The Ecological Risks and Invasive Potential of Introduced Ornamental Plants in China
Abstract
1. Introduction
2. Results
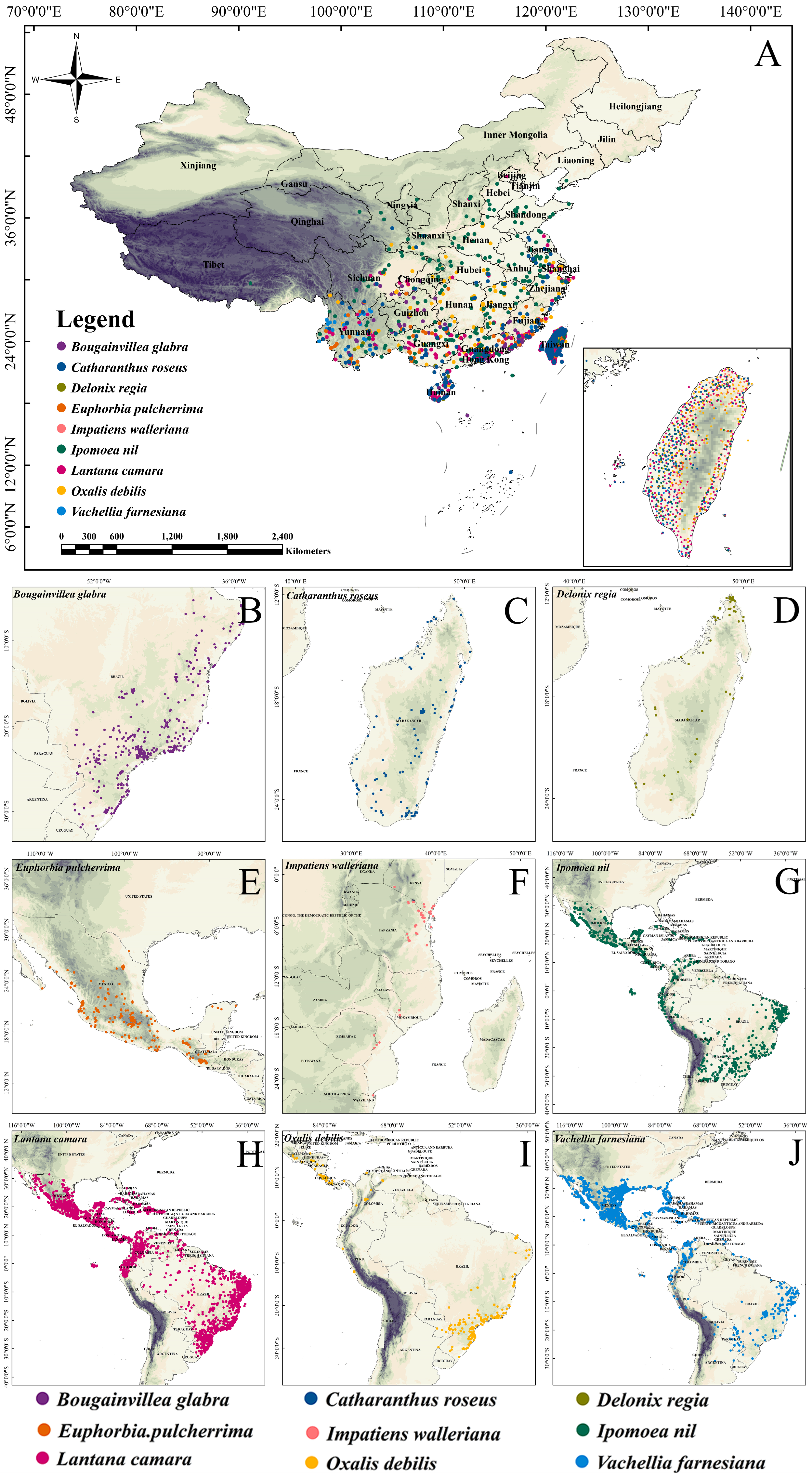
2.1. Climate Niche Comparison of Native vs. Introduced Ranges in Nine IOPCs
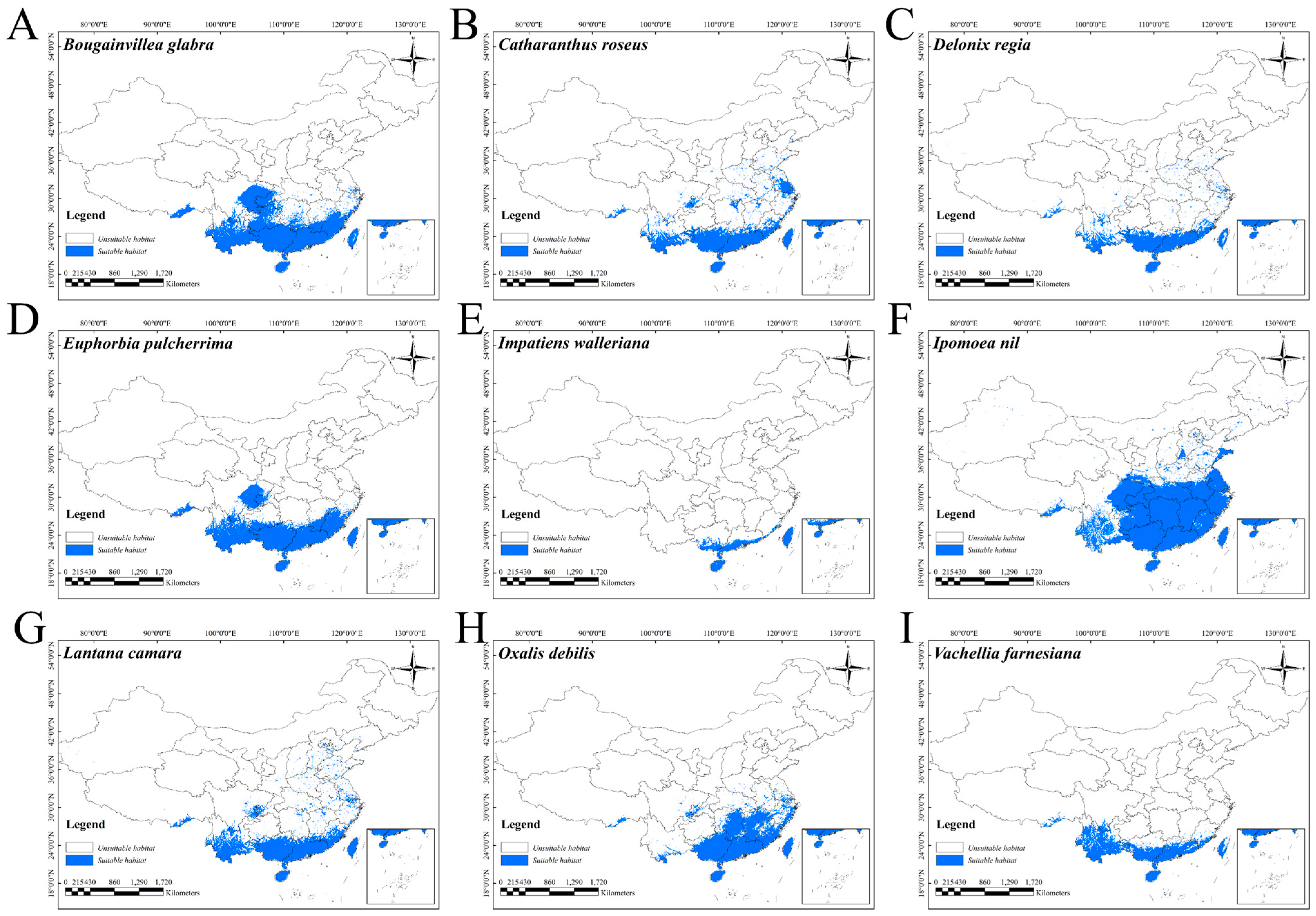
2.2. Changes in Potentially Suitable Areas Under Current Climate Conditions
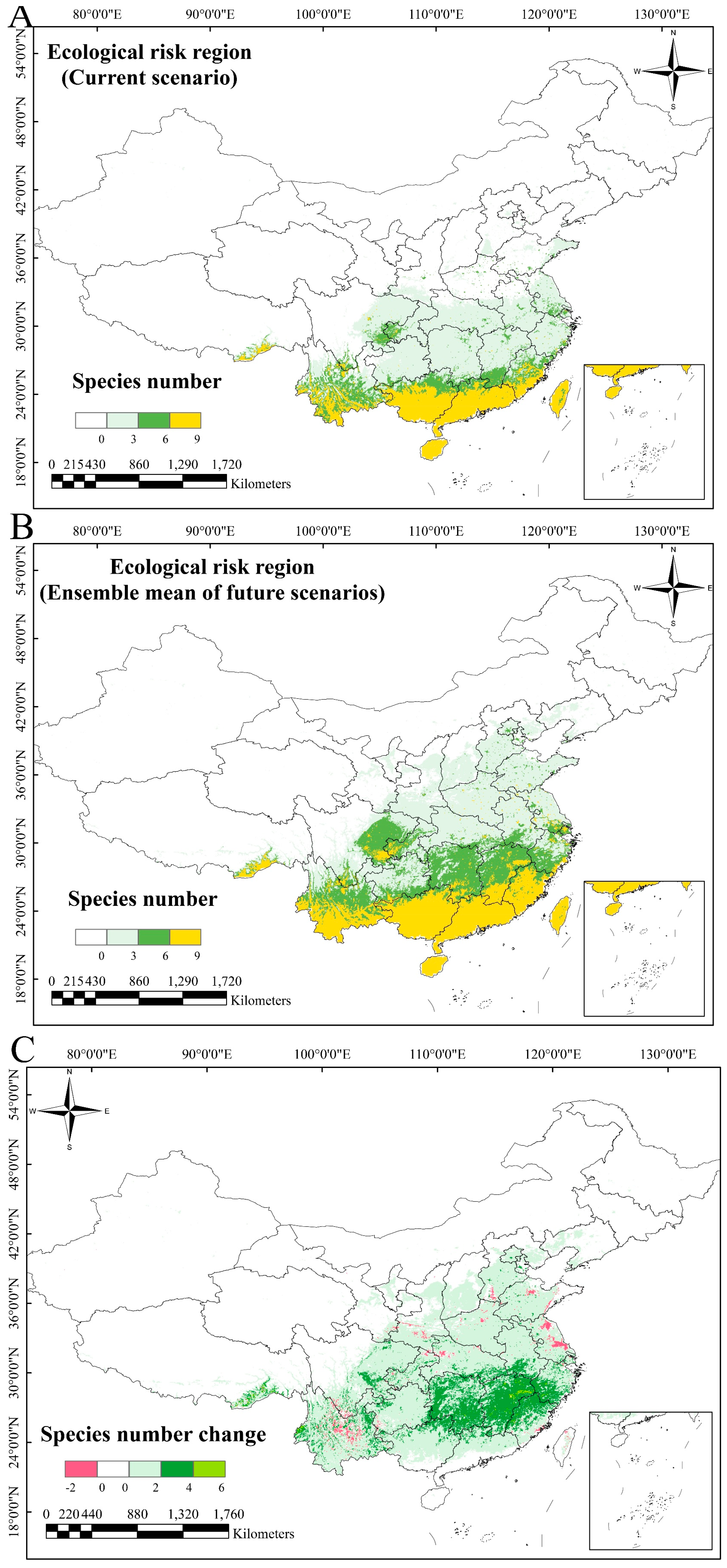
2.3. Predicted Distribution Dynamics of IOPCs Under Climate Change
3. Discussion
3.1. Niche Comparison of the Nine IOPCs
3.2. Implications of IOPC Potentially Suitable Area Predictions
3.3. Ecological Risk Management
4. Materials and Methods
4.1. Occurrence Points of IOPCs
4.2. Environmental Variables
4.3. Construction of the Species Distribution Model
- wi: The weight of the ith model;
- Pi: The prediction probability of the ith model;
- n: The number of individual models used to construct the final ensemble model.
