5-AzaCytidine Promotes Somatic Embryogenesis of Taxodium Hybrid ‘Zhongshanshan’ by Regulating Redox Homeostasis
Abstract
1. Introduction
2. Results
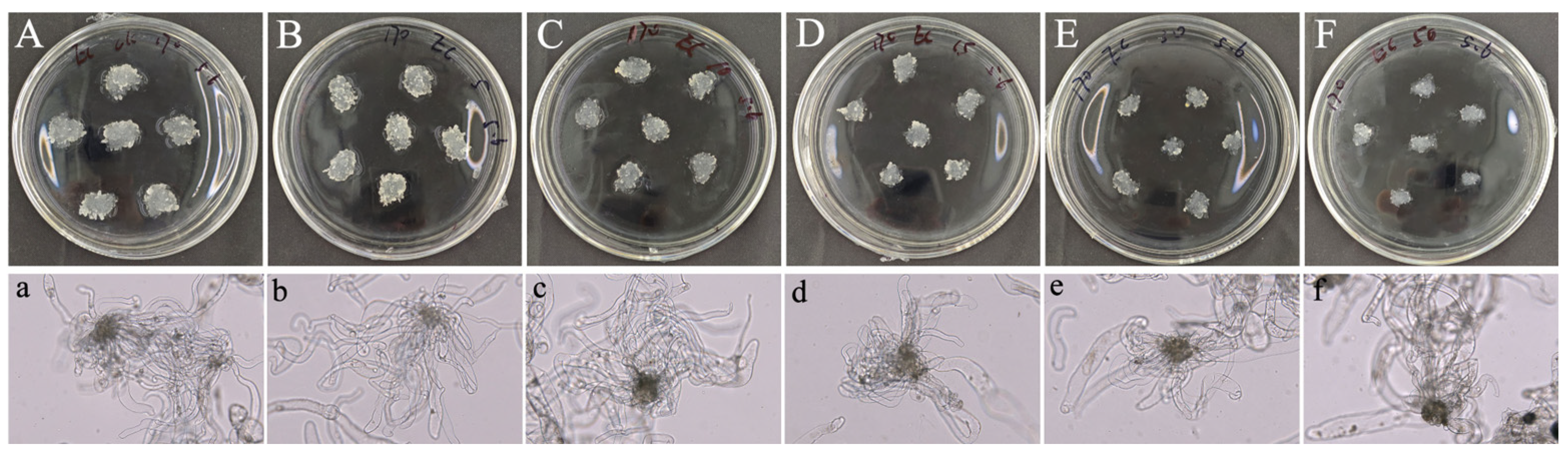
2.1. 5-azaC Inhibited EC Proliferation of Taxodium Hybrid ‘Zhongshanshan’
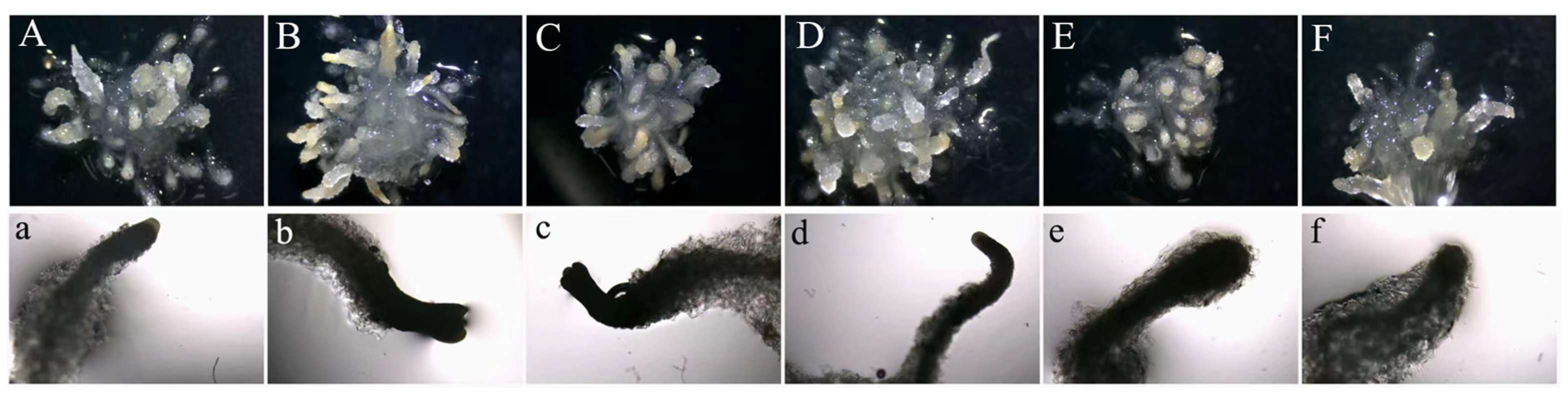
