Foliar Illumination Affects the Severity of Cameraria ohridella Damage Among Horse Chestnut Species
Abstract
1. Introduction
2. Results
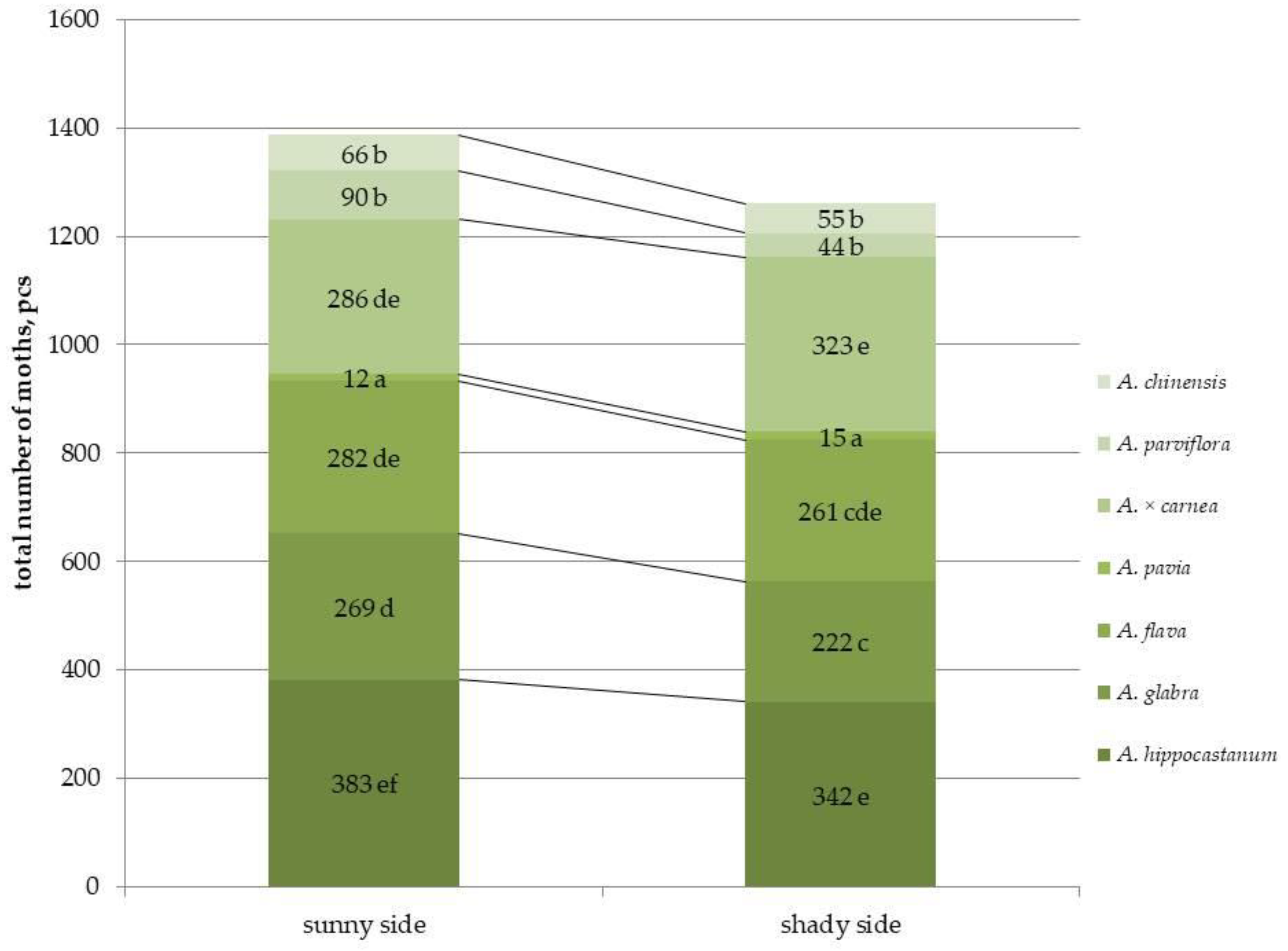
2.1. Determination of the Second Generation Population of C. ohridella
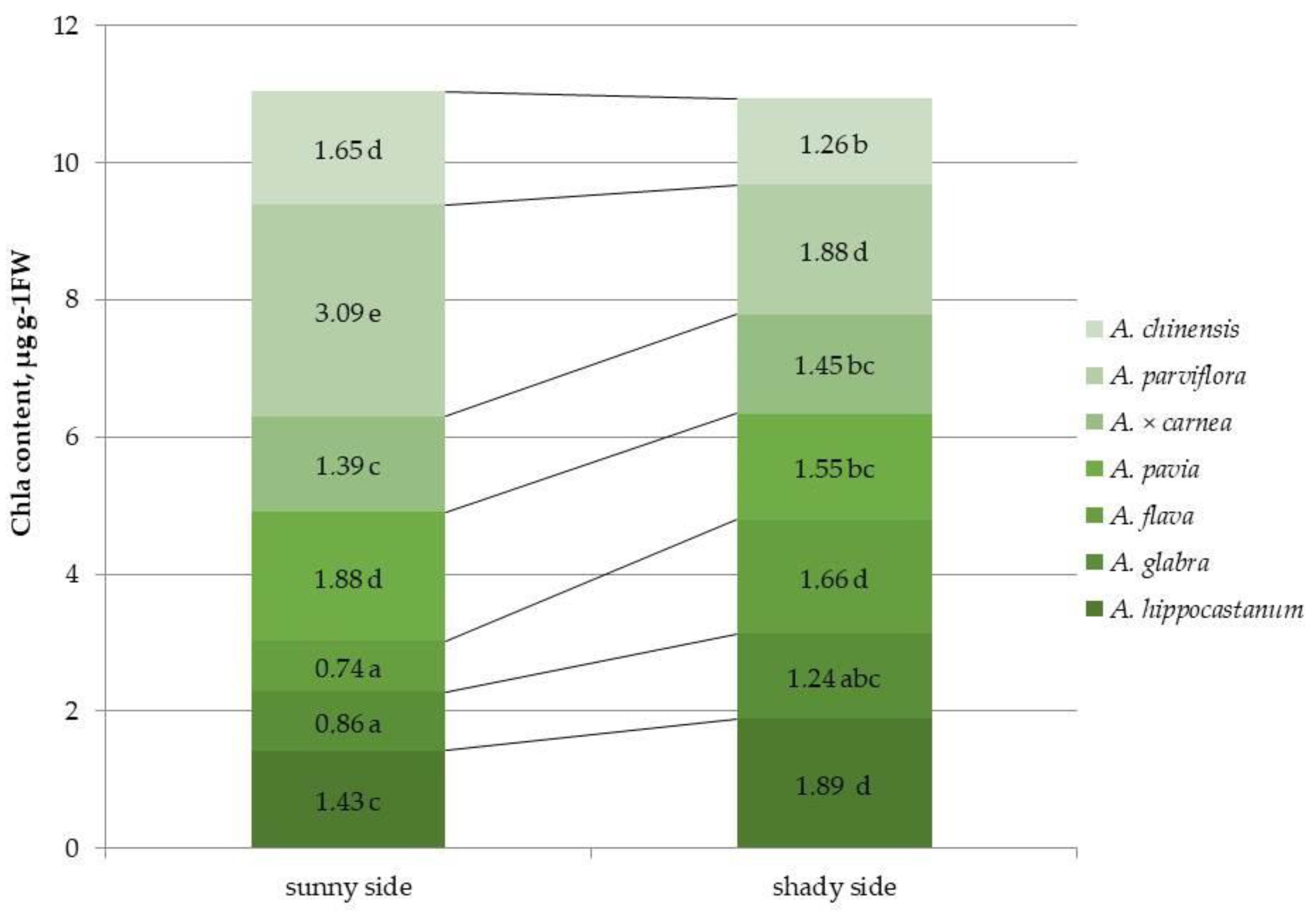
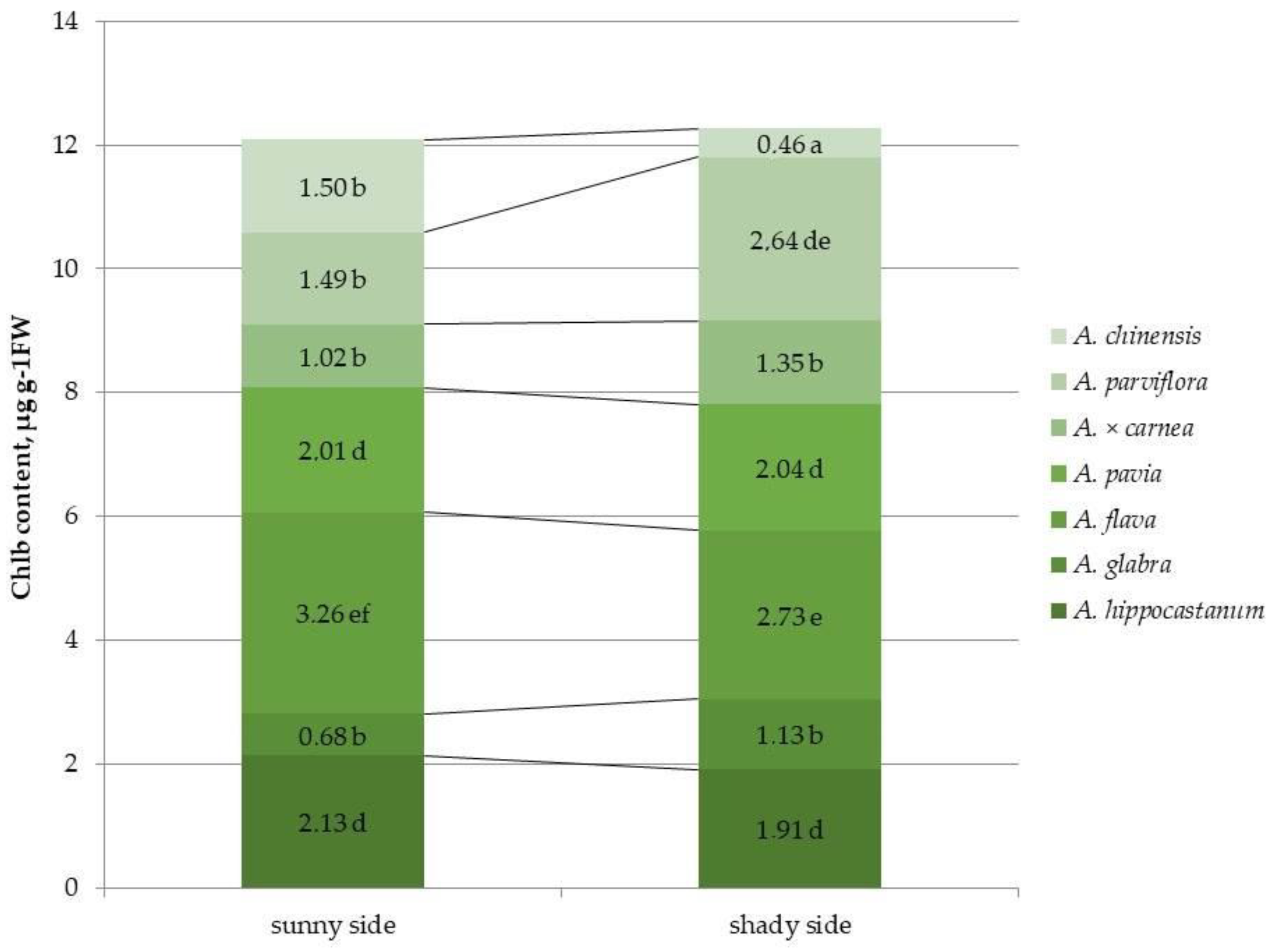
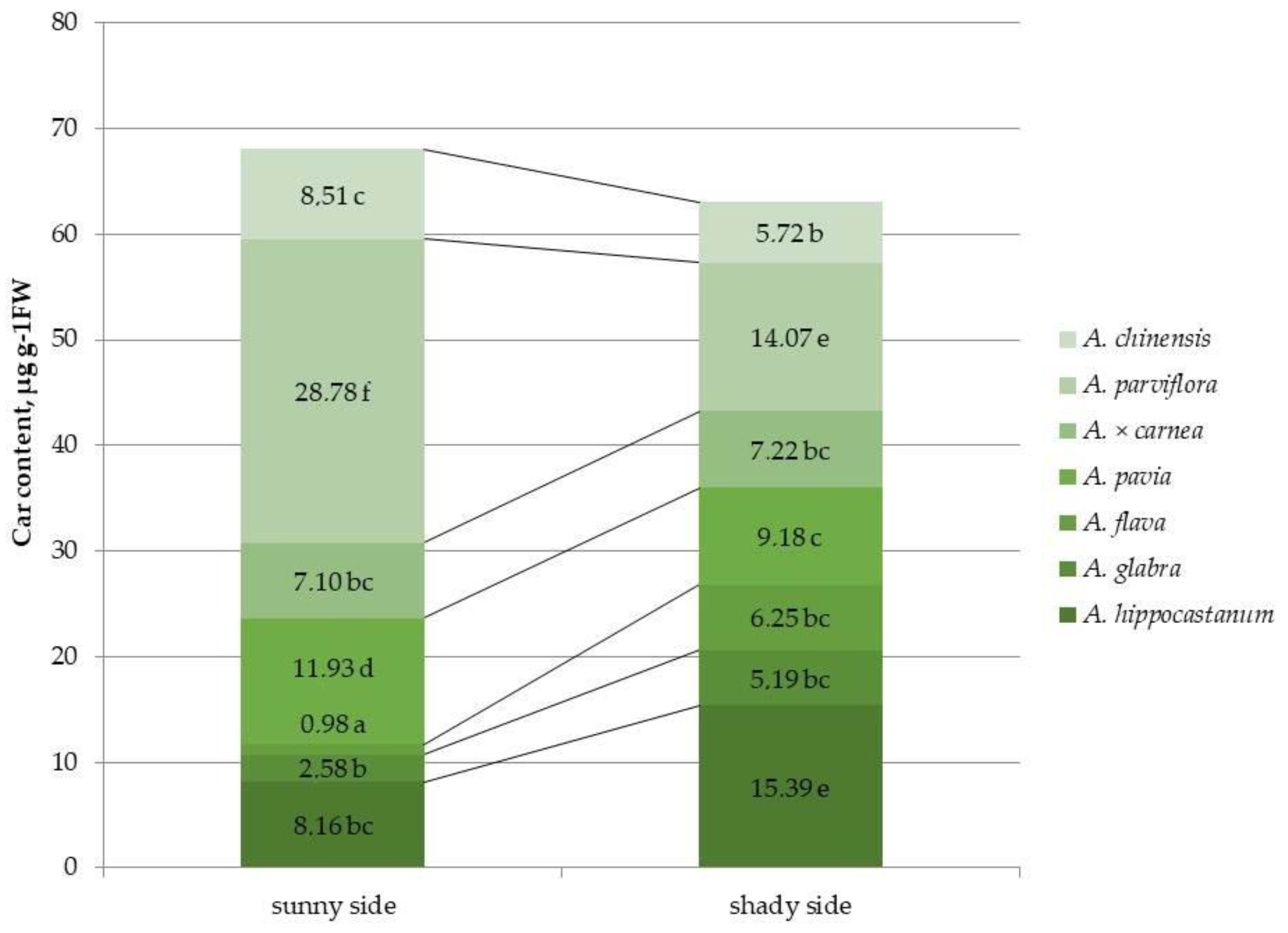
2.2. Determination of Photosynthetic Pigment Content
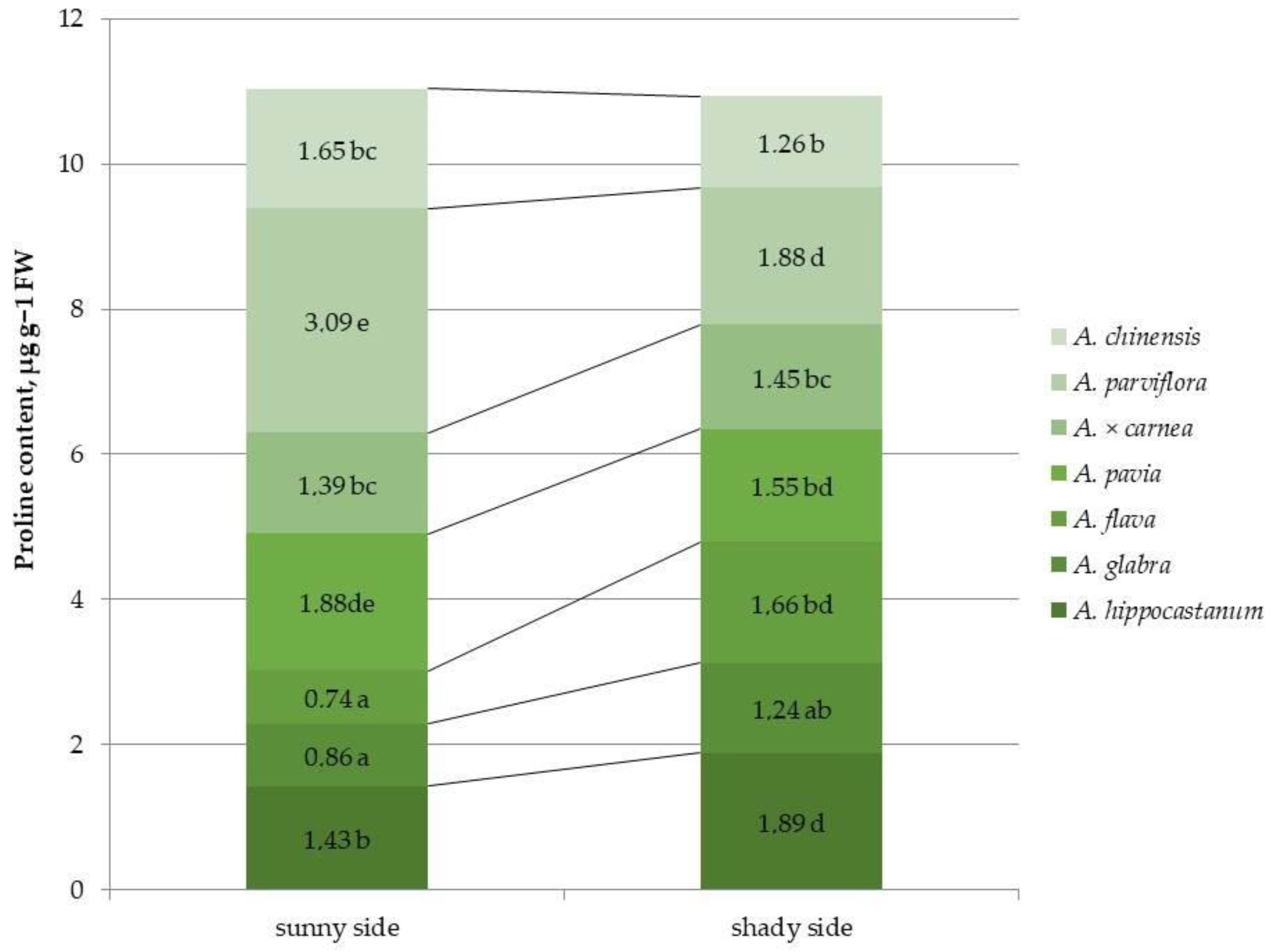
2.3. Determination of Proline Content
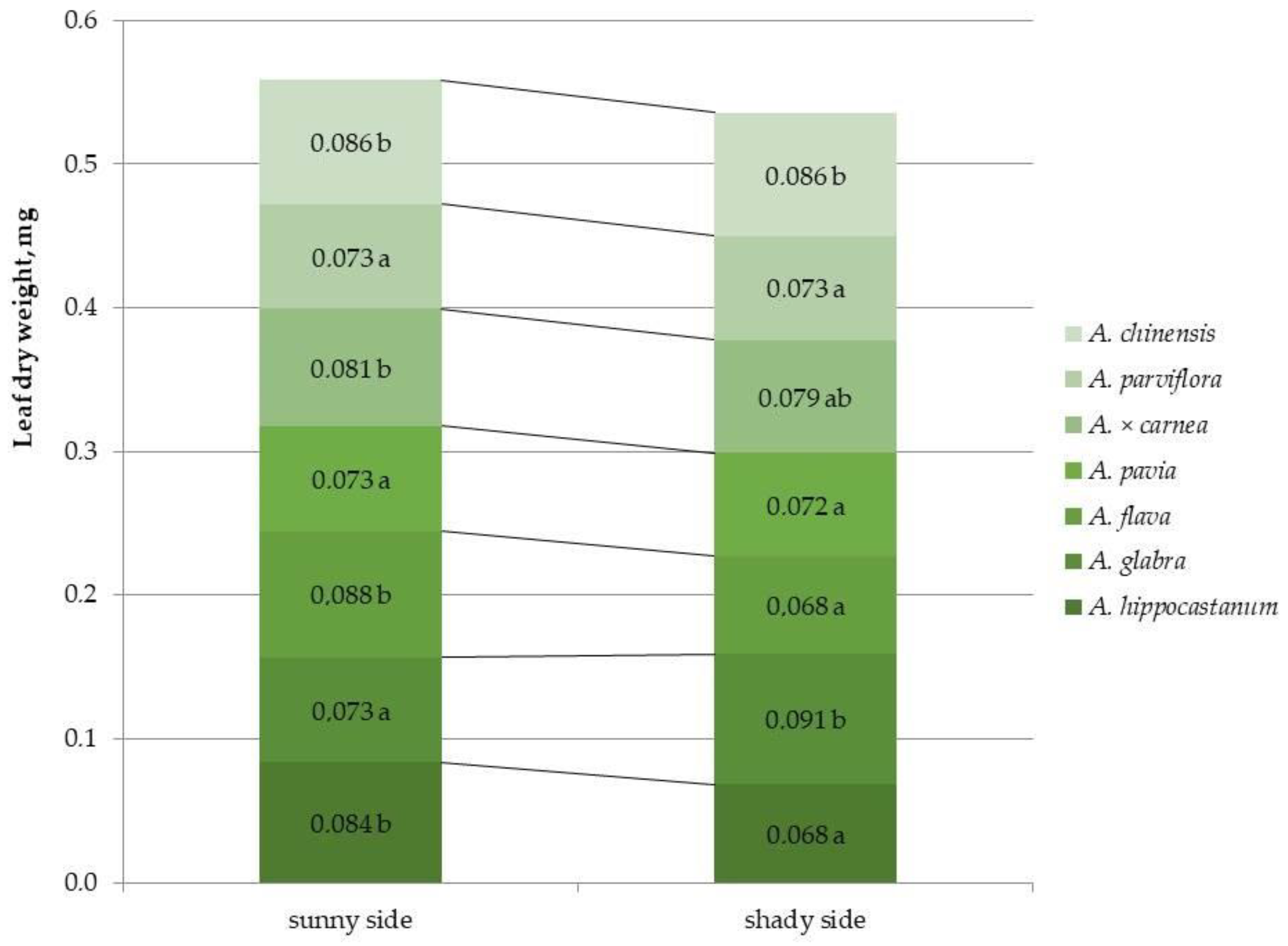
2.4. Determination of Dry Leaf Biomass
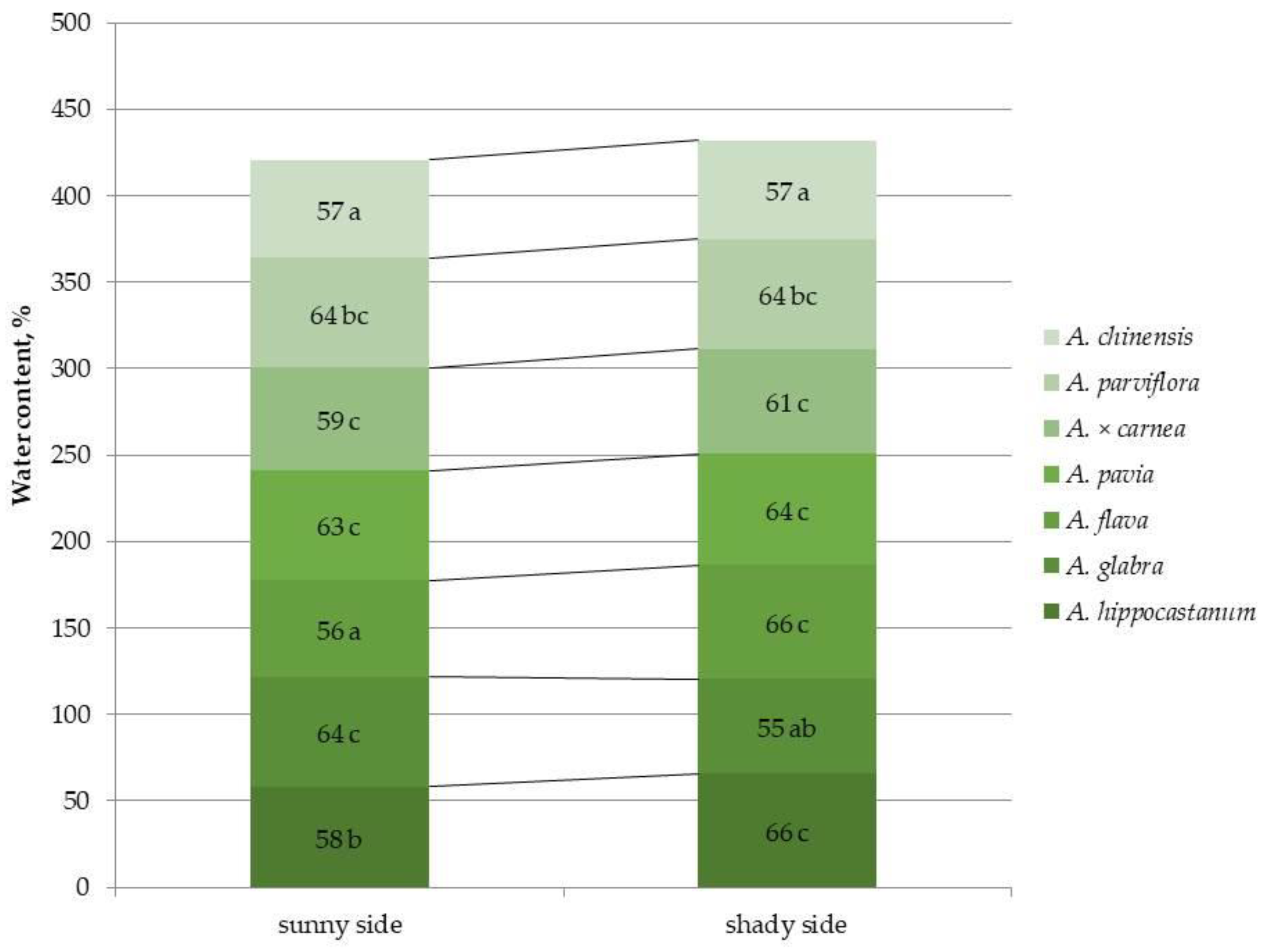
2.5. Detection of Water Content in Leaves of Different Horse Chestnut Species
2.6. Determination of Hyperspectral Indicators
2.7. Determination of Leaf Chlorophyll Fluorescence
3. Discussion
4. Materials and Methods
4.1. Place of Research and Plant Material
4.2. Estimation of Horse Chestnut Miner Abundance Using Pheromone Traps
4.3. Morphometric Indicators
4.4. Determination of Leaf Pigment Content
4.5. Detection of Leaf Proline Content
4.6. Chlorophyll Fluorescence Characteristics
4.7. Determination of Hyperspectral Indices
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| chl a | Chlorophyll a |
| chl b | Chlorophyll b |
| DSWI | Disease Water Stress Index |
| SIPI | Structure Insensitive Pigment Index |
| NDWI | Normalized Difference Water Index |
| F0 | Fluorescence value |
| PAR | The intensity of light driving photosynthesis (actinic radiation) |
| Fv | The greater changeable fluorescence value |
| Fm | The maximum fluorescence value |
| Y(II) | The quantum efficiency of PS II photochemistry |
| ETR | The electron transport rate |
| Car | Carotenoids |
| ROS | Reactive oxygen species |
| NAD+/NADP+ | Nicotinamide adenine dinucleotide/nicotinamide adenine dinucleotide phosphate |
| RWC | Water content |
| FW | Fresh biomass of root or shoot part of seedling |
| DW | Dry biomass of root or shoot part of seedling |
| LED | Light-Emitting Diode |
| Fv/Fm | Maximum quantum efficiency of photosystem II (PSII) |
| NPQ | The regulated energy dissipation in PS II |
| Y(NPQ) | The quantum efficiency of regulated energy dissipation in PS II |
| Y(NO) | The quantum efficiency of non-regulated energy dissipation in PS II |
| r | Correlation |
References
