Genetic Diversity Analysis of Water Lily Germplasms Based on Morphological Traits and SSR Markers
Abstract
1. Introduction
2. Results
2.1. Analysis of Morphological Genetic Diversity in Tropical Water Lily Germplasms
2.1.1. Differences in Plant Morphological Characteristics
2.1.2. Genetic Diversity Analysis of Qualitative Traits
2.1.3. Variant Analysis of the Quantitative Traits
2.2. Comprehensive Evaluation of Morphological Traits of Water Lily Germplasms
2.2.1. Principal Component of Morphological Traits
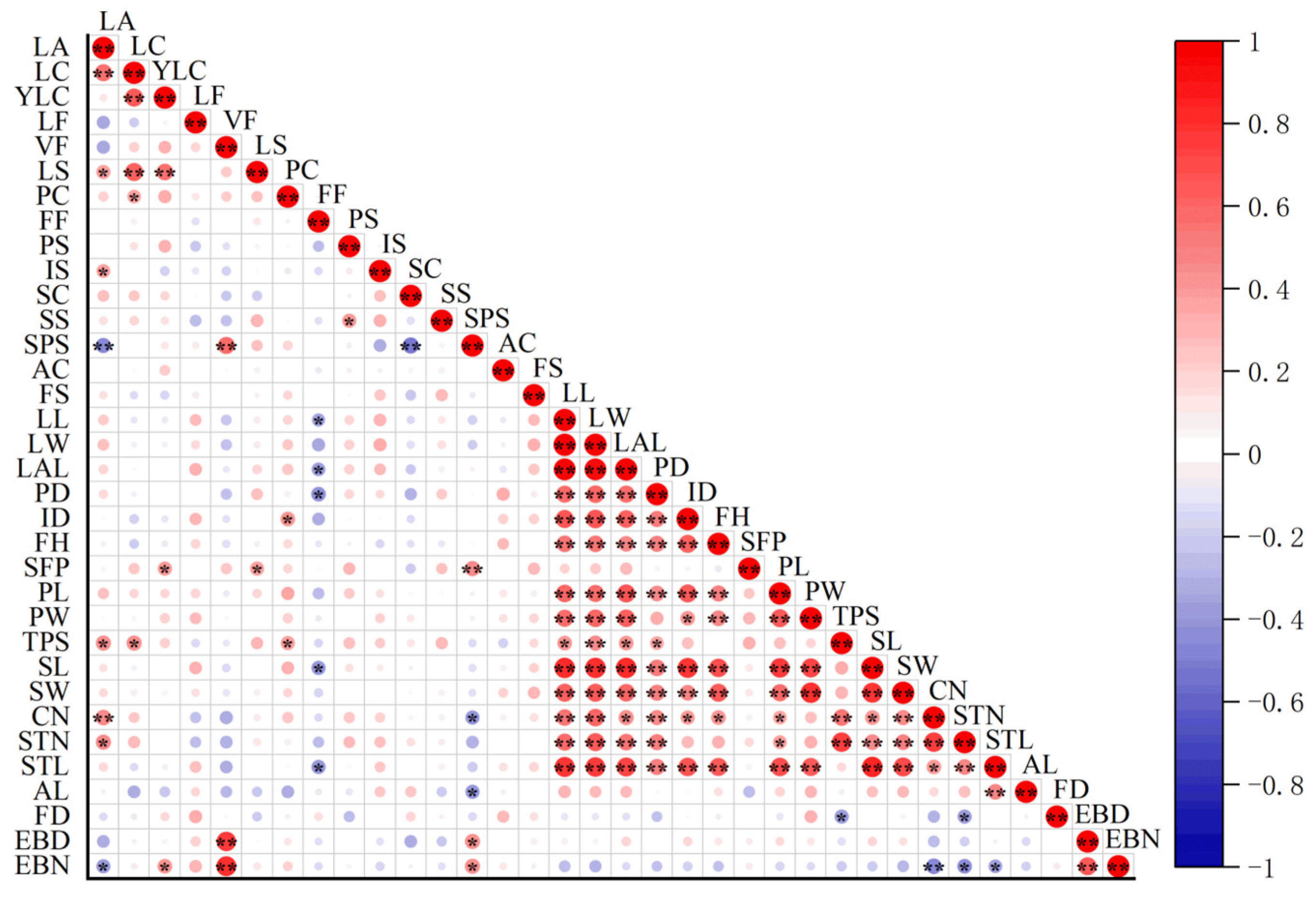
2.2.2. Correlation Analysis of Morphological Traits
2.2.3. Comprehensive Evaluation of Water Lily Germplasms
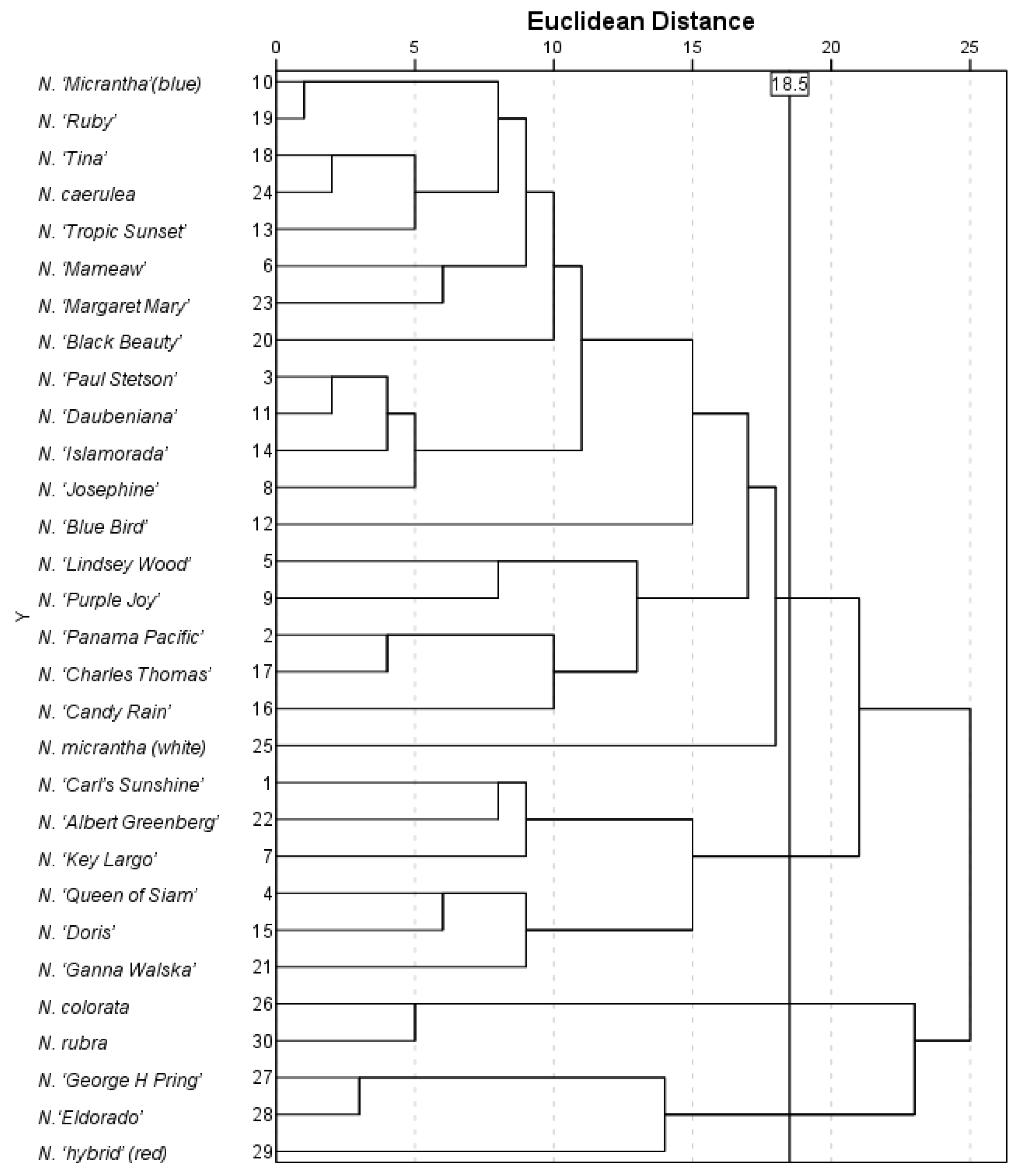
2.2.4. Cluster Analysis of Water Lily Germplasms Based on Morphological Traits
2.3. SSR Genetic Diversity Analysis of Water Lily Germplasms
2.3.1. SSR Polymorphism Analysis
2.3.2. Genetic Similarity Analysis
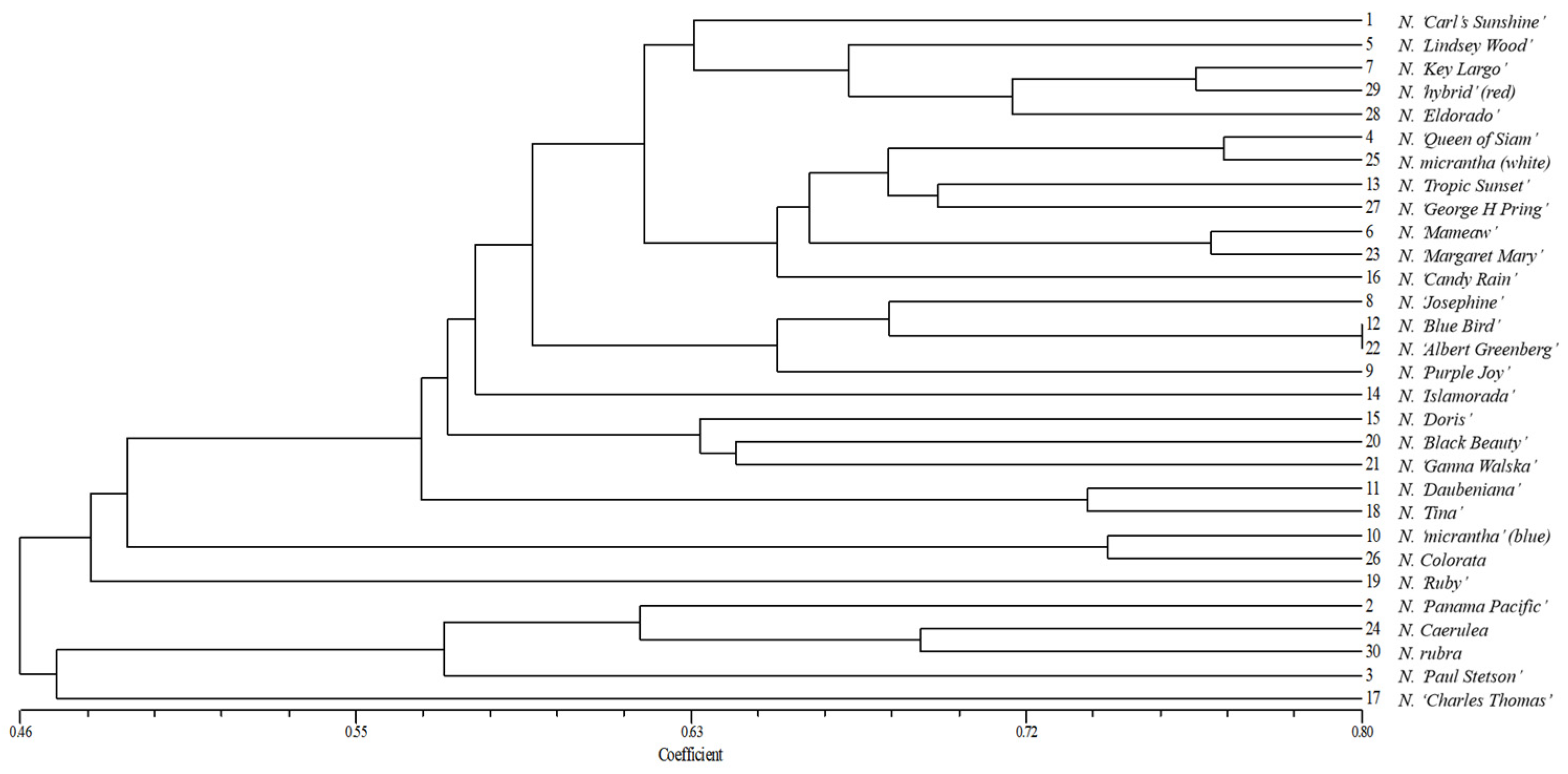
2.3.3. Clustering Analysis of Water Lily Germplasms Based on SSR Markers
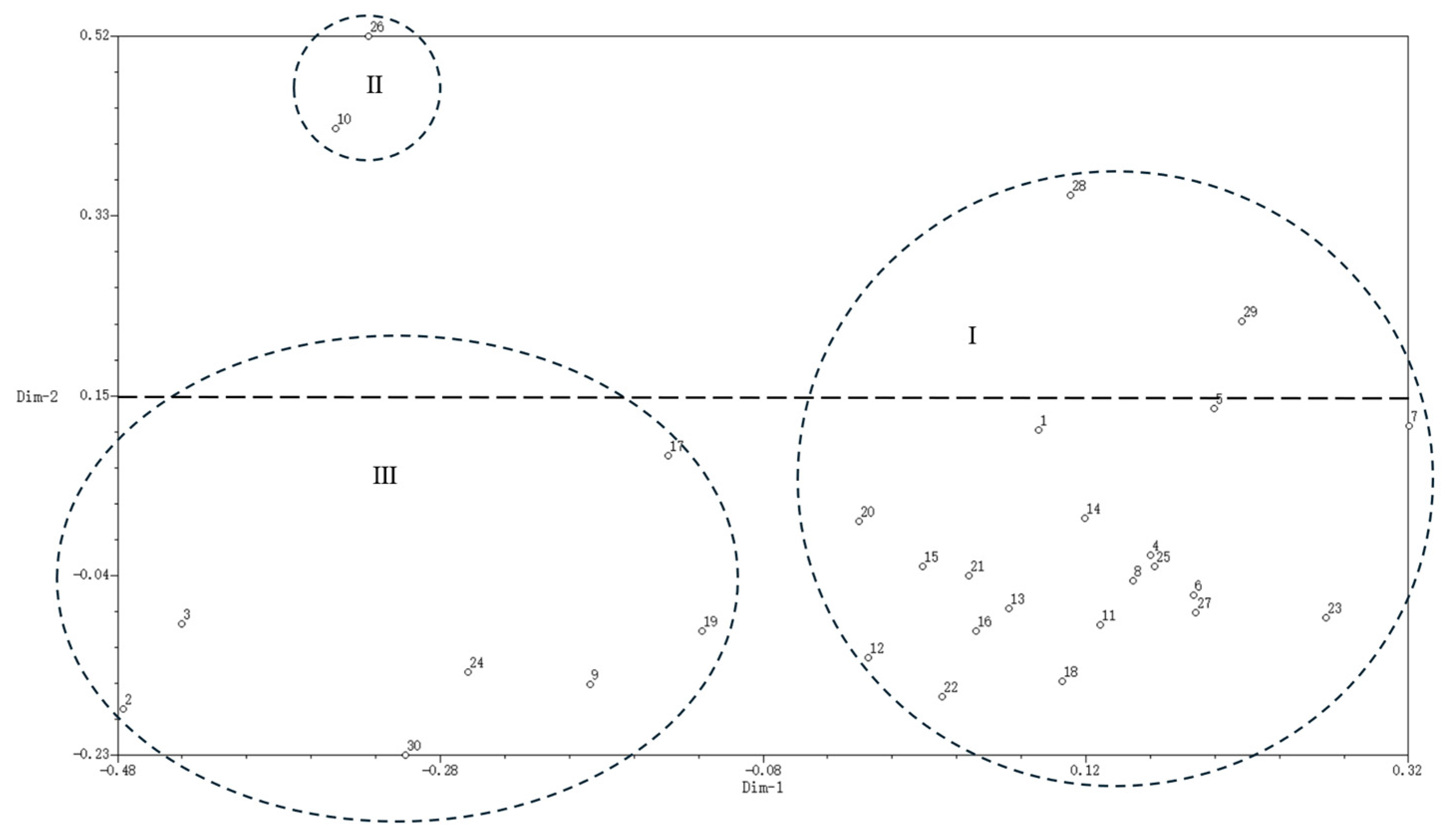
2.3.4. Principal Component Analysis of Water Lily Germplasms Based on SSR Markers
3. Discussion
3.1. Evaluation of Viviparous and Non-Viviparous Water Lily Germplasms Based on Morphological Traits
3.2. Genetic Diversity Analysis of Water Lily Germplasms Based on SSR Molecular Markers
3.3. Cluster Analysis of Water Lily Germplasms Based on Morphological Traits and SSR Molecular Markers
4. Materials and Methods
4.1. Experimental Material
4.2. Experimental Method
4.2.1. Investigation of the Morphological Traits
4.2.2. DNA Extraction and SSR Analysis
4.3. Data Processing and Statistical Analysis
4.3.1. Morphological Analysis
4.3.2. SSR Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, C.; Qiao, G.; Qiu, W.; Yu, D.; Zhou, S.; Shen, Y.; Yu, G.; Jiang, J.; Han, X.; Liu, M.; et al. Molecular breeding of water lily: Engineering cold stress tolerance into tropical water lily. Hortic. Res. 2018, 5, 73. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, T.; Ma, L.; Yan, M.; Gu, Z.; Huang, Y.; Xu, F.; Zhao, Y. Antioxidant and preventive effects of extract from Nymphaea candida flower on in vitro immunological liver injury of rat primary hepatocyte cultures. Evid.-Based Complement. Altern. Med. 2011, 2011, 497673. [Google Scholar] [CrossRef]
- Conard, H.S. The Waterlilies: A Monograph of the Genus Nymphaea; Carnegie Institution of Washington: Washington, DC, USA, 1905; Volume 4. [Google Scholar]
- Xiong, X.H.; Zhang, J.; Yang, Y.Z.; Chen, Y.C.; Su, Q.; Zhao, Y.; Wang, J.; Xia, Z.Q.; Wang, L.S.; Zhang, L.S. Water lily research: Past, present, and future. Trop. Plants 2023, 2, 1. [Google Scholar] [CrossRef]
- Cudalbeanu, M.; Furdui, B.; Cârâc, G.; Barbu, V.; Iancu, A.V.; Marques, F.; Leitão, J.H.; Sousa, S.A.; Dinica, R.M. Antifungal, Antitumoral and Antioxidant Potential of the Danube Delta Nymphaea alba Extracts. Antibiotics 2019, 9, 7. [Google Scholar] [CrossRef]
- Devi, S.A.; Thongam, B.; Handique, P. Nymphaea rubra Roxb. ex Andrews cultivated as an ornamental, food and vegetable in the North Eastern region of India. Genet. Resour. Crop Evol. 2015, 62, 315–320. [Google Scholar] [CrossRef]
- Zhao, Y.; Fan, Y.Y.; Yu, W.G.; Wang, J.; Lu, W.; Song, X.Q. Ultrasound-enhanced subcritical fluid extraction of essential oil from Nymphaea alba var and Its antioxidant activity. J. AOAC Int. 2019, 102, 1448–1454. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Luo, X. Uptake of uranium from aqueous solution by Nymphaea tetragona Georgi: The effect of the accompanying heavy metals. Appl. Radiat. Isot. 2019, 150, 157–163. [Google Scholar] [CrossRef]
- Lu, X.M.; Lu, P.Z.; Chen, J.J. Nitrogen and phosphorus removal and morphological and physiological response in Nymphaea tetragona under various planting densities. Toxicol. Environ. Chem. 2012, 94, 1319–1330. [Google Scholar] [CrossRef]
- Boquete, M.T.; Muyle, A.; Alonso, C. Plant epigenetics: Phenotypic and functional diversity beyond the DNA sequence. Am. J. Bot. 2021, 108, 553–558. [Google Scholar] [CrossRef]
