Root Exudates Mediate the Production of Reactive Oxygen Species in Rhizosphere Soil: Formation Mechanisms and Ecological Effects
Abstract
1. Introduction
2. Detection Method for Rhizosphere ROS
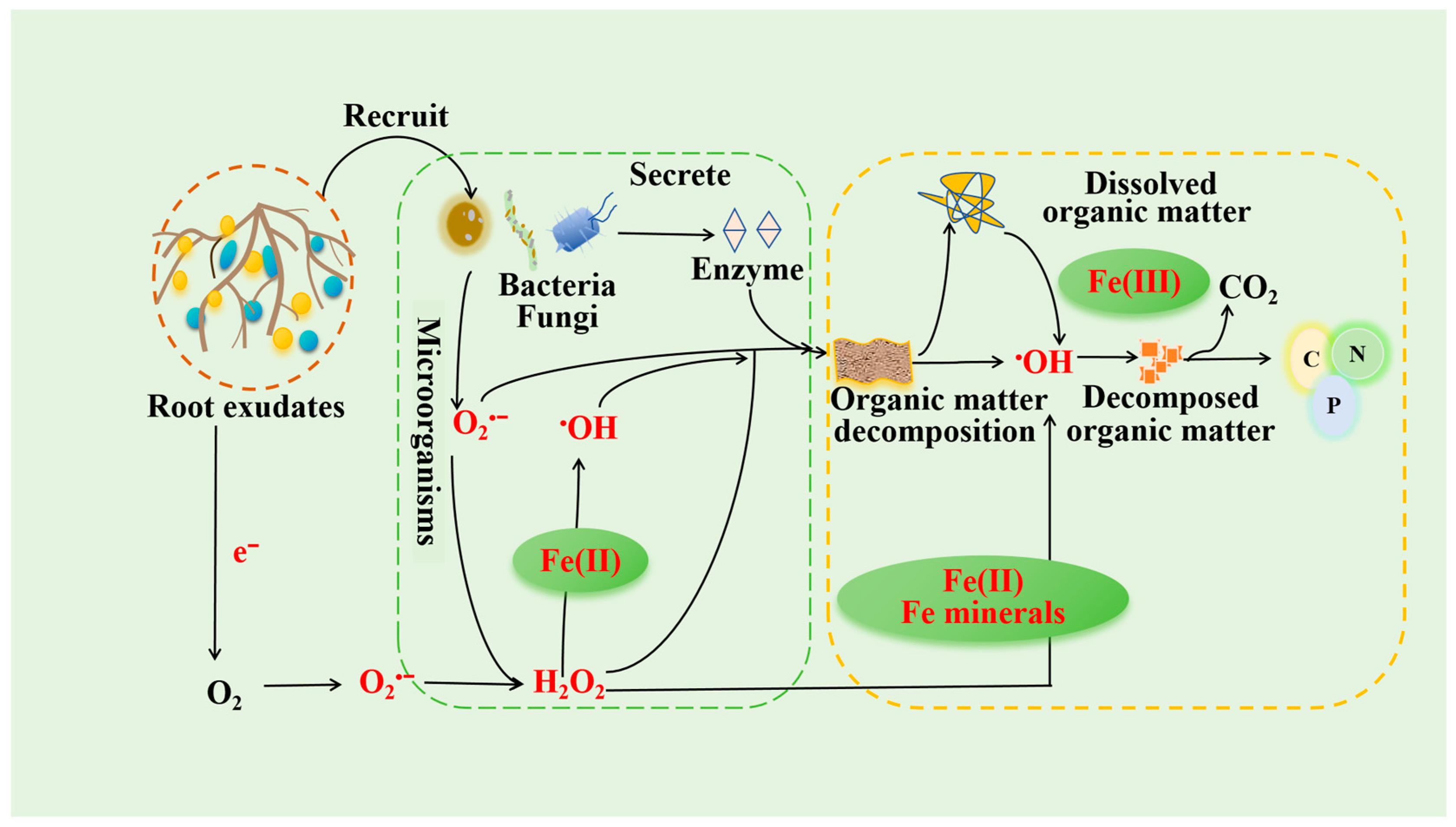
3. Mechanisms of ROS Formation in Rhizosphere
4. Influencing Factors of ROS Production in Rhizosphere
5. Ecological Effects of Root Exudate-Mediated ROS
5.1. Effects on Plant Growth and Health
5.2. Effects on Soil Nutrient Cycling
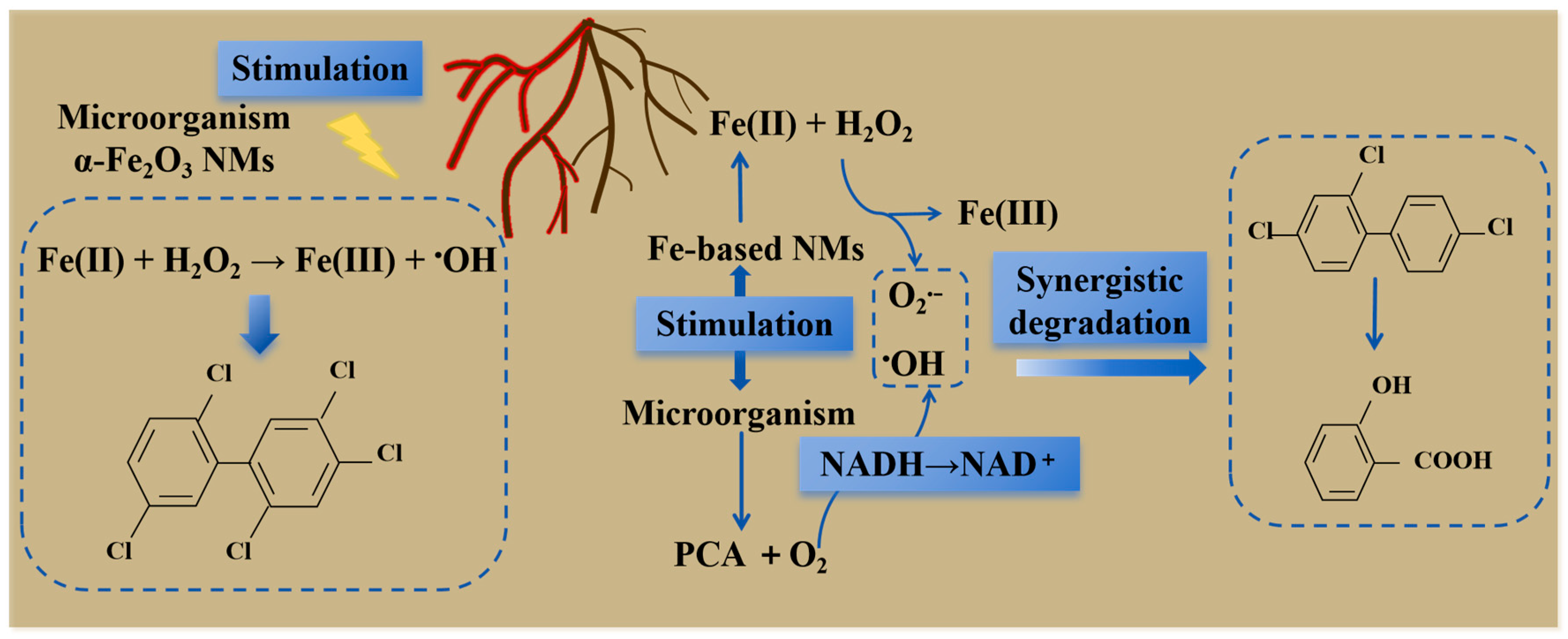
5.3. Effects on Degradation of Soil Pollutants
6. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Apel, K.; Hirt, H. Reactive oxygen species metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Ambuj, B.J.; Rama, S.D.; Pessarakli, M. Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef]
- Vaahtera, L.; Brosché, M.; Wrzaczek, M.; Kangasjärvi, J. Specificity in ROS signaling and transcript signatures. Antioxid. Redox Sign 2014, 21, 1422–1441. [Google Scholar] [CrossRef]
- Del, R.L.A.; Sandalio, L.M.; Corpas, F.J.; Palma, J.M.; Barroso, J.B. Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signaling. Plant Physiol. 2006, 141, 330–335. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Citterico, M.; Kimura, S.; Stevens, D.M.; Wrzaczek, M.; Coaker, G. Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat. Plants 2021, 7, 403–412. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. For. Cell Mol. Biol. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Marcec, J.M.; Gilroy, S.; Poovaiah, B. Mutual interplay of Ca2+ and ROS signaling in plant immune response. Plant Sci. 2019, 283, 343–354. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van, B.F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil. Biol. Biochem. 2015, 83, 184–199. [Google Scholar]
- Jia, Y.; Liu, Z.; Zhou, L.; Liu, X.; Ma, K.; Feng, X. Soil organic carbon sourcing variance in the rhizosphere vs. non-rhizosphere of two mycorrhizal tree species. Soil. Biol. Biochem. 2023, 176, 108884. [Google Scholar] [CrossRef]
- Sutherland, K.M.; Wankel, S.D.; Hansel, C.M. Dark biological superoxide production as a significant flux and sink of marine dissolved oxygen. Proc. Natl. Acad. Sci. USA 2020, 117, 3433–3439. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wang, X.; Liu, X.; Huang, L.; Hu, J.; Chu, R.; Tolic, N.; Dong, H. Mutual interactions between reduced Fe-bearing clay minerals and humic acids under dark, oxygenated condition: Hydroxyl radical generation and humic acid transformation. Environ. Sci. Technol. 2020, 54, 15013–15023. [Google Scholar] [CrossRef]
- Dai, H.; Wu, B.; Chen, B.; Ma, B.; Chu, C. Diel Fluctuation of Extracellular Reactive Oxygen Species Production in the Rhizosphere of Rice. Environ. Sci. Technol. 2022, 56, 9075–9082. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, K.; Zhang, C.; Zhang, X.; Chen, N.; Jia, H. Microscale Spatiotemporal Variation and Generation Mechanisms of Reactive Oxygen Species in the Rhizosphere of Ryegrass: Coupled Biotic-Abiotic Processes. Environ. Sci. Technol. 2022, 56, 16483–16493. [Google Scholar] [CrossRef]
- Huang, D.; Chen, N.; Zhu, C.; Sun, H.; Fang, G.; Zhou, D. Dynamic Production of Hydroxyl Radicals during the Flooding-Drainage Process of Paddy Soil: An In Situ Column Study. Environ. Sci. Technol. 2023, 57, 16340–16347. [Google Scholar] [CrossRef]