- Prada, D.; Velloza, T.M.; Toorop, P.E.; Pridchar, H.W. Genetic population structure in horse chestnut (Aesculus hippocastanum L.): Effect of human-mediated expansion in Europe. Plant Spec. Biol. 2011, 26, 43–50. [Google Scholar] [CrossRef]
- Freise, J.F.; Heitland, W. Neue Aspekte zur Biologie und Ökologie der Roßkastanien-Miniermotte, Cameraria ohridella Deschka & Dimic (1986) (Lep. Gracillariidae), einem neuartigen Schädling an Aesculus hippocastanum. Mitt. Dtsch. Ges. Allg. Angew. Entomol. 2001, 13, 135–139. [Google Scholar]
- Kenis, M.; Girardoz, S.; Avtzis, N.; Kamata, N. Finding the area of origin of the horse-chestnut leaf miner: A Challenge. In Proceedings of the IUFRO Kanazawa 2003 Forest Insect Population Dynamics and Host Influences, Kanazawa, Japan, 14–19 September 2003; pp. 63–66. [Google Scholar]
- Ferracini, C.; Curir, P.; Dolci, M.; Lanzotti, V.; Alma, A. Aesculus pavia foliar saponins: Defensive role against the leafminer Cameraria ohridella. Pest Man. Sci. 2010, 66, 767–772. [Google Scholar] [CrossRef]
- Bogoutdinova, L.R.; Shelepova, O.V.; Konovalova, L.N.; Tkachenko, O.B.; Gulevich, A.A.; Baranova, E.N.; Mitrofanova, I.V. Susceptibility of different Aesculus species to the horse chestnut leaf miner moth: Chemical composition and morphological features of leaves. J. Zool. Bot. Gard. 2024, 5, 691–707. [Google Scholar] [CrossRef]
- Straw, N.A.; Tilbury, C. Host plants of the horse-chestnut leaf-miner (Cameraria ohridella), and the rapid spread of the moth in the UK 2002–2005. Arboricult. J. 2006, 29, 83–99. [Google Scholar] [CrossRef]
- D’Costa, L.; Koricheva, J.; Straw, N.; Simmonds, M.J.S. Oviposition patterns and larval damage by the invasive horse-chestnut leaf miner Cameraria ohridella on different species of Aesculus. Ecol. Entomol. 2013, 38, 456–462. [Google Scholar] [CrossRef]
- Irzykowska, L.; Werner, M.; Bocianowski, J.; Karolewski, Z.; Frużyńska-Jóźwiak, D. Genetic variation of horse chestnut and red horse chestnut and susceptibility on Erysiphe flexuosa and Cameraria ohridella. Biologia 2013, 68, 851–860. [Google Scholar] [CrossRef]
- Popitanu, C.; Lupitu, A.; Copolovici, L.; Bungău, S.; Niinemets, Ü.; Copolovici, D.M. Induced volatile emissions, photosynthetic characteristics, and pigment content in Juglans regia leaves infected with the Erineum-forming mite Aceria erinea. Forests 2021, 12, 920. [Google Scholar] [CrossRef]