- Volkova, P.A.; Choob, V.V.; Shipunov, A.B. The flower organ transition in water lily (Nymphaea alba sl, Nymphaeaceae) under cross-examination with different morphological approaches. Belg. J. Bot. 2007, 140, 60–72. [Google Scholar]
- Volkova, P.A.; Shipunov, A.B. Morphological variation of Nymphaea (Nymphaeaceae) in European Russia. Nord. J. Bot. 2007, 25, 329–338. [Google Scholar] [CrossRef]
- Dkhar, J.; Kumaria, S.; Rao, S.R.; Tandon, P. New insights into character evolution, hybridization and diversity of Indian Nymphaea (Nymphaeaceae): Evidence from molecular and morphological data. Syst. Biodivers. 2013, 11, 77–86. [Google Scholar] [CrossRef]
- Devi, S.A.; Thongam, B.; Handique, P.J. Multivariate analysis of Nymphaea (Nymphaeaceae) taxa in Manipur (India) through morphological variables. Braz. J. Bot. 2016, 39, 359–366. [Google Scholar] [CrossRef]
- Bai, B.B.; Luo, J.J.; Li, H.; Yu, H.Q.; You, J. Preliminary study on the numerical classification of Nymphaea tetragona cultivars. North. Hortic. 2011, 22, 75–78. [Google Scholar]
- Huang, X.; Yang, M.H.; Chu, G.M.; Zhong, M.; Xu, Y.C.; Wang, Y.J. Analysis of variety resources and evaluation of ornamental value in hardy waterlily. Mol. Plant Breed. 2022, 20, 1348–1357. [Google Scholar] [CrossRef]
- Su, Q.; Yahan, Y.; Tian, M.; Zhang, J.; Mao, L.; Tang, Y.; Bu, Z.; Lu, J. Henotypic diversity analysis and comprehensive evaluation of 49 waterlily resources. Southwest. China J. Agric. Sci. 2019, 32, 2670–2681. [Google Scholar] [CrossRef]
- Pan, Q.L.; Fu, Y.G.; Gu, J.; Sheng, Y.H.; Li, Q.X.; Rao, Y.; Zhu, T.L.; Zhou, Y.; Shi, Y.H.; Zhao, Y.; et al. Analysis of phenotypic diversity of Nymphaea L. in Hainan, China. Chin. J. Trop. Crop 2021, 42, 2777–2788. [Google Scholar] [CrossRef]
- Poczai, P.; Mátyás, K.K.; Szabó, I.; Varga, I.; Hyvönen, J.; Cernák, I.; Gorji, A.M.; Decsi, K.; Taller, J. Genetic variability of thermal Nymphaea (Nymphaeaceae) populations based on ISSR markers: Implications on relationships, hybridization, and conservation. Plant Mol. Biol. Rep. 2011, 29, 906–918. [Google Scholar] [CrossRef]
- Mao, L.Y.; Li, H.M.; Long, L.Y.; Huang, Q.W.; Tang, Y.W.; Yu, Y.P.; Huang, X.Y.; Tan, X.H.; Nong, X.H.; Zhu, T.L.; et al. Analyzing the genetic diversity of Nymphaea spp. based on SSR markers. J. Nanjing For. Univ. (Nat. Sci. Ed.) 2024, 48, 57–68. [Google Scholar]
- Huang, X.; Yang, M.H.; Guo, J.X.; Liu, J.C.; Chu, G.M.; Xu, Y.C. Genome-wide survey and analysis of microsatellites in waterlily, and potential for polymorphic marker development. Genes 2022, 13, 1782. [Google Scholar] [CrossRef]
- Mao, L.Y.; Long, L.Y.; Ding, L.Q.; Li, H.M.; Chi, Z.J.; Tang, Y.W.; Su, Q.; Nong, X.H.; Zhu, T.L. Genetic diversity analysis of 147 Nymphaea Linn. plants based on SRAP molecular marker. J. South. Agric. 2023, 54, 454–466. [Google Scholar] [CrossRef]
- Qian, Z.H.; Munywoki, J.M.; Wang, Q.F.; Malombe, I.; Li, Z.Z.; Chen, J.M. Molecular identification of African Nymphaea species (water lily) based on ITS, trnT-trnF and rpl16. Plants 2022, 11, 2431. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.R.; Fu, C.Y.; Yang, C.Y.; Ma, Q.B.; Pan, D.J.; Nian, H. Genetic diversity of wild soybeans from some regions of southern China based on SSR and SRAP markers. Am. J. Plant Sci. 2013, 4, 257–268. [Google Scholar] [CrossRef]
- Zhu, Y.; Liang, D.; Song, Z.; Tan, Y.; Guo, X.; Wang, D. Genetic diversity analysis and core germplasm collection construction of Camellia oleifera based on fruit phenotype and SSR data. Genes 2022, 13, 2351. [Google Scholar] [CrossRef]
- Ali, M.; Rajewski, J.; Baenziger, P.; Gill, K.; Eskridge, K.; Dweikat, I. Assessment of genetic diversity and relationship among a collection of US sweet sorghum germplasm by SSR markers. Mol. Breed. 2008, 21, 497–509. [Google Scholar] [CrossRef]
- Yoshida, T. Genetic diversity among Japanese cultivated sorghum assessed with simple sequence repeats markers. Plant Prod. Sci. 2004, 7, 217–223. [Google Scholar] [CrossRef]
- Chalbi, A.; Chikh-Rouhou, H.; Mezghani, N.; Slim, A.; Fayos, O.; Bel-Kadhi, M.S.; Garces-Claver, A. Genetic diversity analysis of Onion (Allium cepa L.) from the Arid region of Tunisia using phenotypic traits and SSR markers. Horticulturae 2023, 9, 1098. [Google Scholar] [CrossRef]