4.4. Model Accuracy Evaluation
4.5. Niche Comparisons: Niche Overlap and Niche Tests
4.6. Regression Analysis Under Climate Change
4.7. Classification of Ecological Risk Zones
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| IOPC | Introduced ornamental plants in China |
| SDM | Species distribution model |
| PCA | Principal component analysis |
References
- Hu, S.; Jin, C.; Liao, R.; Huang, L.; Zhou, L.; Long, Y.; Luo, M.; Jim, C.Y.; Hu, W.; Lin, D. Herbaceous ornamental plants with conspicuous aesthetic traits contribute to plant invasion risk in subtropical urban parks. J. Environ. Manag. 2023, 347, 119059. [Google Scholar] [CrossRef] [PubMed]
- Lantschner, M.V.; Corley, J.C.; Liebhold, A.M. Drivers of global Scolytinae invasion patterns. Ecol. Appl. 2020, 30, e02103. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Cianfaglione, K.; Nagy, D.U.; Canale, A.; Maggi, F. Evaluation of two invasive plant invaders in Europe (Solidago canadensis and Solidago gigantea) as possible sources of botanical insecticides. J. Pest Sci. 2019, 92, 805–821. [Google Scholar] [CrossRef]
- Pal, R.W.; Chen, S.; Nagy, D.U.; Callaway, R.M. Impacts of Solidago gigantea on other species at home and away. Biol. Invasions 2015, 17, 3317–3325. [Google Scholar] [CrossRef]
- Abhilasha, D.; Quintana, N.; Vivanco, J.; Joshi, J. Do allelopathic compounds in invasive Solidago canadensis sl restrain the native European flora? J. Ecol. 2008, 96, 993–1001. [Google Scholar] [CrossRef]
- Mircea, D.-M.; Li, R.; Blasco Giménez, L.; Vicente, O.; Sestras, A.F.; Sestras, R.E.; Boscaiu, M.; Mir, R. Salt and water stress tolerance in Ipomoea purpurea and Ipomoea tricolor, two ornamentals with invasive potential. Agronomy 2023, 13, 2198. [Google Scholar] [CrossRef]
- Bernich, A.; French, K.; Bedward, M. Assessing the invasion potential of five common exotic vine species in temperate Australian rainforests. NeoBiota 2024, 90, 79–96. [Google Scholar]
- Hao, Q.; Ma, J.-S. Invasive alien plants in China: An update. Plant Divers. 2022, 45, 117. [Google Scholar] [CrossRef]
- Weber, E.; Sun, S.-G.; Li, B. Invasive alien plants in China: Diversity and ecological insights. Biol. Invasions 2008, 10, 1411–1429. [Google Scholar] [CrossRef]
- Liu, J.; Dong, M.; Miao, S.L.; Li, Z.Y.; Song, M.H.; Wang, R.Q. Invasive alien plants in China: Role of clonality and geographical origin. Biol. Invasions 2006, 8, 1461–1470. [Google Scholar] [CrossRef]
- Monnet, A.C.; Vorontsova, M.S.; Govaerts, R.H.; Svenning, J.C.; Sandel, B. Historical legacies and ecological determinants of grass naturalizations worldwide. Ecography 2020, 43, 1373–1385. [Google Scholar] [CrossRef]
- Peterson, A.T. Ecological niche conservatism: A time-structured review of evidence. J. Biogeogr. 2011, 38, 817–827. [Google Scholar] [CrossRef]
- Liu, C.; Wolter, C.; Xian, W.; Jeschke, J.M. Most invasive species largely conserve their climatic niche. Proc. Natl. Acad. Sci. USA 2020, 117, 23643–23651. [Google Scholar] [CrossRef]
- Christina, M.; Limbada, F.; Atlan, A. Climatic niche shift of an invasive shrub (Ulex europaeus): A global scale comparison in native and introduced regions. J. Plant Ecol. 2020, 13, 42–50. [Google Scholar] [CrossRef]