- Chen, N.; Huang, D.; Zeng, Y.; Wang, J.; Liu, G.; Liu, X.; Wu, T.; Gao, Y.; Fang, G.; Wang, Y.; et al. Long-term Application of Agricultural Amendments Regulate Hydroxyl Radicals Production during Oxygenation of Paddy Soils. Environ. Sci. Technol. 2024, 58, 13509–13520. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, X.; Hu, Y.; Sheng, G. New Barrier Role of Iron Plaque: Producing Interfacial Hydroxyl Radicals to Degrade Rhizosphere Pollutants. Environ. Sci. Technol. 2024, 58, 795–804. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Lopes, Â.M.; de Lima, M.F.; Luís, M.R.A.; Araújo, S.A.L.; Nunes, S.H.R.; de Araújo, M.B. Analytical Methods for the Determination of Rosuvastatin in Pharmaceutical Formulations and Biological Fluids: A Critical Review. Crit. Rev. Anal. Chem. 2018, 48, 317–329. [Google Scholar]
- Zhang, W.; Hao, L.; Huang, J.; Xia, L.; Cui, M.; Zhang, X.; Gu, Y.; Wang, P. Chemiluminescence chitosan hydrogels based on the luminol analog L-012 for highly sensitive detection of ROS. Talanta 2019, 201, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Yue, Y.; Yin, C.; Huo, F. Design of dual responsive ROS/RSS fluorescent probes and their application in bioimaging. Chem. Asian J. 2022, 17, e202200907. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; AlNashef, I.M. Superoxide Ion: Generation and Chemical Implications. Chem. Rev. 2016, 116, 3029–3085. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Pathak, J.; Chatterjee, A.; Singh, S.P.; Sinha, R.P. Detection of Reactive Oxygen Species (ROS) in Cyanobacteria Using the Oxidant-sensing Probe 2’,7’-Dichlorodihydrofluorescein Diacetate (DCFH-DA). Bio-Protoc. 2017, 7, e2545. [Google Scholar]
- Yang, H.; Qiu, R.; Tang, Y.; Ye, S.; Wu, S.; Qin, F.; Xiang, L.; Tan, X.; Zeng, G.; Yan, M. Carbonyl and defect of metal-free char trigger electron transfer and O2- in persulfate activation for Aniline aerofloat degradation. Water Res. 2023, 231, 119659. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Detection and characterisation of radicals in biological materials using EPR methodology. BBA General. Subj. 2014, 1840, 708–721. [Google Scholar] [CrossRef]
- Liu, J.; Shen, S.; Zhu, K.; Li, Z.; Chen, N.; Lichtfouse, E.; Jia, H. Novel insights into the factors influencing rhizosphere reactive oxygen species production and their role in polycyclic aromatic hydrocarbons transformation. Soil. Biol. Biochem. 2024, 198, 109562. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Q.; Gong, Y.; Zhang, Y. Enhancing the production of reactive oxygen species in the rhizosphere to promote contaminants degradation in sediments by electrically strengthening microbial extracellular electron transfer. J. Hazard. Mater. 2024, 479, 135644. [Google Scholar] [CrossRef]
- Lin, S.; Ye, C.; Lin, Z.; Huang, L.; Li, D. Recent progress of near-infrared fluorescent probes in the determination of reactive oxygen species for disease diagnosis. Talanta 2024, 268, 125264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, M.; Yuan, Z. Methods for the detection of reactive oxygen species. Anal. Methods-UK 2018, 10, 4625–4638. [Google Scholar] [CrossRef]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Francine, A.F.M.; Oliveira, J.G.; Guimarães, A.O. Electron Paramagnetic Resonance Applied to Free Radicals and Reactive Oxygen Species Detection in Plant Systems. Appl. Magn. Reson. 2023, 55, 335–355. [Google Scholar]