- Holoborodko, K.; Seliutina, O.; Alexeyeva, A.; Brygadyrenko, V.; Ivanko, I.; Shulman, M.; Pakhomov, O.; Loza, I.; Sytnyk, S.; Lovynska, V.; et al. The impact of Cameraria ohridella (Lepidoptera, Gracillariidae) on the state of Aesculus hippocastanum photosynthetic apparatus in the urban environment. Int. J. Plant Biol. 2022, 13, 223–234. [Google Scholar] [CrossRef]
- Peralvarez-Marin, A.; Bourdelande, J.L.; Querol, E.; Padros, E. The role of proline residues in the dynamics of transmembrane helices: The case of bacteriorhodopsin. Mol. Membr. Biol. 2006, 23, 127–135. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Jonas, J.L.; Joern, A. Dietary selection and nutritional regulation in a common mixed-feeding insect herbivore. Entomol. Exp. Appl. 2013, 148, 20–26. [Google Scholar] [CrossRef]
- Paterska, M.; Bandurska, H.; Wysłouch, J.; Molińska-Glura, M.; Moliński, K. Chemical composition of horse-chestnut (Aesculus) leaves and their susceptibility to chestnut leaf miner Cameraria ohridella Deschka & Dimić. Acta Physiol. Plant. 2017, 39, 105. [Google Scholar] [CrossRef]
- Lev-Yadun, S.; Gould, K.S. Role of Anthocyanins in plant defence. In Anthocyanins; Winefield, C., Davies, K., Eds.; Springer: New York, NY, USA, 2008; pp. 22–28. [Google Scholar]
- Kočíková, L.; Miklisová, D.; Čanády, A.; Panigaj, L. Is colour an important factor influencing the behavior of butterflies (Lepidoptera: Hesperioidea, Papilionoidea)? Europ. J. Entomol. 2012, 109, 403–410. [Google Scholar] [CrossRef]
- Karageorgou, P.; Buschmann, C.; Manetas, Y. Red leaf color as a warning signal against insect herbivory: Honest or mimetic? Flora 2008, 203, 648–652. [Google Scholar] [CrossRef]
- Johne, A.B.; Weissbecker, B.; Schütz, S. Reduzierung der Eiablage von C. ohridella (Deschka & Dimic) durch repellents. Nachr. Deut. Pflanz. 2006, 58, 260–261. [Google Scholar]
- Jagiełło, R.; Baraniak, E.; Guzicka, M.; Karolewski, P.; Łukowski, A.; Giertych, M.J. One step closer to understanding the ecology of Cameraria ohridella (Lepidoptera: Gracillariidae): The effects of light conditions. Eur. J. Entomol. 2019, 116, 42–51. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, F.; Ghirardelli, L.A.; Nardini, A.; Salleo, S. Impact of the leaf miner Cameraria ohridella on photosynthesis, water relations and hydraulics of Aesculus hippocastanum leaves. Trees 2003, 17, 376–382. [Google Scholar] [CrossRef]
- Kmieć, K.; Rubinowska, K.; Michałek, W.; Sytykiewicz, H. The effect of galling aphids feeding on photosynthesis photochemistry of elm trees (Ulmus sp.). Photosynthetica 2018, 56, 989–997. [Google Scholar] [CrossRef]