- Atwater, D.Z.; Ervine, C.; Barney, J.N. Climatic niche shifts are common in introduced plants. Nat. Ecol. Evol. 2018, 2, 34–43. [Google Scholar] [CrossRef]
- Schoener, T.W. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 1970, 51, 408–418. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; d’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A. ecospat: An R package to support spatial analyses and modeling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Srivastava, V.; Liang, W.; Keena, M.A.; Roe, A.D.; Hamelin, R.C.; Griess, V.C. Assessing niche shifts and conservatism by comparing the native and post-invasion niches of major forest invasive species. Insects 2020, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Petitpierre, B.; Broennimann, O.; Daehler, C.; Kueffer, C. Unifying niche shift studies: Insights from biological invasions. Trends Ecol. Evol. 2014, 29, 260–269. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Rome, Q.; Villemant, C.; Courchamp, F. Can species distribution models really predict the expansion of invasive species? PLoS ONE 2018, 13, e0193085. [Google Scholar] [CrossRef] [PubMed]
- Jarnevich, C.S.; Engelstad, P.; Williams, D.; Shadwell, K.; Reimer, C.; Henderson, G.; Prevey, J.S.; Pearse, I.S. Predicted occurrence and abundance habitat suitability of invasive plants in the contiguous United States: Updates for the INHABIT web tool. NeoBiota 2024, 96, 261–278. [Google Scholar] [CrossRef]
- García-Gómez, R.E.; Aceves-Medina, G.; Villalobos, H.; Rosenberg, S.P.A.J.; Durazo, R. Predictive performance from abundance distribution models of Vinciguerria lucetia larvae in the southern portion of the California current system using XGBOOST. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2023, 212, 105336. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Modelling species presence-only data with random forests. Ecography 2021, 44, 1731–1742. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Guan, B.-c.; Guo, H.-j.; Chen, S.-s.; Li, D.-m.; Liu, X.; Gong, X.; Ge, G. Shifting ranges of eleven invasive alien plants in China in the face of climate change. Ecol. Inform. 2020, 55, 101024. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Yuan, Y.; Tang, X.; Liu, M.; Liu, X.; Tao, J. Species distribution models of the spartina alterniflora loisel in its origin and invasive country reveal an ecological niche shift. Front. Plant Sci. 2021, 12, 738769. [Google Scholar] [CrossRef]
- Qin, X.; Li, M. Predicting the potential distribution of Oxalis debilis Kunth, an invasive species in China with a maximum entropy model. Plants 2023, 12, 3999. [Google Scholar] [CrossRef]
- Taucare-Ríos, A.; Bizama, G.; Bustamante, R.O. Using global and regional species distribution models (SDM) to infer the invasive stage of Latrodectus geometricus (Araneae: Theridiidae) in the Americas. Environ. Entomol. 2016, 45, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.T.P.; Rodrigues, J.F.M.; Diniz-Filho, J.A.F.; Rangel, T.F. Biogeographical history constrains climatic niche diversification without adaptive forces driving evolution. J. Biogeogr. 2019, 46, 1020–1028. [Google Scholar] [CrossRef]