- Li, S.; Ji, F.F.; Hou, F.; Shi, Q.Q.; Xing, G.M.; Chen, H.; Weng, Y.Q.; Kang, X.P. Morphological, palynological and molecular assessment of Hemerocallis core collection. Sci. Hortic. 2021, 285, 110181. [Google Scholar] [CrossRef]
- Huang, G.Z.; Deng, H.Q.; Li, Z.X.; Li, J. Nymphaea; Chinese Forestry Publishing House: Beijing, China, 2009; pp. 1–10. [Google Scholar]
- Xie, H.; Ai, X.M.; Li, Y.H.; Zhao, C.B.; Sun, Y.Y. Relationship between epiphyllous bud of tropical waterlily (Brachyceras) umbilics and carbohydrate meta-bolism in different parts of leaves. Chin. J. Appl. Ecol. 2022, 33, 2431–2440. [Google Scholar] [CrossRef]
- Sun, J.; Shimizu-Inatsugi, R.; Hofhuis, H.; Shimizu, K.; Hay, A.; Shimizu, K.K.; Sese, J. A recently formed Triploid Cardamine insueta inherits leaf vivipary and submergence tolerance traits of Parents. Front. Genet. 2020, 11, 567262. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, X.M.; Wan, M. Effects of Age on Leaf Growth and Epiphyllous Bud Degeneration in Tropical Viviparous Water Lily of Nymphaea ‘Black Beauty’. J. Nanjing Agric. Univ. 2025, in press. [Google Scholar]
- Zhang, H.P.; Fang, W.M.; Chen, F.D.; Ding, Y.S.; Cui, N.X.; Gu, J.J. Investigation on the morphological diversity of taxa in genus Nymphaea. J. Nanjing Agric. Univ. 2009, 32, 47–52. [Google Scholar]
- He, Z.; Liu, C.; Wang, X.; Wang, R.; Chen, Y.; Tian, Y. Assessment of genetic diversity in Camellia oleifera Abel. accessions using morphological traits and simple sequence repeat (SSR) markers. Breed. Sci. 2020, 70, 586–593. [Google Scholar] [CrossRef]
- Tian, W.; Li, Z.C.; Wang, L.; Sun, S.M.; Wang, D.J.; Wang, K.; Wang, G.Y.; Liu, Z.; Lu, X.M.; Feng, J.R.; et al. Comprehensive evaluation of apple germplasm genetic diversity on the basis of 26 phenotypic traits. Agronomy 2024, 14, 1264. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, Y.F.; Huo, H.H.; Xu, J.Y.; Tian, L.M.; Dong, X.G.; Qi, D.; Liu, C. An assessment of the genetic diversity of pear (Pyrus L.) germplasm resources based on the fruit phenotypic traits. J. Integr. Agric. 2022, 21, 2275–2290. [Google Scholar] [CrossRef]
- Hu, M.; Tian, H.; Yang, K.; Ding, S.; Hao, Y.; Xu, R.; Zhang, F.; Liu, H.; Zhang, D. Comprehensive evaluation and selection of 192 Maize accessions from different sources. Plants 2024, 13, 1397. [Google Scholar] [CrossRef]
- Herrera-González, M.P.; Zamora-Jerez, A.; Cifuentes-Velasquez, R.; Arévalo-Rodríguez, L.A.; Pereira-Lorenzo, S. Comprehensive evaluation and selection of cardamom (Elettaria cardamomum (L.) Maton) germplasm using morphological traits. Plants 2024, 13, 2786. [Google Scholar] [CrossRef]
- Liu, D.L.; Wang, X.Q.; Li, W.S.; Li, J.J.; Tan, W.B.; Xing, W. Genetic diversity analysis of the phenotypic traits of 215 sugar beet germplasm resources. Sugar Tech 2022, 24, 1790–1800. [Google Scholar] [CrossRef]
- Chen, F.; Liu, X.; Yu, C.; Chen, Y.; Tang, H.; Zhang, L. Water lilies as emerging models for Darwin’s abominable mystery. Hortic. Res. 2017, 4, 17051. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Zhang, X.; Li, Z.; Zhao, Y.; Lohaus, R.; Chang, X.; Dong, W.; Ho, S.Y.W.; Liu, X.; et al. The water lily genome and the early evolution of flowering plants. Nature 2020, 577, 79–84. [Google Scholar] [CrossRef]
- Su, Q.; Wang, H.; Liu, J.; Li, C.; Bu, Z.; Lin, Y.; Lu, J.; Lai, Z. Construction of core collection of Nymphaea based on SSR fluorescent markers. Acta Hortic. Sin. 2023, 50, 2128–2138. [Google Scholar] [CrossRef]
- Wilson, A.C.; Carlson, S.S.; White, T.J. Biochemical evolution. Annu. Rev. Biochem. 1977, 46, 573–639. [Google Scholar] [CrossRef]
- Lekgari, A.; Dweikat, I. Assessment of genetic variability of 142 sweet sorghum germplasm of diverse origin with molecular and morphological markers. Open J. Ecol. 2014, 4, 371–393. [Google Scholar] [CrossRef]
- Manco, R.; Basile, B.; Capuozzo, C.; Scognamiglio, P.; Forlani, M.; Rao, R.; Corrado, G. Molecular and phenotypic diversity of traditional European plum (Prunus domestica L.) germplasm of Southern Italy. Sustainability 2019, 11, 4112. [Google Scholar] [CrossRef]