- Ksas, B.; Havaux, M. Determination of ROS-Induced Lipid Peroxidation by HPLC-Based Quantification of Hydroxy Polyunsaturated Fatty Acids. Methods Mol. Biol. 2022, 2526, 181–189. [Google Scholar]
- Zhu, S.; Jiang, Z.; Jiang, Y.; Dong, Y.; Li, J.; Shi, L. The successive reduction of iodate to iodide driven by iron redox cycling. J. Hazard. Mater. 2024, 480, 136436. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil. Biol. Biochem. 2009, 42, 391–404. [Google Scholar] [CrossRef]
- Diaz, J.M.; Hansel, C.M.; Voelker, B.M.; Mendes, C.M.; Andeer, P.F.; Zhang, T. Widespread Production of Extracellular Superoxide by Heterotrophic Bacteria. Science 2013, 340, 1223–1226. [Google Scholar] [CrossRef]
- Zhang, P.; Yuan, S.; Liao, P. Mechanisms of hydroxyl radical production from abiotic oxidation of pyrite under acidic conditions. Geochim. Cosmochim. Acta 2016, 172, 444–457. [Google Scholar] [CrossRef]
- Jeewani, P.H.; Gunina, A.; Tao, L.; Zhu, Z.; Kuzyakov, Y.; Van Zwieten, L.; Guggenberger, G.; Shen, C.; Yu, G.; Singh, B.P.; et al. Rusty sink of rhizodeposits and associated keystone microbiomes. Soil. Biol. Biochem. 2020, 147, 107840. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Kuzyakov, Y.; Zhan, P.; Wang, Q.; Hettenhausen, C.; Xiao, D.; Qi, J.; Zhang, Z. Differentiating microbial taxonomic and functional responses to physical disturbance in bulk and rhizosphere soils. Land. Degrad. Dev. 2020, 31, 2858–2871. [Google Scholar] [CrossRef]
- Hui, Y.G.; Yakov, K. Fenton chemistry and reactive oxygen species in soil: Abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth-Sci. Rev. 2021, 214, 103525. [Google Scholar]
- Košnář, Z.; Částková, T.; Wiesnerová, L.; Praus, L.; Jablonský, I.; Koudela, M.; Tlustoš, P. Comparing the removal of polycyclic aromatic hydrocarbons in soil after different bioremediation approaches in relationto the extracellular enzyme activities. J. Environ. Sci. 2019, 76, 249–258. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Z.; Zhang, R.; Liu, J.; Liu, J.; Dai, Y.; Zhang, C.; Jia, H. Interfacial reaction between organic acids and iron-containing clay minerals: Hydroxyl radical generation and phenolic compounds degradation. Sci. Total Environ. 2021, 783, 147025. [Google Scholar] [CrossRef] [PubMed]
- Zhalnina, K.; Louie, K.B.; Hao, Z.; Mansoori, N.; Da Rocha, U.N.; Shi, S.; Cho, H.; Karaoz, U.; Loqué, D.; Bowen, B.P.; et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018, 3, 470–480. [Google Scholar] [CrossRef]
- Hansel, C.M.; Zeiner, C.A.; Santelli, C.M.; Webb, S.M. Mn(II) oxidation by an ascomycete fungus is linked to superoxide production during asexual reproduction. Proc. Natl. Acad. Sci. USA 2012, 109, 12621–12625. [Google Scholar] [CrossRef]
- Tong, M.; Yuan, S.; Ma, S.; Jin, M.; Deng, L.; Dong, C.; Xixiang, L.; Yiqun, G.; Yanxin, W. Production of Abundant Hydroxyl Radicals from Oxygenation of Subsurface Sediments. Environ. Sci. Technol. 2016, 50, 214–221. [Google Scholar] [CrossRef]
- Firoz, S.; Markus, G.; Susan, N.; De Beeck Michiel, O.; Luigi, G.; Dirk, H.; Per, P.; Anders, T. Secretion of Iron(III)-Reducing Metabolites during Protein Acquisition by the Ectomycorrhizal Fungus Paxillus involutus. Microorganisms 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Kim, H.K.; Park, H.M.; Park, Y.; Park, K.; Seo, J.; Kim, K.H. Mimicking the Fenton reaction-induced wood decay by fungi for pretreatment of lignocellulose. Bioresour. Technol. 2015, 179, 467–472. [Google Scholar] [CrossRef]