- Melton, A.E.; Clinton, M.H.; Wasoff, D.N.; Lu, L.; Hu, H.; Chen, Z.; Ma, K.; Soltis, D.E.; Soltis, P.S. Climatic niche comparisons of eastern North American and eastern Asian disjunct plant genera. Glob. Ecol. Biogeogr. 2022, 31, 1290–1302. [Google Scholar] [CrossRef]
- Bates, O.K.; Bertelsmeier, C. Climatic niche shifts in introduced species. Curr. Biol. 2021, 31, R1252–R1266. [Google Scholar] [CrossRef]
- Broennimann, O.; Mráz, P.; Petitpierre, B.; Guisan, A.; Müller-Schärer, H. Contrasting spatio-temporal climatic niche dynamics during the eastern and western invasions of spotted knapweed in North America. J. Biogeogr. 2014, 41, 1126–1136. [Google Scholar] [CrossRef]
- Callen, S.T.; Miller, A.J. Signatures of niche conservatism and niche shift in the North American kudzu (Pueraria montana) invasion. Divers. Distrib. 2015, 21, 853–863. [Google Scholar] [CrossRef]
- Sax, D.F.; Stachowicz, J.J.; Brown, J.H.; Bruno, J.F.; Dawson, M.N.; Gaines, S.D.; Grosberg, R.K.; Hastings, A.; Holt, R.D.; Mayfield, M.M. Ecological and evolutionary insights from species invasions. Trends Ecol. Evol. 2007, 22, 465–471. [Google Scholar] [CrossRef]
- Goncalves, E.; Herrera, I.; Duarte, M.; Bustamante, R.O.; Lampo, M.; Velasquez, G.; Sharma, G.P.; García-Rangel, S. Global invasion of Lantana camara: Has the climatic niche been conserved across continents? PLoS ONE 2014, 9, e111468. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.J.; Randin, C.; Zimmermann, N.E. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Rota, C.T.; Fletcher Jr, R.J.; Evans, J.M.; Hutto, R.L. Does accounting for imperfect detection improve species distribution models? Ecography 2011, 34, 659–670. [Google Scholar] [CrossRef]
- Guélat, J.; Kéry, M. Effects of spatial autocorrelation and imperfect detection on species distribution models. Methods Ecol. Evol. 2018, 9, 1614–1625. [Google Scholar] [CrossRef]
- Legendre, P. Spatial autocorrelation: Trouble or new paradigm? Ecology 1993, 74, 1659–1673. [Google Scholar] [CrossRef]
- Petitpierre, B.; Broennimann, O.; Kueffer, C.; Daehler, C.; Guisan, A. Selecting predictors to maximize the transferability of species distribution models: Lessons from cross-continental plant invasions. Glob. Ecol. Biogeogr. 2017, 26, 275–287. [Google Scholar] [CrossRef]
- Steen, V.A.; Tingley, M.W.; Paton, P.W.; Elphick, C.S. Spatial thinning and class balancing: Key choices lead to variation in the performance of species distribution models with citizen science data. Methods Ecol. Evol. 2021, 12, 216–226. [Google Scholar]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar]
- Liu, C.; Newell, G.; White, M. On the selection of thresholds for predicting species occurrence with presence-only data. Ecol. Evol. 2016, 6, 337–348. [Google Scholar] [CrossRef]
- Lobo, J.M.; Jiménez-Valverde, A.; Real, R. AUC: A misleading measure of the performance of predictive distribution models. Glob. Ecol. Biogeogr. 2008, 17, 145–151. [Google Scholar] [CrossRef]