- Hong, Y.B.; Pandey, M.K.; Lu, Q.; Liu, H.; Gangurde, S.S.; Li, S.X.; Liu, H.Y.; Li, H.F.; Liang, X.Q.; Varshney, R.K. Genetic diversity and distinctness based on morphological and SSR markers in peanut. Agron. J. 2021, 113, 4648–4660. [Google Scholar] [CrossRef]
- Maccaferri, M.; Stefanelli, S.; Rotondo, F.; Tuberosa, R.; Sanguineti, M.C. Relationships among durum wheat accessions. I. Comparative analysis of SSR, AFLP, and phenotypic data. Genome 2007, 50, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Petrović, S.; Marić, S.; Čupić, T.; Rebekić, A.; Rukavina, I. Assessment of molecular and phenotypic diversity among winter wheat cultivars. Genetika 2017, 49, 583–598. [Google Scholar] [CrossRef]
- Ben-Ayed, R.; Sans-Grout, C.; Moreau, F.; Grati-Kamoun, N.; Rebai, A. Genetic similarity among Tunisian olive cultivars and two unknown feral olive trees estimated through SSR markers. Biochem. Genet. 2014, 52, 258–268. [Google Scholar] [CrossRef]
- Sonnante, G.; Carluccio, A.V.; De Paolis, A.; Pignone, D. Identification of artichoke SSR markers: Molecular variation and patterns of diversity in genetically cohesive taxa and wild allies. Genet. Resour. Crop Evol. 2008, 55, 1029–1046. [Google Scholar] [CrossRef]
- Kotchoni, S.O.; Gachomo, E.W. A rapid and hazardous reagent free protocol for genomic DNA extraction suitable for genetic studies in plants. Mol. Biol. Rep. 2009, 36, 1633–1636. [Google Scholar] [CrossRef]
- Su, Q.; Tian, M.; Liu, J.; Wang, L.; Li, C.; Li, X.; Huang, Z.; Wang, h. SSR loci characteristic analysis of water lily based on bio-informatics methodology. Southwest. China J. Agric. Sci. 2021, 34, 2076–2083. [Google Scholar] [CrossRef]
- Yang, Y.C.; Tan, Z.M.; Liang, S.; Cheng, W.; Sun, Y.H.; Cheng, Y.X.; Song, Y.; Wang, Y.M.; Wu, J.L.; Wang, Q. Genetic diversity analysis and comprehensive evaluation of “M82” in EMS-mutagenized tomato. Genes 2025, 16, 179. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Feng, M.S.; Li, X.; Huang, X.Y.; Chen, G.; Bai, W.Y.; Xu, X.J.; Li, J.Y.; Li, X.H.; Leng, B. Phenotypic variation analysis and excellent clone selection of Alnus cremastogyne from different provenances. Plants 2023, 12, 3259. [Google Scholar] [CrossRef]
- Tang, G.M.; Li, W.D.; Zhou, Y.X.; Kong, Y.H.; Xiao, X.l.; Peng, Y.S.; Zhang, L.; Fu, H.Y.; Liu, Y.; Huang, G.L. Genetic diversity analysis of Cymbidium faberi germplasm resources based on phenotypic traits. Sci. Agric. Sin. 2025, 58, 339–354. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, W.M.; Yue, Z.H.; Gong, B.C.; Yang, X.; Wu, K.Y.; Liu, C.Y.; Xu, Y. Phenotypic diversity and relationships of fruit traits in persimmon (Diospyros kaki Thunb.) germplasm resources. Agriculture 2023, 13, 1804. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, F.; Tang, X.H.; Yang, Y.X.; Zhou, T.; Liu, H.Y. Morphology and SSR markers-based genetic diversity analysis of sesame (Sesamum indicum L.) cultivars released in China. Agriculture 2023, 13, 1885. [Google Scholar] [CrossRef]
- Wang, Z.H.; Peng, H.; Yue, C.N.; Ye, C.; Li, W.J.; Yang, P.X. Molecular markers and phenotypic identification reveal the genetic diversity and structure of four local tea plant populations in China. Genet. Resour. Crop Evol. 2024, 71, 635–649. [Google Scholar] [CrossRef]
- Anderson, J.A.; Churchill, G.; Autrique, J.; Tanksley, S.; Sorrells, M. Optimizing parental selection for genetic linkage maps. Genome 1993, 36, 181–186. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, Y.Y.; Xu, W.L.; Wang, C.J.; Cui, C.S.; Qu, S.P. Genetic diversity of pumpkin based on morphological and SSR markers. Pak. J. Bot. 2020, 52, 477–487. [Google Scholar] [CrossRef]
- Peng, L.; Ru, M.; Wang, B.Q.; Wang, Y.; Li, B.; Yu, J.; Liang, Z.S. Genetic diversity assessment of a germplasm collection of Salvia miltiorrhiza Bunge. based on morphology, ISSR and SRAP markers. Biochem. Syst. Ecol. 2014, 55, 84–92. [Google Scholar] [CrossRef]
- Cheng, L.T.; Sun, X.Y.; Xie, F.C.; Song, H.; Jiang, J.; Zhu, H.S.; Bai, X.M.; Chen, Y.J. Genetic diversity analysis and molecular ID construction of kentucky bluegrass based on phenotypic traits and SSR molecular markers. Chin. J. Grassl. 2022, 44, 1–10. [Google Scholar] [CrossRef]