- Tsing, B.; Anand, R.R.; Kaksonen, A.H.; Ignacio, G.; Anais, P.; Ryan, R.P.N.; Lintern, M.J.; Spinks, S.C.; Zhuang, X. The role of fungi in the biogeochemical cycling of supergene gold and satellite transition metals: A potential new exploration tool. Ore Geol. Rev. 2022, 140, 104595. [Google Scholar]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root Exudation of Primary Metabolites: Mechanisms and Their Roles in Plant Responses to Environmental Stimuli. Front. Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, X.; Zhang, J.; Cai, Z.; Jiang, K.; Chang, Y. Deciphering the relative importance of soil and plant traits on the development of rhizosphere microbial communities. Soil. Biol. Biochem. 2020, 148, 107909. [Google Scholar] [CrossRef]
- Laurent, P.; Raaijmakers, J.M.; Philippe, L.; van der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar]
- Hannes, S.; Thilo, E. Detection and quantification of native microbial populations on soil-grown rice roots by catalyzed reporter deposition-fluorescence in situ hybridization. Fems Microbiol. Ecol. 2014, 87, 390–402. [Google Scholar]
- Tiziani, R.; Pii, Y.; Celletti, S.; Cesco, S.; Mimmo, T. Phosphorus deficiency changes carbon isotope fractionation and triggers exudate reacquisition in tomato plants. Sci. Rep. 2020, 10, 15970. [Google Scholar] [CrossRef]
- Wang, C.; Fu, B.; Zhang, L.; Xu, Z. Soil moisture–plant interactions: An ecohydrological review. J. Soil. Sediment. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Liu, X.; Cao, L.; Wang, Q.; Zhang, X.; Hu, X. Effect of tea saponin on phytoremediation of Cd and pyrene in contaminated soils by Lolium multiflorum. Environ. Sci. Pollut. Res. Int. 2017, 24, 18946–18952. [Google Scholar] [CrossRef]
- Bonke, S.A.; Risse, T.; Schnegg, A.; Brueckner, A. In situ electron paramagnetic resonance spectroscopy for catalysis. Nat. Rev. Methods Primers 2021, 1, 33. [Google Scholar] [CrossRef]
- Ndez, J.A.H.; Ferrer, M.A.; Nez, A.J.; Barcelo, A.R.; Sevilla, F. Antioxidant Systems and ·-O2·-/H2O2 Production in the Apoplast of Pea Leaves. Its Relation with Salt-Induced Necrotic Lesions in Minor Veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Daniell, H. Engineering Cytoplasmic Male Sterility via the Chloroplast Genome by Expression of b-Ketothiolase. Breakthr. Technol. 2005, 138, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative Modifications to Cellular Components in Plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, C.G.; Pastori, G.M.; Foyer, C.H. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000, 123, 335–344. [Google Scholar] [CrossRef]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Damia, H.; Cigudosa, J.C.; Miguel, U.; Javier, B.; et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.; Li, J.; Liang, Y. Role of ROS signaling in the plant defense against vascular pathogens. Curr. Opin. Plant Biol. 2024, 81, 102617. [Google Scholar]
- Su, J.; Liu, Y.; Han, F.; Gao, F.; Gan, F.; Huang, K.; Li, Z. ROS, an Important Plant Growth Regulator in Root Growth and Development: Functional Genes and Mechanism. Biology 2024, 13, 1033. [Google Scholar] [CrossRef]
- Venkidasamy, B.; Ghorbanpour, M.; Thiruvengadam, M. Reactive oxygen and nitrogen species in plant defense mechanisms. Plant Cell Rep. 2024, 43, 298. [Google Scholar] [CrossRef]