- Kearney, M.; Porter, W. Mechanistic niche modelling: Combining physiological and spatial data to predict species’ ranges. Ecol. Lett. 2009, 12, 334–350. [Google Scholar]
- Grace, J.B.; Anderson, T.M.; Olff, H.; Scheiner, S.M. On the specification of structural equation models for ecological systems. Ecol. Monogr. 2010, 80, 67–87. [Google Scholar] [CrossRef]
- Tu, W.; Xiong, Q.; Qiu, X.; Zhang, Y. Dynamics of invasive alien plant species in China under climate change scenarios. Ecol. Indic. 2021, 129, 107919. [Google Scholar] [CrossRef]
- Wan, J.Z.; Wang, C.J.; Tan, J.F.; Yu, F.H. Climatic niche divergence and habitat suitability of eight alien invasive weeds in China under climate change. Ecol. Evol. 2017, 7, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Buisson, L.; Thuiller, W.; Casajus, N.; Lek, S.; Grenouillet, G. Uncertainty in ensemble forecasting of species distribution. Glob. Change Biol. 2010, 16, 1145–1157. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.; Xiao, C.; Liu, L.; Wang, T.; Shen, C. Evaluation of the temperature downscaling performance of PRECIS to the BCC-CSM2-MR model over China. Clim. Dyn. 2022, 59, 1143–1159. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, Y.; Zhu, F.; Jiang, Y. Prediction of the impact of climate change on fast-growing timber trees in China. For. Ecol. Manag. 2021, 501, 119653. [Google Scholar] [CrossRef]
- Bufford, J.L.; Dawes, T.N.; Hulme, P.E. Species that invade natural areas have shorter lag times than other naturalized ornamental species. Ecography 2025, e07721. [Google Scholar] [CrossRef]
- Pergl, J.; Sádlo, J.; Petrusek, A.; Laštůvka, Z.; Musil, J.; Perglová, I.; Šanda, R.; Šefrová, H.; Šíma, J.; Vohralík, V. Black, Grey and Watch Lists of alien species in the Czech Republic based on environmental impacts and management strategy. NeoBiota 2016, 28, 1–37. [Google Scholar] [CrossRef]
- Lodge, D.M.; Simonin, P.W.; Burgiel, S.W.; Keller, R.P.; Bossenbroek, J.M.; Jerde, C.L.; Kramer, A.M.; Rutherford, E.S.; Barnes, M.A.; Wittmann, M.E. Risk analysis and bioeconomics of invasive species to inform policy and management. Annu. Rev. Environ. Resour. 2016, 41, 453–488. [Google Scholar] [CrossRef]
- Epanchin-Niell, R.S. Economics of invasive species policy and management. Biol. Invasions 2017, 19, 3333–3354. [Google Scholar] [CrossRef]
- Lampert, A.; Liebhold, A.M. Optimizing the use of suppression zones for containment of invasive species. Ecol. Appl. 2023, 33, e2797. [Google Scholar] [CrossRef]
- Schuster, M.J.; Wragg, P.D.; Reich, P.B. Using revegetation to suppress invasive plants in grasslands and forests. J. Appl. Ecol. 2018, 55, 2362–2373. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Z.; Smith, W.A.; Ellis, D.R.; Chen, Y.; Zheng, X.; Pei, Y.; Luo, K.; Zhao, D.; Yao, Q. Invasive ornamental plants: Problems, challenges, and molecular tools to neutralize their invasiveness. Crit. Rev. Plant Sci. 2004, 23, 381–389. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Xie, G.L.; Wang, W.K. Assessing the Potential Distribution of Pseudoechthistatus (Coleoptera: Cerambycidae) in China Under Climate Change Using Species Distribution Models. Ecol. Evol. 2025, 15, e71303. [Google Scholar] [CrossRef] [PubMed]