- Marić, S.; Bolarić, S.; Martinčić, J.; Pejić, I.; Kozumplik, V. Genetic diversity of hexaploid wheat cultivars estimated by RAPD markers, morphological traits and coefficients of parentage. Plant Breed. 2004, 123, 366–369. [Google Scholar] [CrossRef]


| No. | Traits | Frequency Distribution (%) | Genetic Diversity Index (H’) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| 1 | Leaf auricle | 53.33 | 6.67 | 40.00 | -- | -- | -- | 0.888 |
| 2 | Leaf color | 80.00 | 3.33 | 16.67 | -- | -- | -- | 0.585 |
| 3 | Young leaf color | 46.67 | 26.67 | 26.67 | -- | -- | -- | 1.065 |
| 4 | Leaf form | 30.00 | 30.00 | 23.33 | 16.67 | -- | -- | 1.366 |
| 5 | Viviparity of leaf | 16.67 | 83.33 | -- | -- | -- | -- | 0.456 |
| 6 | Leaf spot | 60.00 | 16.67 | 23.33 | -- | -- | -- | 0.950 |
| 7 | Petal color | 10.00 | 20.00 | 13.33 | 33.33 | 6.67 | 16.67 | 1.681 |
| 8 | Flower fragrance | 36.67 | 40.00 | 23.33 | -- | -- | -- | 1.062 |
| 9 | Petal shape | 46.67 | 30.00 | 23.33 | -- | -- | -- | 1.072 |
| 10 | Inflorescence shape | 40.00 | 60.00 | -- | -- | -- | -- | 0.673 |
| 11 | Sepal color | 20.00 | 80.00 | -- | -- | -- | -- | 0.500 |
| 12 | Sepal shape | 43.33 | 6.67 | 50.00 | -- | -- | -- | 0.901 |
| 13 | Sepal and petal spot | 30.00 | 56.67 | 13.33 | -- | -- | -- | 0.957 |
| 14 | Anther color | 6.67 | 26.67 | 6.67 | 46.67 | 13.33 | -- | 1.346 |
| 15 | Fruit shape | 13.33 | 53.33 | 33.33 | -- | -- | -- | 0.969 |
| No. | Traits | Mean | Max | Min | SD | CV (%) | Genetic Diversity Index (H’) |
|---|---|---|---|---|---|---|---|
| 1 | Leaf length/cm | 18.43 | 26.19 | 10.46 | 4.34 | 23.54 | 1.916 |
| 2 | Leaf width/cm | 16.13 | 21.91 | 9.35 | 3.79 | 23.52 | 1.972 |
| 3 | Leaf auricle length/cm | 8.23 | 11.19 | 4.98 | 1.96 | 23.85 | 1.868 |
| 4 | Petiole diameter/cm | 0.50 | 0.71 | 0.29 | 0.12 | 23.67 | 2.007 |
| 5 | Inflorescence diameter/cm | 8.07 | 11.83 | 4.60 | 1.57 | 19.43 | 1.532 |
| 6 | Flower height/cm | 9.00 | 16.46 | 5.35 | 3.05 | 33.84 | 1.846 |
| 7 | Single flowering period/d | 5.18 | 7.60 | 3.50 | 1.02 | 19.76 | 1.949 |
| 8 | Petal length/cm | 4.50 | 6.29 | 2.90 | 0.80 | 17.73 | 1.844 |
| 9 | Petal width/cm | 1.21 | 1.67 | 0.70 | 0.23 | 18.92 | 1.996 |
| 10 | Sepal length/cm | 19.72 | 33.30 | 12.10 | 4.82 | 24.43 | 1.932 |
| 11 | Sepal width/cm | 4.88 | 6.95 | 3.00 | 0.83 | 16.9 | 1.963 |
| 12 | Total number of petals | 1.87 | 2.64 | 1.10 | 0.37 | 19.9 | 1.775 |
| 13 | Number of carpels | 18.22 | 22.90 | 14.40 | 2.21 | 12.11 | 1.913 |
| 14 | Number of stamens | 111.26 | 164.10 | 73.60 | 27.33 | 24.56 | 1.762 |
| 15 | Stamen length/cm | 1.83 | 2.31 | 1.35 | 0.25 | 13.71 | 1.924 |
| 16 | Anther length/cm | 0.87 | 1.16 | 0.53 | 0.16 | 18.48 | 1.950 |
| 17 | Flower density/20d | 4.47 | 7.93 | 2.54 | 1.23 | 27.51 | 2.024 |
| 18 | Diameter of epiphyllous buds/cm | 0.56 | 1.02 | 0.00 | 0.33 | 58.88 | 1.710 |
| 19 | Number of epiphyllous buds/cm | 2.28 | 4.60 | 0.00 | 1.29 | 56.56 | 1.629 |
| Average | -- | -- | -- | 2.93 | 25.12 | 1.870 | |
| Traits | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 |
|---|---|---|---|---|---|---|---|
| Leaf auricle | 0.117 | −0.303 | 0.198 | 0.173 | 0.021 | −0.123 | −0.182 |
| Leaf color | 0.016 | −0.079 | 0.414 | 0.246 | −0.104 | −0.040 | −0.006 |
| Young leaf color | 0.003 | 0.126 | 0.360 | 0.274 | −0.064 | 0.198 | 0.026 |
| Leaf form | 0.045 | 0.271 | −0.153 | 0.213 | 0.089 | −0.027 | 0.174 |
| Leaf viviparity | −0.114 | 0.344 | 0.166 | 0.028 | −0.083 | −0.050 | 0.207 |
| Leaf spot | 0.048 | 0.032 | 0.369 | 0.018 | 0.061 | −0.062 | −0.130 |
| Petal color | 0.094 | 0.126 | 0.220 | 0.116 | −0.117 | −0.247 | −0.052 |
| Flower fragrance | −0.130 | −0.028 | 0.053 | 0.136 | 0.120 | −0.478 | −0.033 |
| Petal shape | 0.087 | −0.083 | 0.153 | −0.186 | −0.058 | 0.411 | 0.144 |
| Inflorescence shape | 0.101 | −0.218 | −0.015 | 0.056 | 0.341 | 0.196 | 0.137 |
| Sepal color | −0.009 | −0.194 | −0.020 | 0.472 | −0.123 | 0.152 | 0.158 |
| Sepal shape | 0.058 | −0.084 | 0.202 | −0.190 | 0.413 | 0.230 | −0.113 |
| Sepal and petal spot | −0.083 | 0.319 | 0.143 | −0.262 | 0.041 | −0.067 | −0.078 |
| Anther color | 0.030 | 0.123 | 0.044 | 0.147 | −0.026 | 0.321 | −0.526 |
| Fruit shape | 0.089 | 0.037 | −0.014 | −0.049 | 0.493 | −0.185 | 0.003 |