- Pantelis, L.; Basil, G.; Hartmut, Q.; Panagiotis, A. Disturbance of reactive oxygen species homeostasis induces atypical tubulin polymer formation and affects mitosis in root-tip cells of Triticum turgidum and Arabidopsis thaliana. Cytoskeleton 2012, 69, 1–21. [Google Scholar]
- Xu, Q.T.; Yang, L.; Zhou, Z.Q.; Mei, F.Z.; Qu, L.H.; Zhou, G.S. Process of aerenchyma formation and reactive oxygen species induced by waterlogging in wheat seminal roots. Planta 2013, 238, 969–982. [Google Scholar] [CrossRef]
- Takaki, Y.; Imene, R.; Mikio, N. Lysigenous aerenchyma formation in maize root is confined to cortical cells by regulation of genes related to generation and scavenging of reactive oxygen species. Plant Signal Behav. 2011, 6, 759–761. [Google Scholar]
- Drew, M.C.; He, C.; Morgan, P.W. Programmed cell death and aerenchyma formation in roots. Trends Plant Sci. 2000, 5, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Gunawardena, A.H.; Pearce, D.M.; Jackson, M.B.; Hawes, C.R.; Evans, D.E. Characterisation of programmed cell death during aerenchyma formation induced by ethylene or hypoxia in roots of maize (Zea mays L.). Planta 2001, 212, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, E.; Mendes, R.; Bakker, P.A.H.M.; Raaijmakers, J.M. Fungal invasion of the rhizosphere microbiome. ISME J. 2016, 10, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, Y. Toward understanding the genetic bases underlying plant-mediated “cry for help” to the microbiota. Imeta 2022, 1, e8. [Google Scholar] [CrossRef]
- Frank, W.; Beate, A.; Helmut, B.; József, F.; Katja, B.; Marina, F.; Tobias, H.; Ralph, H.; Christina, N.; von Wettstein, D.; et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc. Natl. Acad. Sci. USA 2005, 102, 13386–13391. [Google Scholar]
- Song, Y.; Wilson, A.J.; Zhang, X.; Thoms, D.; Sohrabi, R.; Song, S.; Geissmann, Q.; Liu, Y.; Walgren, L.; Sheng, Y.; et al. FERONIA restricts Pseudomonas in the rhizosphere microbiome via regulation of reactive oxygen species. Nat. Plants 2021, 7, 644–654. [Google Scholar] [CrossRef]
- Wen, T.; Ding, Z.; Thomashow, L.S.; Hale, L.; Yang, S.; Xie, P.; Liu, X.; Wang, H.; Shen, Q.; Yuan, J. Deciphering the mechanism of fungal pathogen-induced disease-suppressive soil. New Phytol. 2023, 238, 2634–2650. [Google Scholar] [CrossRef]
- Maarten, M.; Tjakko, A. Primary and secondary oxidative stress in Bacillus. Environ. Microbiol. 2011, 13, 1387–1394. [Google Scholar]
- Bilal, S.; Khan, A.L.; Shahzad, R.; Kim, Y.; Imran, M.; Khan, M.J.; Al-Harrasi, A.; Kim, T.H.; Lee, I. Mechanisms of Cr(VI) resistance by endophytic Sphingomonas sp. LK11 and its Cr(VI) phytotoxic mitigating effects in soybean ( Glycine max L.). Ecotoxicol. Environ. Safe 2018, 164, 648–658. [Google Scholar] [CrossRef]
- Duhamel, S.; Diaz, J.M.; Adams, J.C.; Djaoudi, K.; Steck, V.; Waggoner, E.M. Phosphorus as an integral component of global marine biogeochemistry. Nat. Geosci. 2021, 14, 359–368. [Google Scholar] [CrossRef]
- Han, R.; Wang, Z.; Lv, J.; Zhu, Z.; Yu, G.; Li, G.; Zhu, Y. Multiple Effects of Humic Components on Microbially Mediated Iron Redox Processes and Production of Hydroxyl Radicals. Environ. Sci. Technol. 2022, 56, 16419–16427. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Liu, J.; Wang, Z.; Zhu, K.; Jia, B.; Yang, H.; Qin, J.; Xie, J.; Latif, J.; Liu, F.; et al. Spatiotemporal dynamics of reactive oxygen species in the detritusphere and their critical roles in organic carbon mineralisation. Soil. Biol. Biochem. 2025, 202, 109700. [Google Scholar] [CrossRef]