- Movahedi, A.; Almasi, Z.Y.A.; Wei, H.; Rutland, P.; Sun, W.; Mousavi, M.; Li, D.; Zhuge, Q. Plant secondary metabolites with an overview of Populus. Int. J. Mol. Sci. 2021, 22, 6890. [Google Scholar] [CrossRef]
- Lackner, S.; Lackus, N.D.; Paetz, C.; Köllner, T.G.; Unsicker, S.B. Aboveground phytochemical responses to belowground herbivory in poplar trees and the consequence for leaf herbivore preference. Plant Cell Environ. 2019, 42, 3293–3307. [Google Scholar] [CrossRef] [PubMed]
- Caretto, S.; Linsalata, V.; Colella, G.; Mita, G.; Lattanzio, V. Carbon fluxes between primary metabolism and phenolic pathway in plant tissues under stress. Int. J. Mol. Sci. 2015, 16, 26378–26394. [Google Scholar] [CrossRef]
- Jang, G.; Kim, J.; Yu, J.-K.; Kim, H.-J.; Kim, Y.; Kim, D.-W.; Kim, K.-H.; Lee, C.W.; Chung, Y.S. Review: Cost-effective unmanned aerial vehicle (UAV) platform for field plant breeding application. Remote Sens. 2020, 12, 998. [Google Scholar] [CrossRef]
- Savin, I.Y.; Shishkonakova, E.A.; Prudnikova, E.Y.; Vindeker, G.V.; Grubina, P.G. About effect of weeds on spectral reflectance properties of winter wheat canopy. Sel’skokhozyaistvennaya Biol. 2020, 55, 53–65. [Google Scholar] [CrossRef]
- Shpanev, A.M.; Smuk, V.V. Changes in the spectral characteristics of cultivated and weed plants under the influence of mineral fertilizers in agrocenoses of the North-West of Russia. Sovr. Probl. DZZ Kosm. 2022, 19, 165–177. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Shpanev, A.M.; Rusakov, D.V. Application of spectral indices to assess the influence of crop weeds and nitrogen nutrition on the activity of the photosynthetic apparatus of plants and the yield of winter triticale. Sovr. Probl. DZZ Kosm. 2024, 21, 235–247. [Google Scholar] [CrossRef]
- Bogoutdinova, L.R.; Tkacheva, E.V.; Konovalova, L.N.; Tkachenko, O.B.; Olekhnovich, L.S.; Gulevich, A.A.; Baranova, E.N.; Shelepova, O.V. Effect of sun exposure of the horse chestnut (Aesculus hippocastanum L.) on the occurrence and number of Cameraria ohridella (Lepidoptera: Gracillariidae). Forests 2023, 14, 1079. [Google Scholar] [CrossRef]