| Leaf length/cm | 0.299 | 0.036 | −0.057 | −0.065 | 0.074 | −0.029 | 0.141 |
| Leaf width/cm | 0.303 | 0.014 | −0.035 | −0.066 | 0.090 | −0.036 | 0.108 |
| Leaf auricle length/cm | 0.290 | 0.093 | −0.015 | −0.070 | 0.073 | −0.014 | 0.184 |
| Petiole diameter/cm | 0.231 | 0.053 | 0.057 | −0.226 | −0.066 | 0.174 | −0.189 |
| Inflorescence diameter/cm | 0.238 | 0.143 | −0.078 | −0.048 | −0.068 | −0.063 | −0.199 |
| Flower height/cm | 0.208 | 0.154 | −0.128 | −0.018 | −0.242 | −0.102 | −0.243 |
| Single flowering period/d | 0.068 | 0.120 | 0.274 | −0.066 | 0.315 | −0.035 | 0.085 |
| Petal length/cm | 0.246 | 0.104 | 0.048 | 0.110 | −0.076 | 0.029 | −0.038 |
| Petal width/cm | 0.225 | 0.155 | −0.022 | 0.267 | 0.142 | −0.007 | 0.073 |
| Total number of petals | 0.292 | 0.129 | −0.073 | 0.063 | −0.044 | −0.008 | −0.002 |
| Sepal length/cm | 0.255 | 0.113 | −0.017 | 0.121 | 0.001 | −0.097 | −0.057 |
| Width of sepal/cm | 0.172 | −0.127 | 0.270 | −0.062 | −0.049 | −0.122 | 0.270 |
| Number of carpels | 0.223 | −0.176 | 0.098 | −0.034 | −0.171 | −0.094 | −0.034 |
| Number of stamens | 0.234 | −0.185 | 0.115 | −0.056 | −0.184 | −0.004 | 0.164 |
| Stamen length/cm | 0.280 | 0.027 | −0.147 | 0.074 | 0.027 | 0.082 | −0.103 |
| Anther length/cm | 0.100 | −0.095 | −0.273 | 0.145 | −0.044 | 0.103 | 0.250 |
| Flower density/20d | −0.047 | 0.128 | −0.117 | 0.398 | 0.341 | 0.068 | −0.158 |
| Diameter of epiphyllous buds/cm | −0.006 | 0.367 | 0.009 | −0.073 | −0.080 | 0.025 | 0.178 |
| Number of epiphyllous buds | −0.135 | 0.313 | 0.120 | 0.085 | −0.053 | 0.265 | 0.210 |
| Eigenvalue | 9.501 | 4.238 | 3.979 | 2.350 | 1.829 | 1.751 | 1.565 |
| Contribution rate/% | 27.943 | 12.465 | 11.703 | 6.911 | 5.380 | 5.151 | 4.603 |
| Cumulative contribution rate/% | 27.943 | 40.408 | 52.111 | 59.022 | 64.403 | 69.554 | 74.157 |
| Germplasm | Principal Component Value | F-Value | Ranking | ||||||
|---|---|---|---|---|---|---|---|---|---|
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | |||
| N. ‘Purple Joy’ | 5.26 | 3.99 | 1.49 | −2.30 | 0.42 | −0.43 | −1.21 | 2.60 | 1 |
| N. ‘Key Largo’ | 3.95 | 1.92 | 0.27 | 1.71 | 0.95 | 0.57 | 0.80 | 2.17 | 2 |
| N. ‘Eldorado’ | 6.62 | −2.06 | −1.36 | −0.38 | 0.44 | −0.68 | −0.02 | 1.88 | 3 |
| N. ‘Carl’s Sunshine’ | 1.91 | −0.15 | 3.75 | 1.96 | 2.46 | −1.46 | 1.58 | 1.64 | 4 |
| N. ‘Albert Greenberg’ | 2.92 | −1.15 | 3.04 | 0.70 | −0.68 | 1.12 | 0.80 | 1.53 | 5 |
| N. ‘Blue Bird’ | 4.88 | −0.82 | −1.07 | 0.44 | −1.37 | −2.22 | 0.51 | 1.35 | 6 |
| N. ‘Panama Pacific’ | 2.50 | 2.32 | −0.17 | −2.27 | 1.70 | −0.25 | −0.48 | 1.17 | 7 |
| N. ‘Tropic Sunset’ | 1.18 | 0.66 | 1.42 | 1.15 | −0.32 | −0.58 | 1.73 | 0.93 | 8 |
| N. ‘Ruby’ | 1.82 | 1.26 | −2.17 | 2.06 | 0.82 | 0.99 | 0.87 | 0.93 | 9 |
| N. ‘Lindsey Wood’ | 0.09 | 2.74 | 2.06 | −2.70 | −0.18 | 1.25 | 0.02 | 0.64 | 10 |
| N. ‘hybrid’ (red) | 3.65 | −3.64 | −1.84 | −0.22 | −2.20 | 1.03 | −1.59 | 0.27 | 11 |
| N. ‘Queen of Siam’ | −0.44 | −1.07 | 3.89 | 0.03 | −0.58 | 1.23 | −0.87 | 0.26 | 12 |
| N. ‘Margaret Mary’ | −0.40 | 2.07 | −1.57 | 0.34 | 0.77 | 2.40 | 0.46 | 0.23 | 13 |
| N. ‘Ganna Walska’ | −0.31 | 0.82 | 2.53 | 1.72 | −2.87 | −1.12 | −1.64 | 0.19 | 14 |
| N. ‘Micrantha’(blue) | −1.19 | 2.26 | −0.53 | 1.36 | 1.09 | −0.98 | 0.28 | 0.00 | 15 |
| N. ‘George H Pring’ | 2.17 | −4.71 | −1.47 | −1.73 | 0.23 | 0.98 | 1.15 | −0.21 | 16 |
| N. caerulea | 0.04 | 0.25 | −1.13 | −0.80 | −1.45 | −0.64 | 1.26 | −0.27 | 17 |
| N. ‘Doris’ | −2.43 | −1.15 | 2.94 | 1.64 | −0.36 | 0.48 | −1.32 | −0.57 | 18 |
| N. rubra | 0.17 | −2.24 | −1.11 | 0.43 | −0.22 | 0.27 | −2.11 | −0.58 | 19 |
| N. ‘Black Beauty’ | −2.50 | 1.18 | −0.57 | 1.51 | −1.25 | 2.06 | 0.29 | −0.62 | 20 |
| N. ‘Tina’ | −1.74 | 0.20 | −0.99 | 0.12 | 0.09 | 0.88 | 1.07 | −0.64 | 21 |
| N. micrantha (white) | −1.18 | 1.32 | −1.91 | −0.99 | −0.71 | −2.12 | 0.83 | −0.76 | 22 |
| N. ‘Paul Stetson’ | −1.99 | −0.15 | −1.68 | 0.62 | −0.08 | 0.16 | 0.65 | −0.94 | 23 |
| N. ‘Mameaw’ | −2.71 | −0.69 | −0.35 | −1.52 | 0.24 | 1.18 | 0.76 | −1.19 | 24 |
| N. ‘Charles Thomas’ | −2.74 | −0.66 | 1.22 | −2.88 | 1.75 | −0.43 | −2.29 | −1.26 | 25 |