- Yu, C.; Lu, Y.; Zhang, Y.; Qian, A.; Zhang, P.; Tong, M.; Yuan, S. Significant Contribution of Solid Organic Matter for Hydroxyl Radical Production during Oxygenation. Environ. Sci. Technol. 2022, 56, 11878–11887. [Google Scholar] [CrossRef]
- Goranov, A.I.; Chen, H.; Duan, J.; Myneni, S.C.B.; Hatcher, P.G. Potentially Massive and Global Non-Pyrogenic Production of Condensed “Black” Carbon through Biomass Oxidation. Environ. Sci. Technol. 2024, 58, 2750–2761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Q.; Hu, H.; Zhu, J.; Liu, Y. Effects of Fe(II) on As(III) oxidation in Fe(II)-As(III) co-oxidation: Limiting and driving roles. J. Hazard. Mater. 2023, 447, 130790. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Dong, L.; Zhang, G.; Bai, X.; Wang, J.; Li, Y.; Hu, S.; Yu, X. Interactions between root and rhizosphere microbes mediate phosphorus acquisition in Pinus tabulaeformis. Ind. Crops Prod. 2024, 215, 118624. [Google Scholar] [CrossRef]
- Barrow, N.J.; Lambers, H. Phosphate-solubilising microorganisms mainly increase plant phosphate uptake by effects of pH on root physiology. Plant Soil. 2022, 476, 1–6. [Google Scholar] [CrossRef]
- Gerke, J. Improving Phosphate Acquisition from Soil via Higher Plants While Approaching Peak Phosphorus Worldwide: A Critical Review of Current Concepts and Misconceptions. Plants 2024, 13, 3478. [Google Scholar] [CrossRef]
- Bi, Z.; Wang, W.; Zhao, L.; Wang, X.; Xing, D.; Zhou, Y.; Lee, D.J.; Ren, N.; Chen, C. The generation and transformation mechanisms of reactive oxygen species in the environment and their implications for pollution control processes: A review. Environ. Res. 2024, 260, 119592. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, X.; Cai, L.; Wu, J. Responses of Soil Aggregate Stability and SOC to Different Tillage Modes and Straw Input Level. Sustainability 2025, 17, 893. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Zheng, T.; Dai, Y.; Li, Z.; Lin, D. Fe-Based Nanomaterials and Plant Growth Promoting Rhizobacteria Synergistically Degrade Polychlorinated Biphenyls by Producing Extracellular Reactive Oxygen Species. Environ. Sci. Technol. 2023, 57, 12771–12781. [Google Scholar] [CrossRef]
- Zheng, T.; Hou, J.; Wu, T.; Jin, H.; Dai, Y.; Xu, J.; Yang, K.; Lin, D. Ferric Oxide Nanomaterials and Plant-Rhizobacteria Symbionts Cogenerate Iron Plaque for Removing Highly Chlorinated Contaminants in Dryland Soils. Environ. Sci. Technol. 2024, 58, 11063–11073. [Google Scholar] [CrossRef]
- Jia, H.; Shi, Y.; Nie, X.; Zhao, S.; Wang, T.; Sharma, V.K. Persistent free radicals in humin under redox conditions and their impact in transforming polycyclic aromatic hydrocarbons. Front. Environ. Sci. Eng. 2020, 14, 6962–6984. [Google Scholar] [CrossRef]
- Zhan, M.; Yang, X.; Xian, Q.; Kong, L. Photosensitized degradation of bisphenol A involving reactive oxygen species in the presence of humic substances. Chemosphere 2006, 63, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Liu, X.; Duan, L.; Zhang, X.; Dong, L.; Liu, X.; Li, P.; Li, B.; Xue, M.; et al. The Overlooked Role of Humin in Dark Hydroxyl Radical Production during Oxygenation. Environ. Sci. Technol. 2024, 58, 18033–18040. [Google Scholar] [CrossRef]
- Chen, N.; Huang, D.; Liu, G.; Chu, L.; Fang, G.; Zhu, C.; Zhou, D.; Gao, J. Active iron species driven hydroxyl radicals formation in oxygenation of different paddy soils: Implications to polycyclic aromatic hydrocarbons degradation. Water Res. 2021, 203, 117484. [Google Scholar] [CrossRef]