- Sinitambirivoutin, M.; Milne, E.; Schiettecatte, L.-S.; Tzamtzis, I.; Dionisio, D.; Henry, M.; Brierley, I.; Salvatore, M.; Bernoux, M. An updated IPCC major soil types map derived from the harmonized world soil database v2. 0. Catena 2024, 244, 108258. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Meng, S.; Jiang, Q.; Hu, G.; Zhang, L.; Yao, X. Potential distribution under climate change and ecological niche differences between Actinidia chinensis complex. Sci. Hortic. 2024, 337, 113533. [Google Scholar] [CrossRef]
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD–a platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, X.; Zhang, J.; Zhao, J.; Ge, Z.; Zhang, Z. Predicting the potential suitable distribution of Larix principis-rupprechtii Mayr under climate change scenarios. Forests 2022, 13, 1428. [Google Scholar] [CrossRef]
- Collart, F.; Hedenäs, L.; Broennimann, O.; Guisan, A.; Vanderpoorten, A. Intraspecific differentiation: Implications for niche and distribution modelling. J. Biogeogr. 2021, 48, 415–426. [Google Scholar] [CrossRef]
- Calenge, C. Exploring habitat selection by wildlife with adehabitat. J. Stat. Softw. 2007, 22, 6. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhu, S.; Wu, X.; Yang, S.; Yan, Y.; Wen, Y. Multiple Ecological Niche Modeling Reveals Niche Conservatism and Divergence in East Asian Yew (Taxus). Plants 2025, 14, 1094. [Google Scholar] [CrossRef] [PubMed]
- Petitpierre, B.; Kueffer, C.; Broennimann, O.; Randin, C.; Daehler, C.; Guisan, A. Climatic niche shifts are rare among terrestrial plant invaders. Science 2012, 335, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
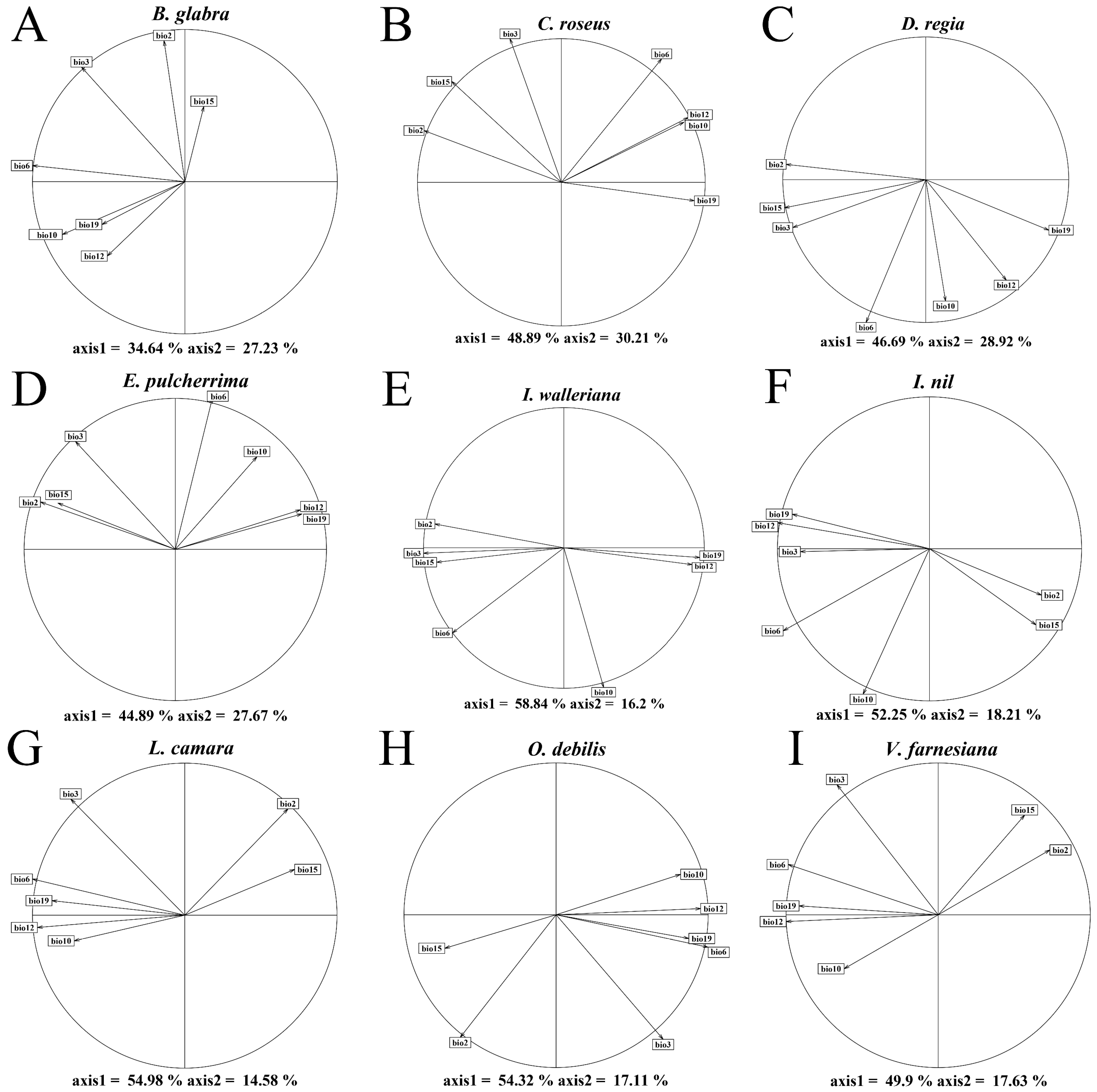

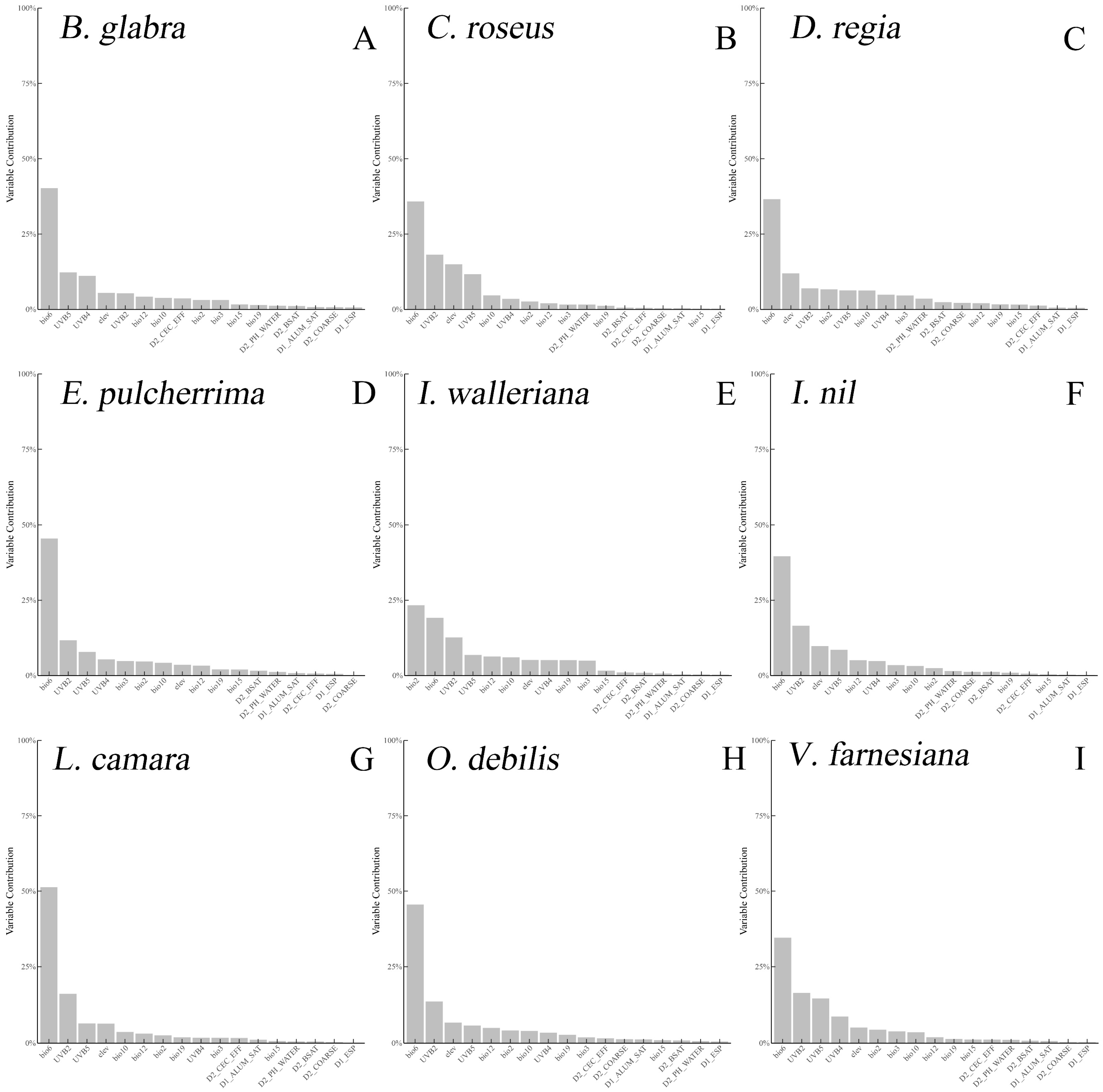

| IOPC | Niche Overlap (D) | Niche Similarity (p) | Niche Equivalency (p) | Unfilling | Stability | Expansion |
|---|---|---|---|---|---|---|
| Bougainvillea glabra | 0.2153 | 0.0350 | 0.0099 | 0.3277 | 0.4880 | 0.5120 |
| Catharanthus roseus | 0.0665 | 0.1538 | 1.0000 | 0.6758 | 0.2874 | 0.7126 |
| Delonix regia | 0.0838 | 0.1928 | 1.0000 | 0.7202 | 0.5459 | 0.4541 |
| Euphorbia pulcherrima | 0.0697 | 0.0759 | 0.0099 | 0.8614 | 0.3355 | 0.6645 |
| Impatiens walleriana | 0.0069 | 0.6244 | 1.0000 | 0.8613 | 0.0561 | 0.9439 |
| Ipomoea nil | 0.0462 | 0.3417 | 1.0000 | 0.3775 | 0.9635 | 0.0365 |
| Lantana camara | 0.0478 | 0.1908 | 1.0000 | 0.8856 | 0.5750 | 0.4250 |
| Oxalis debilis | 0.0506 | 0.2727 | 1.0000 | 0.8321 | 0.2138 | 0.7862 |
| Vachellia farnesiana | 0.1393 | 0.0919 | 0.0495 | 0.5565 | 0.9982 | 0.0018 |
| IOPC | R2 | Regression Coefficient (b, 1 × 106 km2/°C) | p |
|---|---|---|---|
| B. glabra | 0.93 | 26.72 | 4.17 × 10−10 |
| C. roseus | 0.94 | 15.23 | 2.48 × 10−10 |
| D. regia | 0.94 | 13.13 | 1.55 × 10−10 |
| E. pulcherrima | 0.86 | 27.36 | 9.58 × 10−8 |
| I. walleriana | 0.86 | 8.93 | 9.03 × 10−8 |
| I. nil | 0.85 | 14.78 | 1.73 × 10−7 |
| L. camara | 0.94 | 22.59 | 1.29 × 10−10 |
| O. debilis | 0.66 | 9.40 | 8.28 × 10−5 |
