zhang, Y.; Huang, Y.; Pei, X. An efficient manganese-oxidizing fungus Cladosporium halotolerans strain XM01: Mn(II) oxidization and Cd adsorption behavior. Chemosphere 2022, 287, 132026. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Choudhury, P.P. Aspergillus niger-mediated degradation of orthosulfamuron in rice soil. Environ. Monit. Assess. 2020, 192, 813. [Google Scholar] [CrossRef]
- Yuan, X.; Hong, S.; Xiong, W.; Raza, W.; Shen, Z.; Wang, B.; Li, R.; Ruan, Y.; Shen, Q.; Dini-Andreote, F. Development of fungal-mediated soil suppressiveness against Fusarium wilt disease via plant residue manipulation. Microbiome 2021, 9, 200. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, X.; Garcia-Lemos, A.M.; Nunes, I.; Nicolaisen, M.H.; Nybroe, O. Different effects of soil fertilization on bacterial community composition in the Penicillium canescens hyphosphere and in bulk soil. Appl. Environ. Microbiol. 2020, 86, e02969-19. [Google Scholar] [CrossRef]
- Liu, Q.; Kong, W.; Cui, X.; Hu, S.; Shi, Z.; Wu, J.; Zhang, Y.; Qiu, L. Dynamic succession of microbial compost communities and functions during Pleurotus ostreatus mushroom cropping on a short composting substrate. Front. Microbiol. 2022, 13, 946777. [Google Scholar] [CrossRef]
- O, S.; Park, S.K. Global ecosystem responses to flash droughts are modulated by background climate and vegetation conditions. Commun. Earth Environ. 2024, 5, 88. [Google Scholar] [CrossRef]
- Huang, L.; Bao, W.; Kuzyakov, Y.; Hu, H.; Zhang, H.; Li, F. Enzyme stoichiometry reveals microbial nitrogen limitation in stony soils. Sci. Total. Environ. 2024, 946, 174124. [Google Scholar] [CrossRef] [PubMed]
- Standish, R.J.; Parkhurst, T. Interventions for resilient nature-based solutions: An ecological perspective. J. Ecol. 2024, 112, 2502–2509. [Google Scholar] [CrossRef]
- Qu, X.; Li, X.; Bardgett, R.D.; Kuzyakov, Y.; Revillini, D.; Sonne, C.; Xia, C.; Ruan, H.; Liu, Y.; Cao, F.; et al. Deforestation impacts soil biodiversity and ecosystem services worldwide. Proc. Natl. Acad. Sci. USA 2024, 121, e2318475121. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, S.; Li, Y.; Lambers, H.; Zhang, W.; Zhang, S.; Dong, X.; Yang, G.; Wang, R.; Yan, C.; et al. Increasing phosphorus availability reduces grassland soil N2O emission: Plants and microbes move from mutualism to self-reliance. Agric. Ecosyst. Environ. 2025, 389, 109695. [Google Scholar] [CrossRef]
- Muhammad, M.; Wahab, A.; Waheed, A.; Hakeem, K.R.; Mohamed, H.I.; Basit, A.; Toor, M.D.; Liu, Y.; Li, L.; Li, W. Navigating climate change: Exploring the dynamics between plant–soil microbiomes and their impact on plant growth and productivity. Glob. Chang. Biol. 2025, 31, e70057. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Zhang, X.; Yang, M.; Liu, Y.; Ye, J.; Tangyu, J.; Gao, J.; Liu, W.; Fan, Y. Soil Microbes Mediate Productivity Differences Between Natural and Plantation Forests. Plants 2026, 15, 98. https://doi.org/10.3390/plants15010098
Zhang X, Yang M, Liu Y, Ye J, Tangyu J, Gao J, Liu W, Fan Y. Soil Microbes Mediate Productivity Differences Between Natural and Plantation Forests. Plants. 2026; 15(1):98. https://doi.org/10.3390/plants15010098
Chicago/Turabian StyleZhang, Xing, Mengya Yang, Yangyang Liu, Jinkun Ye, Jiechen Tangyu, Jie Gao, Weiguo Liu, and Yuchuan Fan. 2026. "Soil Microbes Mediate Productivity Differences Between Natural and Plantation Forests" Plants 15, no. 1: 98. https://doi.org/10.3390/plants15010098
APA StyleZhang, X., Yang, M., Liu, Y., Ye, J., Tangyu, J., Gao, J., Liu, W., & Fan, Y. (2026). Soil Microbes Mediate Productivity Differences Between Natural and Plantation Forests. Plants, 15(1), 98. https://doi.org/10.3390/plants15010098