- Yang, X.; Cai, H.; Bao, M.; Yu, J.; Lu, J.; Li, Y. Insight into the highly efficient degradation of PAHs in water over graphene oxide/Ag 3 PO 4 composites under visible light irradiation. Chem. Eng. J. 2018, 334, 355–376. [Google Scholar] [CrossRef]
- Liao, W.; Zhang, M.; Huang, G.; Yu, J. Ca2+ and CaM are Involved in NO- and H2O2-Induced Adventitious Root Development in Marigold. J. Plant Growth Regul. 2012, 31, 253–264. [Google Scholar] [CrossRef]
- Stroiński, A.; Giżewska, K.; Zielezińska, M. Abscisic acid is required in transduction of cadmium signal to potato roots. Biol. Plant. 2013, 57, 121–127. [Google Scholar] [CrossRef]
- Cuypers, A.; Plusquin, M.; Remans, T.; Jozefczak, M.; Keunen, E.; Gielen, H.; Opdenakker, K.; Ambily, R.N.; Munters, E.; Artois, T.J.; et al. Cadmium stress: An oxidative challenge. Biometals An. Int. J. On. Role Metal. Ions Biol. Biochem. Med. 2010, 23, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Eapen, D.S.; Souza, S.F. Cadmium accumulation and its influence on lipid peroxidation and antioxidative system in an aquatic plant, Bacopa monnieri L. Chemosphere 2006, 62, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Greipsson, S.; Crowder, A.A. Amelioration of copper and nickel toxicity by iron plaque on roots of rice (Oryza sativa). Can. J. Bot. 1992, 70, 824–830. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhu, Y.G.; Smith, F.A.; Smith, S.E. Do Phosphorus Nutrition and Iron Plaque Alter Arsenate (As) Uptake by Rice Seedlings in Hydroponic Culture? New Phytol. 2004, 162, 481–488. [Google Scholar] [CrossRef]
- Liu, H.J.; Zhang, J.L.; Zhang, F.S. Role of iron plaque in Cd uptake by and translocation within rice ( Oryza sativa L.) seedlings grown in solution culture. Environ. Exp. Bot. 2006, 59, 314–320. [Google Scholar] [CrossRef]
- Miao, F.; Zhang, X.; Fu, Q.; Hu, H.; Islam, M.S.; Fang, L.; Zhu, J. Sulfur enhances iron plaque formation and stress resistance to reduce the transfer of Cd and As in the soil-rice system. Sci. Total Environ. 2024, 927, 171689. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Wang, Q.; Hu, M.; Zhang, X.; Liu, T. Controlling factors of iron plaque formation and its adsorption of cadmium and arsenic throughout the entire life cycle of rice plants. Sci. Total Environ. 2024, 953, 176106. [Google Scholar] [CrossRef]
- Manzoor, N.; Ahmed, T.; Noman, M.; Shahid, M.; Nazir, M.M.; Ali, L.; Alnusaire, T.S.; Li, B.; Schulin, R.; Wang, G. Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ. 2021, 769, 145221. [Google Scholar] [CrossRef] [PubMed]
- Elanchezhian, R.; Kumar, D.; Ramesh, K.; Biswas, A.K.; Guhey, A.; Patra, A.K. Morpho-physiological and biochemical response of maize (Zea mays L.) plants fertilized with nano-iron (Fe3O4) micronutrient. J. Plant Nutr. 2017, 40, 1969–1977. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wan, J.; Zhang, C.; Cheng, M.; Qin, X.; Xue, W. Stabilized Nanoscale Zerovalent Iron Mediated Cadmium Accumulation and Oxidative Damage of Boehmeria nivea (L.) Gaudich Cultivated in Cadmium Contaminated Sediments. Environ. Sci. Technol. 2017, 51, 11308–11316. [Google Scholar] [CrossRef]