- Xu, T.T.; Wu, X.; Luo, Z.B.; Tang, L.D.; Gao, J.Y.; Zang, L.S. Light intensity differentially mediates the life cycle of lepidopteran leaf feeders and stem borers. Pest Manag. Sci. 2024, 80, 4216–4222. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, L.E. Resistance and Susceptibility to the Invasive Leaf Miner Cameraria ohridella Within the Genus Aesculus. Ph.D. Thesis, University of London, London, UK, 2014; pp. 495–505. [Google Scholar]
- Johne, A.B.; Weissbecker, B.; Schütz, S. Volatile emissions from Aesculus hippocastanum induced by mining of larval stages of Cameraria ohridella influence oviposition by conspecific females. J. Chem. Ecol. 2006, 32, 2303–2319. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tazoe, Y.; Vyas, P.; Yano, S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 2006, 57, 343–354. [Google Scholar] [CrossRef]
- Pastierovič, F.; Kalyniukova, A.; Hradecký, J.; Dvořák, O.; Vítámvás, J.; Mogilicherla, K.; Tomášková, I. Biochemical responses in Populus tremula: Defending against sucking and leaf-chewing insect herbivores. Plants 2024, 13, 1243. [Google Scholar] [CrossRef]
- Chavan, S.B.; Rawale, G.B.; Pradhan, A.; Uthappa, A.R.; Kakade, V.D.; Morade, A.S.; Reddy, K.S. Optimizing tree shade gradients in Emblica officinalis-based agroforestry systems: Impacts on soybean physio-biochemical traits and yield under degraded soils. Agrofor. Syst. 2025, 99, 21. [Google Scholar] [CrossRef]
- Kaur, H.; Salh, P.K.; Singh, B. Role of defence enzymes and phenolics in resistance of wheat crop (Triticum aestivum L.) towards aphid complex. J. Plant Interact. 2017, 12, 304–311. [Google Scholar] [CrossRef]
- Lu, Y.H.; Li, X.H.; Xue, W.J.; Yang, H.Y.; Liu, Y.; Wang, F.; Yu, Y.S.; Yang, U.Z. Impacts of four biochemicals on population development of Aphis gossypii Glover. J.-Yangzhou Univ. Agric. Life Sci. Ed. 2005, 26, 83–87. [Google Scholar]
- Gely, C.; Laurance, S.G.W.; Stork, N.E. How do herbivorous insects respond to drought stress in trees? Biol. Rev. 2020, 95, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Hillabrand, R.M.; Hacke, U.G.; Lieffers, V.J. Defoliation constrains xylem and phloem functionality. Tree Physiol. 2019, 39, 1099–1108. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Cochard, H.; Delzon, S.; Boivin, T.; Burlett, R.; Cailleret, M.; Corso, D.; Delmas, C.E.L.; De Caceres, M.; Diaz-Espejo, A.; et al. Plant hydraulics at the heart of plant, crops and ecosystem functions in the face of climate change. New Phytol. 2024, 241, 984–999. [Google Scholar] [CrossRef] [PubMed]