| N. colorata | −1.80 | −3.25 | −1.68 | 1.91 | 3.09 | −1.01 | −2.36 | −1.30 | 26 |
| N. ‘Candy Rain’ | −3.17 | −0.28 | −0.85 | −2.31 | −0.22 | −1.16 | 1.30 | −1.61 | 27 |
| N. ‘Islamorada’ | −4.38 | 1.31 | −0.29 | 0.10 | −1.47 | 0.12 | −1.02 | −1.63 | 28 |
| N. ‘Daubeniana’ | −4.11 | 0.45 | −1.59 | 0.28 | −1.71 | −2.16 | −0.18 | −1.98 | 29 |
| N. ‘Josephine’ | −6.06 | −0.70 | −0.29 | 0.03 | 1.63 | 0.52 | 0.77 | −2.24 | 30 |
| Primer | Repeat Motif | Primer Sequence (5′-3′) | Tm/°C | Na | Ne | H | I | Ho | He | PIC |
|---|---|---|---|---|---|---|---|---|---|---|
| NtG006 | (GAA)5 | F:GGTCTTGCAGACGTCCAAGA R:GATCATCGTCGGCGTCTTCT | 58.5 | 9 | 7.08 | 0.43 | 1.75 | 0.05 | 0.45 | 0.82 |
| NtG011 | (AGG)6 | F:CTCTCATGGCCGACTCATCC R:CGTCTCGTCGACATCAGGAG | 58 | 4 | 2.01 | 0.42 | 1.03 | 0.12 | 0.56 | 0.66 |
| NtG012 | (AGG)5 | F:CGGTTGGGTGAAGATCGGAA R:CGCCGAACTGAAAGACGAAC | 59 | 4 | 2.11 | 0.29 | 1.23 | 0.23 | 0.75 | 0.69 |
| NtG014 | (CTC)7 | F:GGAGACCCAAATGGCCGATT R:CGATCCTTCGTCCTCCGATG | 58.5 | 10 | 7.48 | 0.29 | 1.86 | 0.32 | 0.49 | 0.90 |
| NtG036 | (AGA)5 | F:AGGCCAGAATGCTGTGTTGT R:TGGACATGGATCAGGTGCTT | 61 | 6 | 4.59 | 0.20 | 1.45 | 0.35 | 0.75 | 0.79 |
| NtG042 | (CCT)6 | F:CGCAAAGAGGGAACAATGGC R:CTGTTGCATGCCGGTTATCG | 58.5 | 6 | 4.65 | 0.39 | 1.32 | 0.22 | 0.53 | 0.75 |
| NtG051 | (CCA)6 | F:GAACATGCCTCCACCCATCA R:AGGGAGTTGATGAACAGCGG | 58 | 8 | 6.88 | 0.31 | 1.67 | 0.08 | 0.71 | 0.85 |
| NtG071 | (ATG)8 | F:ATCGCAGATCGGCAGAAGAG R:TTTGCTTGCGTCTCCTCCTT | 58.5 | 7 | 6.01 | 0.37 | 1.87 | 0.12 | 0.62 | 0.79 |
| NtG106 | (TCC)7 | F:AGCAGAACTCAACTCACCGG R:GACCTGCTGGACTTGTCGAA | 58 | 6 | 5.23 | 0.36 | 1.65 | 0.32 | 0.76 | 0.76 |
| NtG107 | (CTT)5 | F:CAGAGACTTACCGCGCTAGG R:GCAGGCAGTTGCGATAGTGA | 58 | 4 | 2.01 | 0.47 | 1.14 | 0.42 | 0.57 | 0.74 |
| NtG111 | (ATA)25 | F:ATTCGAGTGATGGCATGCCT R:AGCCAGCTCGAAGTGACAAA | 58 | 5 | 3.28 | 0.27 | 1.35 | 0.14 | 0.73 | 0.57 |
| NtG115 | (GGT)5 | F:GCAGCAAGTGGTCTCTGTCT R:CTGCTGCTCTGACACCATGA | 61 | 10 | 7.59 | 0.26 | 2.12 | 0.41 | 0.81 | 0.90 |
| NtG146 | (TTA)8 | F:GCCACACCAGCCCACTAAAT R:ATTGAACAGTGGTGGAGCCA | 58 | 7 | 6.56 | 0.30 | 1.85 | 0.25 | 0.95 | 0.75 |
| NtG152 | (GTC)5 | F:GTCCATGTAGTCGTCCAGCC R:GGAAGCGTCGATCAGTGGAT | 61.5 | 9 | 7.56 | 0.30 | 1.87 | 0.43 | 0.81 | 0.85 |
| NtG156 | (TCA)5 | F:ATTTCTCCAACAGCAGGCCA R:CTTACAGGACGTGGGTAGGC | 58 | 9 | 7.86 | 0.24 | 1.95 | 0.22 | 0.62 | 0.80 |
| NtG188 | (AGA)5 | F:ACATGGACGCCAAGCAACTA R:GGAAGCACAAACAGGATGGC | 59 | 10 | 8.02 | 0.31 | 2.25 | 0.32 | 0.75 | 0.86 |
| Mean | 7.19 | 5.56 | 0.33 | 1.65 | 0.25 | 0.68 | 0.78 | |||
| No. | Latin Name of Variety | Attribute | Source | Type |
|---|---|---|---|---|
| 1 | N. ‘Carl’s Sunshine’ | horticultural species | Nanning, Guangxi | V |
| 2 | N. ‘Panama Pacific’ | horticultural species | Nanjing, Jiangsu | V |
| 3 | N. ‘Paul Stetson | horticultural species | Nanjing, Jiangsu | V |
| 4 | N. ‘Queen of Siam’ | horticultural species | Haikou, Hainan | V |
| 5 | N. ‘Lindsey Wood’ | horticultural species | Nanning, Guangxi | V |
| 6 | N. ‘Mameaw’ | horticultural species | Nanning, Guangxi | V |
| 7 | N. ‘Key Largo’ | horticultural species | Haikou, Hainan | V |
| 8 | N. ‘Josephine’ | horticultural species | Haikou, Hainan | V |
| 9 | N. ‘Purple Joy’ | horticultural species | Haikou, Hainan | V |
| 10 | N. ‘micrantha’ (blue) | horticultural species | Nanjing, Jiangsu | V |
| 11 | N. ‘Daubeniana’ | horticultural species | Kunming, Yunnan | V |
| 12 | N. ‘Blue Bird’ | horticultural species | Haikou, Hainan | V |
| 13 | N. ‘Tropic Sunset’ | horticultural species | Haikou, Hainan | V |
| 14 | N. ‘Islamorada’ | horticultural species | Kunming, Yunnan | V |
| 15 | N. ‘Doris’ | horticultural species | Haikou, Hainan | V |
| 16 | N. ‘Candy Rain’ | horticultural species | Haikou, Hainan | V |
| 17 | N. ‘Charles Thomas’ | horticultural species | Nanjing, Jiangsu | V |
| 18 | N. ‘Tina’ | horticultural species | Haikou, Hainan | V |
| 19 | N. ‘Ruby’ | horticultural species | Nanjing, Jiangsu | V |
| 20 | N. ‘Black Beauty’ | horticultural species | Haikou, Hainan | V |
| 21 | N. ‘Ganna Walska’ | horticultural species | Haikou, Hainan | V |
| 22 | N. ‘Albert Greenberg’ | horticultural species | Haikou, Hainan | V |