- Blute, N.K.; Brabander, D.J.; Hemond, H.F.; Sutton, S.R.; Newville, M.G.; Rivers, M.L. Arsenic sequestration by ferric iron plaque on cattail roots. Environ. Sci. Technol. 2004, 38, 6074–6077. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, F.; Zhang, Q.; Lan, Q.; Liu, C.; Guo, X.; Cai, Q.; Chen, Y.; Wang, G.; Ding, J. Influence of iron plaque on the uptake and accumulation of chromium by rice (Oryza sativa L.) seedlings: Insights from hydroponic and soil cultivation. Ecotoxicol. Environ. Safe 2018, 162, 51–58. [Google Scholar] [CrossRef] [PubMed]

| Methods | Applicable Scenarios | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Chemiluminescence | Assess the degradation mechanism of soil pollutants | High sensitivity; real-time monitoring of ROS production | Low specificity; vulnerable to environmental factors | [29,30] |
| Fluorescence probe | Study the interaction between plant roots and microorganisms | Simple operation; low cost; detect ROS at low concentrations | Limitation of the probe stability; easily affected by organic matter or minerals | [31] |
| Spectrophotometry | Assess the redox status of rhizosphere soil; detect the soil microbial activity | Simple operation; low cost; carry out quantitative determination; wide range of applications | Real-time monitoring cannot be achieved; limited sensitivity | [32] |
| Electron paramagnetic resonance spectroscopy | Assess the soil redox status; analyze the plant responses to environmental stresses | High specificity; no marking required; suitable for complex environments; real-time monitoring | High cost of instruments; high requirements for the uniformity and stability of samples | [33,34] |
| High-performance liquid chromatography | Detect the oxidation products in rhizosphere soil; evaluate the metabolic activity of soil microorganisms | High sensitivity and high accuracy; complete the analysis in a short time; wide applicability | High cost of instruments; complex operation; not suitable for large-scale sample detection | [35] |
| In vivo imaging technology | Study the temporal and spatial variation of ROS in the rhizosphere | Real time and dynamic; high resolution | High cost of instruments; complex operation | [29] |
| Iodine reduction titration method | Evaluate redox changes during soil pollution remediation | Simple operation; low cost; wide range of application; fast detection speed | Cannot distinguish different types of ROS; unable to perform real-time monitoring; high reaction conditions | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Y.; Tian, X.; Guo, J.; Luan, Y.; Wang, D. Root Exudates Mediate the Production of Reactive Oxygen Species in Rhizosphere Soil: Formation Mechanisms and Ecological Effects. Plants 2025, 14, 1395. https://doi.org/10.3390/plants14091395
Wang X, Liu Y, Tian X, Guo J, Luan Y, Wang D. Root Exudates Mediate the Production of Reactive Oxygen Species in Rhizosphere Soil: Formation Mechanisms and Ecological Effects. Plants. 2025; 14(9):1395. https://doi.org/10.3390/plants14091395
Chicago/Turabian StyleWang, Xuqin, Yalei Liu, Xiaoyan Tian, Juan Guo, Yaning Luan, and Dengzhi Wang. 2025. "Root Exudates Mediate the Production of Reactive Oxygen Species in Rhizosphere Soil: Formation Mechanisms and Ecological Effects" Plants 14, no. 9: 1395. https://doi.org/10.3390/plants14091395
APA StyleWang, X., Liu, Y., Tian, X., Guo, J., Luan, Y., & Wang, D. (2025). Root Exudates Mediate the Production of Reactive Oxygen Species in Rhizosphere Soil: Formation Mechanisms and Ecological Effects. Plants, 14(9), 1395. https://doi.org/10.3390/plants14091395