- Raimondo, F.; Trifilò, P.; Gullo, M.A.L. Does citrus leaf miner impair hydraulics and fitness of citrus host plants? Tree Physiol. 2013, 33, 1319–1327. [Google Scholar] [CrossRef]
- Sellin, A.; Õunapuu, E.; Kupper, P. Effects of light intensity and duration on leaf hydraulic conductance and distribution of resistance in shoots of silver birch (Betula pendula). Physiol. Plant. 2008, 134, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Õunapuu-Pikas, E.; Sellin, A. Plasticity and light sensitivity of leaf hydraulic conductance to fast changes in irradiance in common hazel (Corylus avellana L.). Plant Sci. 2020, 290, 110299. [Google Scholar] [CrossRef] [PubMed]
- Augustin, S.; Guichard, S.; Heitland, W.; Freise, J.; Svatoš, A.; Gilbert, M. Monitoring and dispersal of the invading Gracillariidae Cameraria ohridella. J. Appl. Entomol. 2009, 133, 58–66. [Google Scholar] [CrossRef]
- Niakan, M.; Ahmadi, A. Effects of foliar spraying kinetin on growth parameters and photosynthesis of tomato under different levels of drought stress. Iran J. Plant Physiol. 2014, 4, 939–947. [Google Scholar]
- Che’Ya, N.N.; Mohidem, N.A.; Roslin, N.A.; Saberioon, M.; Tarmidi, M.Z.; Arif Shah, J.; Fazlil Ilahi, W.F.; Man, N. Mobile computing for pest and disease management using spectral signature analysis: A Review. Agronomy 2022, 12, 967. [Google Scholar] [CrossRef]
- Mandi, S.S. Natural UV Radiation in Enhancing Survival Value and Quality of Plants; Springer: New Delhi, India, 2016. [Google Scholar]
- Ashok, A.; Rani, H.P.; Jayakumar, K.V. Monitoring of dynamic wetland changes using NDVI and NDWI based Landsat imagery. Remote Sens. Appl. Soc. Environ. 2021, 23, 100547. [Google Scholar] [CrossRef]
- Bochenek, Z.; Ziolkowski, D.; Bartold, M.; Orlowska, K.; Ochtyra, A. Monitoring forest biodiversity and the impact of climate on forest environment using high-resolution satellite images. Eur. J. Remote Sens. 2017, 51, 166–181. [Google Scholar] [CrossRef]
- Hardin, J.W. A Revision of the American Hippocastanaceae-II. Brittonia 1957, 9, 173–195. [Google Scholar] [CrossRef]
- Hardin, J.W. Studies in the Hippocastanaceae, V. Species of the Old World. Brittonia 1960, 12, 26–38. [Google Scholar] [CrossRef]