| V. farnesiana | 0.93 | 4.47 | 4.46 × 10−10 |
| Climate Change Scenario | Average Temperature Rise (°C) | Potential Distribution Area Change Rate (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. glabra | C. roseus | D. regia | E. pulcherrima | I. walleriana | I. nil | L. camara | O. debilis | V. farnesiana | ||
| SSP370_2021-2040 | 0.998 | 22.756 | 11.035 | 15.374 | 22.070 | 14.027 | 52.360 | 17.016 | 29.037 | 13.214 |
| SSP126_2021-2040 | 1.248 | 33.781 | 21.494 | 28.044 | 29.847 | 18.645 | 71.936 | 28.743 | 29.439 | 19.882 |
| SSP245_2021-2040 | 1.312 | 34.421 | 29.611 | 25.050 | 32.564 | 21.696 | 106.551 | 35.691 | 45.773 | 15.530 |
| SSP585_2021-2040 | 1.368 | 49.386 | 42.653 | 36.627 | 56.979 | 22.862 | 145.976 | 52.993 | 53.341 | 18.765 |
| SSP126_2061-2080 | 1.727 | 25.630 | 18.199 | 21.041 | 31.758 | 12.817 | 67.591 | 24.468 | 36.144 | 13.403 |
| SSP126_2081-2100 | 1.818 | 48.713 | 32.012 | 37.807 | 53.283 | 21.797 | 105.789 | 45.734 | 47.129 | 23.651 |
| SSP126_2041-2060 | 1.881 | 67.324 | 45.796 | 55.602 | 75.720 | 26.964 | 127.359 | 66.612 | 45.840 | 23.004 |
| SSP370_2041-2060 | 1.979 | 73.158 | 57.963 | 66.780 | 97.248 | 32.609 | 126.906 | 85.187 | 53.528 | 30.352 |
| SSP245_2041-2060 | 2.025 | 20.031 | 9.891 | 12.122 | 18.316 | 11.466 | 57.776 | 16.173 | 28.307 | 10.759 |
| SSP585_2041-2060 | 2.583 | 50.871 | 34.051 | 47.152 | 42.442 | 19.524 | 115.074 | 46.262 | 37.853 | 21.227 |
| SSP245_2061-2080 | 2.616 | 76.294 | 65.691 | 78.227 | 98.149 | 30.499 | 188.221 | 91.744 | 52.342 | 32.540 |
| SSP245_2081-2100 | 2.967 | 80.989 | 71.255 | 75.678 | 96.870 | 32.924 | 165.934 | 98.889 | 54.837 | 32.297 |
| SSP370_2061-2080 | 3.172 | 30.038 | 23.812 | 21.533 | 34.333 | 17.277 | 86.086 | 30.551 | 44.966 | 13.765 |
| SSP585_2061-2080 | 3.606 | 59.222 | 43.127 | 43.947 | 59.958 | 23.398 | 121.233 | 62.470 | 46.661 | 23.145 |
| SSP370_2081-2100 | 3.991 | 78.963 | 76.965 | 82.498 | 81.238 | 29.265 | 229.735 | 105.311 | 69.339 | 31.075 |
| SSP585_2081-2100 | 4.691 | 87.784 | 82.332 | 93.810 | 95.650 | 31.096 | 241.512 | 111.736 | 55.320 | 35.777 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Yang, M.; Ma, X.; Hu, Q.; Feng, L.; Hu, Y.; He, J.; Zhang, X.; Zheng, J. The Ecological Risks and Invasive Potential of Introduced Ornamental Plants in China. Plants 2025, 14, 1361. https://doi.org/10.3390/plants14091361
Wang H, Yang M, Ma X, Hu Q, Feng L, Hu Y, He J, Zhang X, Zheng J. The Ecological Risks and Invasive Potential of Introduced Ornamental Plants in China. Plants. 2025; 14(9):1361. https://doi.org/10.3390/plants14091361
Chicago/Turabian StyleWang, Haoyu, Min Yang, Xiaohua Ma, Qingdi Hu, Lei Feng, Yaping Hu, Jiehui He, Xule Zhang, and Jian Zheng. 2025. "The Ecological Risks and Invasive Potential of Introduced Ornamental Plants in China" Plants 14, no. 9: 1361. https://doi.org/10.3390/plants14091361
APA StyleWang, H., Yang, M., Ma, X., Hu, Q., Feng, L., Hu, Y., He, J., Zhang, X., & Zheng, J. (2025). The Ecological Risks and Invasive Potential of Introduced Ornamental Plants in China. Plants, 14(9), 1361. https://doi.org/10.3390/plants14091361