| 23 | N. ‘Margaret Mary’ | horticultural species | Kunming, Yunnan | V |
| 24 | N. caerulea | initial species | Nanjing, Jiangsu | V |
| 25 | N. micrantha (white) | initial species | Haikou, Hainan | V |
| 26 | N. colorata | horticultural species | Nanjing, Jiangsu | NV |
| 27 | N. ‘George H Pring’ | horticultural species | Haikou, Hainan | NV |
| 28 | N. ‘Eldorado’ | horticultural species | Nanning, Guangxi | NV |
| 29 | N. ‘hybrid’ (red) | horticultural species | Nanning, Guangxi | NV |
| 30 | N. rubra | horticultural species | Nanjing, Jiangsu | NV |
| No. | Traits | Abbreviation | Criteria for Documenting |
|---|---|---|---|
| 1 | Leaf auricle | LA | Separated = 1, closed = 2, covered = 3 |
| 2 | Leaf color | LC | Green = 1, brown = 2, green and brown = 3 |
| 3 | Young leaf color | YLC | Green = 1, brown = 2, leaves with brown fleck = 3 |
| 4 | Leaf form | LF | Rotundity = 1, elliptic = 2, oval = 3, broad oval shape = 4 |
| 5 | Viviparity of leaf | VF | No = 1, yes = 2 |
| 6 | Leaf spot | LS | No = 1, general = 2, many = 3 |
| 7 | Petal color | PC | White = 1, amaranth = 2, blue = 3, violet = 4, yellow = 5, compound color = 6 |
| 8 | Flower fragrance | FF | Strong = 1, refreshing = 2, slight = 3 |
| 9 | Petal shape | PS | Lance-shaped = 1, oval = 2, spoon-shaped = 3 |
| 10 | Inflorescence shape | IS | Radial-shaped = 1, cup-shaped = 2 |
| 11 | Sepal color | SC | Similar = 1, different = 2 |
| 12 | Sepal shape | SS | Lance-shaped = 1, oval = 2, obovate = 3 |
| 13 | Sepal and petal spot | SPS | No fleck = 1, fleck of sepal = 2, fleck of sepal and petal = 3 |
| 14 | Anther color | AC | White = 1, yellow = 2, pink = 3, purple = 4, red = 5 |
| 15 | Fruit shape | FS | Pineapple-shaped = 1, apple-shaped = 2, peach-shaped = 3 |
| 16 | Leaf length/cm | LL | Average length per leaf |
| 17 | Leaf width/cm | LW | Average width per leaf |
| 18 | Leaf auricle length/cm | LAL | Average length of delamination per leaf |
| 19 | Petiole diameter/cm | PD | Average diameter of petiole per leaf |
| 20 | Inflorescence diameter/cm | ID | Average diameter per flower |
| 21 | Flower height/cm | FH | Average height per flower |
| 22 | Single flowering period/d | SFP | Average single flower period per flower |
| 23 | Petal length/cm | PL | Average length of outer and inner petals per flower |
| 24 | Petal width/cm | PW | Average width of outer and inner petals per flower |
| 25 | Total number of petals | TPS | Average number of petals per flower |
| 26 | Sepal length/cm | SL | Average length of sepals per flower |
| 27 | Sepal width/cm | SW | Average width of sepals per flower |
| 28 | Number of carpels | CN | Average number of carpels per flower |
| 29 | Number of stamens | STN | Average number of stamens per flower |
| 30 | Stamen length/cm | STL | Average length of stamens per flower |
| 31 | Anther length/cm | AL | Average length of anthers per flower |
| 32 | Flower density/20d | FD | Average flower density every 20 days |
| 33 | Diameter of epiphyllous buds/cm | EBD | Average diameter of epiphyllous buds per leaf |
| 34 | Number of epiphyllous buds/cm | EBN | Average number of epiphyllous buds per flower leaf |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, M.; Lu, W.; Gao, L.; Li, C.; Liu, H.; Zhao, C.; Ai, X. Genetic Diversity Analysis of Water Lily Germplasms Based on Morphological Traits and SSR Markers. Plants 2025, 14, 1365. https://doi.org/10.3390/plants14091365
Wan M, Lu W, Gao L, Li C, Liu H, Zhao C, Ai X. Genetic Diversity Analysis of Water Lily Germplasms Based on Morphological Traits and SSR Markers. Plants. 2025; 14(9):1365. https://doi.org/10.3390/plants14091365
Chicago/Turabian StyleWan, Min, Wei Lu, Luxue Gao, Cuiping Li, Hanli Liu, Caibao Zhao, and Xingmei Ai. 2025. "Genetic Diversity Analysis of Water Lily Germplasms Based on Morphological Traits and SSR Markers" Plants 14, no. 9: 1365. https://doi.org/10.3390/plants14091365
APA StyleWan, M., Lu, W., Gao, L., Li, C., Liu, H., Zhao, C., & Ai, X. (2025). Genetic Diversity Analysis of Water Lily Germplasms Based on Morphological Traits and SSR Markers. Plants, 14(9), 1365. https://doi.org/10.3390/plants14091365