- Shlyk, A.A. Definition of a Chlorophyll and Carotenoids in Extracts of Green Leaves. In Biochemical Methods in Physiology of Plants; Nauka: Moscow, Russia, 1971. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Bogoutdinova, L.R.; Khaliluev, M.R.; Chaban, I.A.; Gulevich, A.A.; Shelepova, O.V.; Baranova, E.N. Salt tolerance assessment of different tomato varieties at the seedling stage. Horticulturae 2024, 10, 598. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Shelepova, O.V.; Baranova, E.N.; Sudarikov, K.A.; Savostyanova, L.I.; Mitrofanova, I.V. Parameters of photosynthetic activity as markers of biotic stress in Chrysanthemum coreanunum (H. Lév. & Vaniot) Nakai ex T. Mori. Russ. J. Plant Physiol. 2025, 72, 7. [Google Scholar] [CrossRef]

| Species | Side | RGB | Index | ||||
|---|---|---|---|---|---|---|---|
| R | G | B | NDWI | DSWI | SIPI | ||
| A. hippocastanum | sunny | 39.75 ± 1.25 | 74.25 ± 1.60 | 37.75 ± 1.11 | 0.999 ± 0.0012 | 2.519 ± 0.001 | 1.250 ± 0.0011 |
| shady | 24.4 ± 2.04 | 52.4 ± 1.50 | 24.2 ± 0.73 | 0.998 ± 0.001 | 2.629 ± 0.0014 | 1.222 ± 0.0011 | |
| A. glabra | sunny | 51.8 ± 2.20 | 101 ± 4.25 | 35.8 ± 1.46 | 1.002 ± 0.0013 | 3.284 ± 0.0012 | 1.403 ± 0.0011 |
| shady | 46.8 ± 1.91 | 82.6 ± 1.86 | 43.6 ± 1.57 | 1.000 ± 0.001 | 2.866 ± 0.0013 | 1.218 ± 0.0011 | |
| A. flava | sunny | 72.6 ± 1.75 | 113.4 ± 2.77 | 67.4 ± 2.84 | 1.000 ± 0.0013 | 3.191 ± 0.0011 | 1.372 ± 0.0011 |
| shady | 64.8 ± 4.39 | 107.6 ± 3.68 | 55.6 ± 3.43 | 0.998 ± 0.0012 | 3.036 ± 0.001 | 1.383 ± 0.0011 | |
| A. pavia | sunny | 57 ± 1.30 | 85 ± 2.21 | 53 ± 2.68 | 0.997 ± 0.0012 | 2.973 ± 0.001 | 1.365 ± 0.0011 |
| shady | 46.2 ± 2.60 | 70.2 ± 2.78 | 35.2 ± 2.67 | 1.001 ± 0.0015 | 2.416 ± 0.0012 | 1.248 ± 0.0011 | |
| A. × carnea | sunny | 37.8 ± 1.59 | 66.4 ± 2.01 | 36.2 ± 1.46 | 1.003 ± 0.0013 | 2.204 ± 0.001 | 1.157 ± 0.0011 |
| shady | 29.6 ± 1.08 | 64.6 ± 1.69 | 24.8 ± 0.66 | 1.002 ± 0.0012 | 2.178 ± 0.001 | 1.211 ± 0.0011 | |
| A. parviflora | sunny | 41.2 ± 2.01 | 68 ± 2.28 | 40 ± 1.95 | 1.000 ± 0.0009 | 2.004 ± 0.0011 | 1.156 ± 0.001 |
| shady | 47.2 ± 2.15 | 74.2 ± 1.32 | 50.2 ± 1.62 | 0.998 ± 0.0013 | 1.888 ± 0.0011 | 1.133 ± 0.0012 | |
| A. chinensis | sunny | 52.2 ± 5.15 | 80.4 ± 2.93 | 37.8 ± 2.24 | 1.001 ± 0.0011 | 2.786 ± 0.0012 | 1.331 ± 0.0011 |
| shady | 54.2 ± 1.85 | 78.4 ± 1.33 | 32.6 ± 0.93 | 0.997 ± 0.0012 | 3.364 ± 0.001 | 1.418 ± 0.0011 | |
| Foliage in the Light | Fv/Fm | NPQ | Y(NPQ) | Y(NO) | ETR |
|---|---|---|---|---|---|
| A. hippocastanum | 0.333 ± 0.0012 | 0.178 ± 0.0012 | 0.13 ± 0.0012 | 0.729 ± 0.0013 | 11.3 ± 0.3 |
| A. glabra | 0.491 ± 0.0014 | 0.173 ± 0.0014 | 0.149 ± 0.0013 | 0.754 ± 0.0018 | 11.5 ± 0.19 |
| A. flava | 0.371 ± 0.0015 | 0.244 ± 0.0015 | 0.153 ± 0.0014 | 0.703 ± 0.0013 | 11.5 ± 0.21 |
| A. pavia | 0.491 ± 0.0011 | 0.384 ± 0.0013 | 0.159 ± 0.0013 | 0.688 ± 0.001 | 13.5 ± 0.53 |
| A. × carnea | 0.652 ± 0.0011 | 0.437 ± 0.0012 | 0.193 ± 0.001 | 0.631 ± 0.001 | 14.8 ± 0.21 |
| A. parviflora | 0.682 ± 0.001 | 0.491 ± 0.001 | 0.198 ± 0.001 | 0.654 ± 0.001 | 16.9 ± 0.71 |
| A. chinensis | 0.717 ± 0.0012 | 0.521 ± 0.0011 | 0.326 ± 0.0011 | 0.589 ± 0.0018 | 19.1 ± 0.22 |
| Foliage in the Shade | Fv/Fm | NPQ | Y(NPQ) | Y(NO) | ETR |
|---|---|---|---|---|---|
| A. hippocastanum | 0.337 ± 0.0011 | 0.104 ± 0.0013 | 0.087 ± 0.0013 | 0.843 ± 0.0012 | 5.6 ± 0.23 |
| A. glabra | 0.441 ± 0.0012 | 0.149 ± 0.0014 | 0.117 ± 0.0018 | 0.701 ± 0.0014 | 10.1 ± 0.72 |
| A. flava | 0.358 ± 0.0013 | 0.219 ± 0.001 | 0.115 ± 0.0018 | 0.682 ± 0.001 | 7.7 ± 0.85 |
| A. pavia | 0.484 ± 0.0014 | 0.399 ± 0.001 | 0.139 ± 0.001 | 0.663 ± 0.0014 | 12.8 ± 0.35 |
| A. × carnea | 0.595 ± 0.0014 | 0.448 ± 0.0018 | 0.187 ± 0.001 | 0.648 ± 0.001 | 12.8 ± 0.11 |
| A. parviflora | 0.654 ± 0.0017 | 0.398 ± 0.0016 | 0.189 ± 0.0012 | 0.661 ± 0.0012 | 11.1 ± 0.43 |
| A. chinensis | 0.652 ± 0.0018 | 0.558 ± 0.0012 | 0.293 ± 0.0013 | 0.599 ± 0.001 | 16.3 ± 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Bogoutdinova, L.R.; Shelepova, O.V.; Rostovtseva, H.I.; Raldugina, G.N.; Baranova, E.N.; Gulevich, A.A. Foliar Illumination Affects the Severity of Cameraria ohridella Damage Among Horse Chestnut Species. Plants 2026, 15, 86. https://doi.org/10.3390/plants15010086
Bogoutdinova LR, Shelepova OV, Rostovtseva HI, Raldugina GN, Baranova EN, Gulevich AA. Foliar Illumination Affects the Severity of Cameraria ohridella Damage Among Horse Chestnut Species. Plants. 2026; 15(1):86. https://doi.org/10.3390/plants15010086
Chicago/Turabian StyleBogoutdinova, Liliya R., Olga V. Shelepova, Helen I. Rostovtseva, Galina N. Raldugina, Ekaterina N. Baranova, and Alexander A. Gulevich. 2026. "Foliar Illumination Affects the Severity of Cameraria ohridella Damage Among Horse Chestnut Species" Plants 15, no. 1: 86. https://doi.org/10.3390/plants15010086
APA StyleBogoutdinova, L. R., Shelepova, O. V., Rostovtseva, H. I., Raldugina, G. N., Baranova, E. N., & Gulevich, A. A. (2026). Foliar Illumination Affects the Severity of Cameraria ohridella Damage Among Horse Chestnut Species. Plants, 15(1), 86. https://doi.org/10.3390/plants15010086