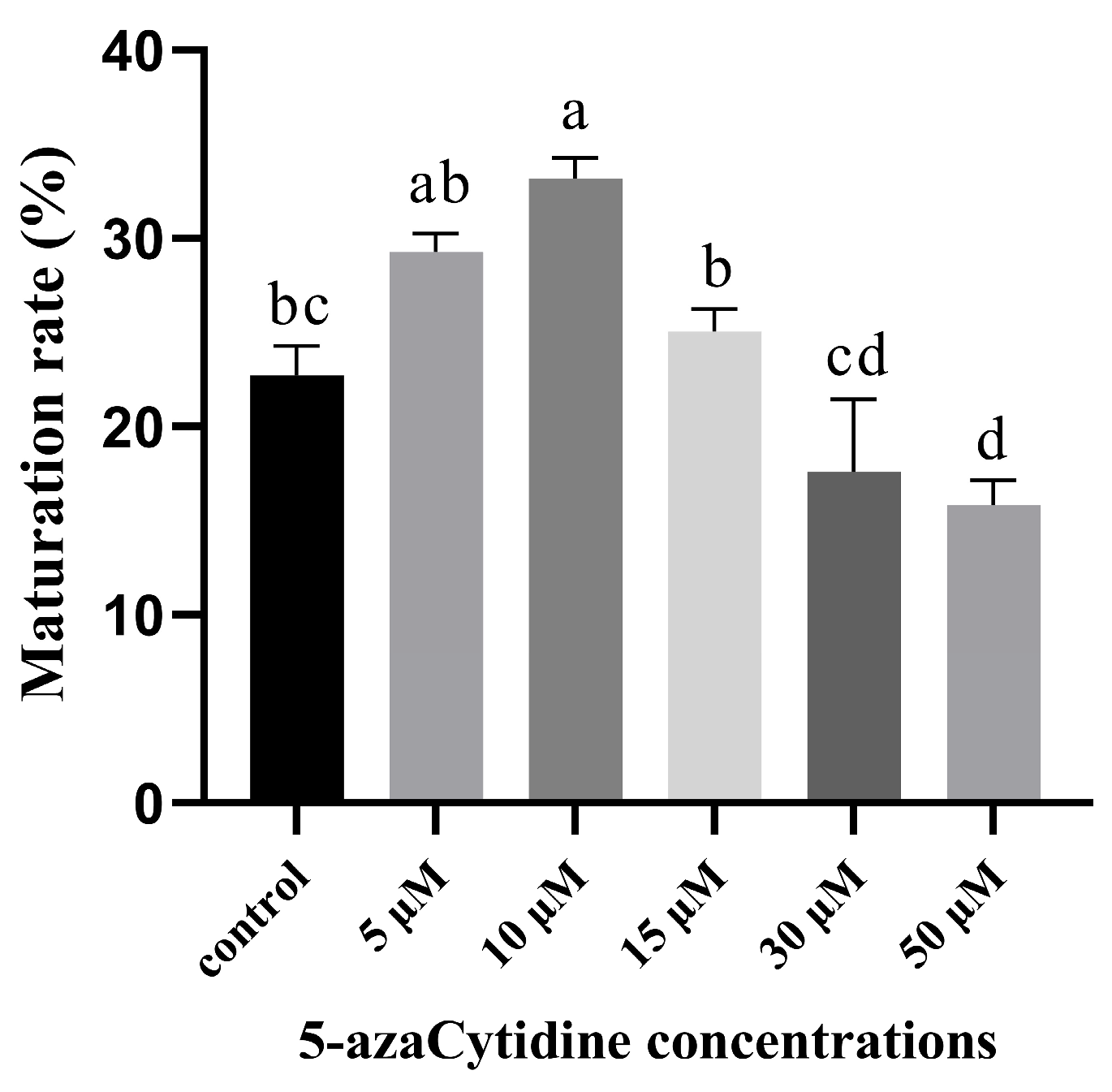
2.2. 5-azaC Promoted Embryogenic Capacity of Taxodium Hybrid ‘Zhongshanshan’
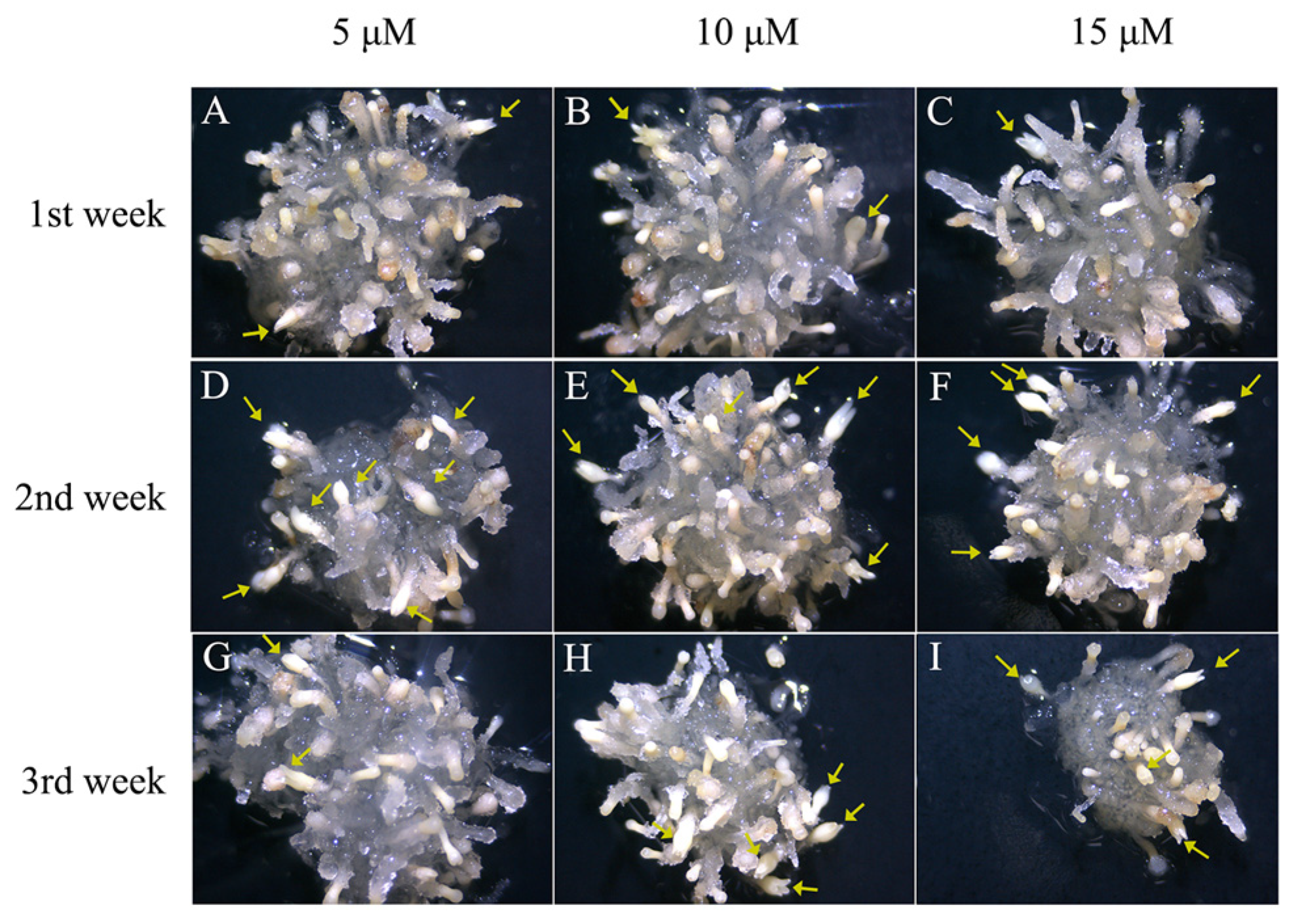
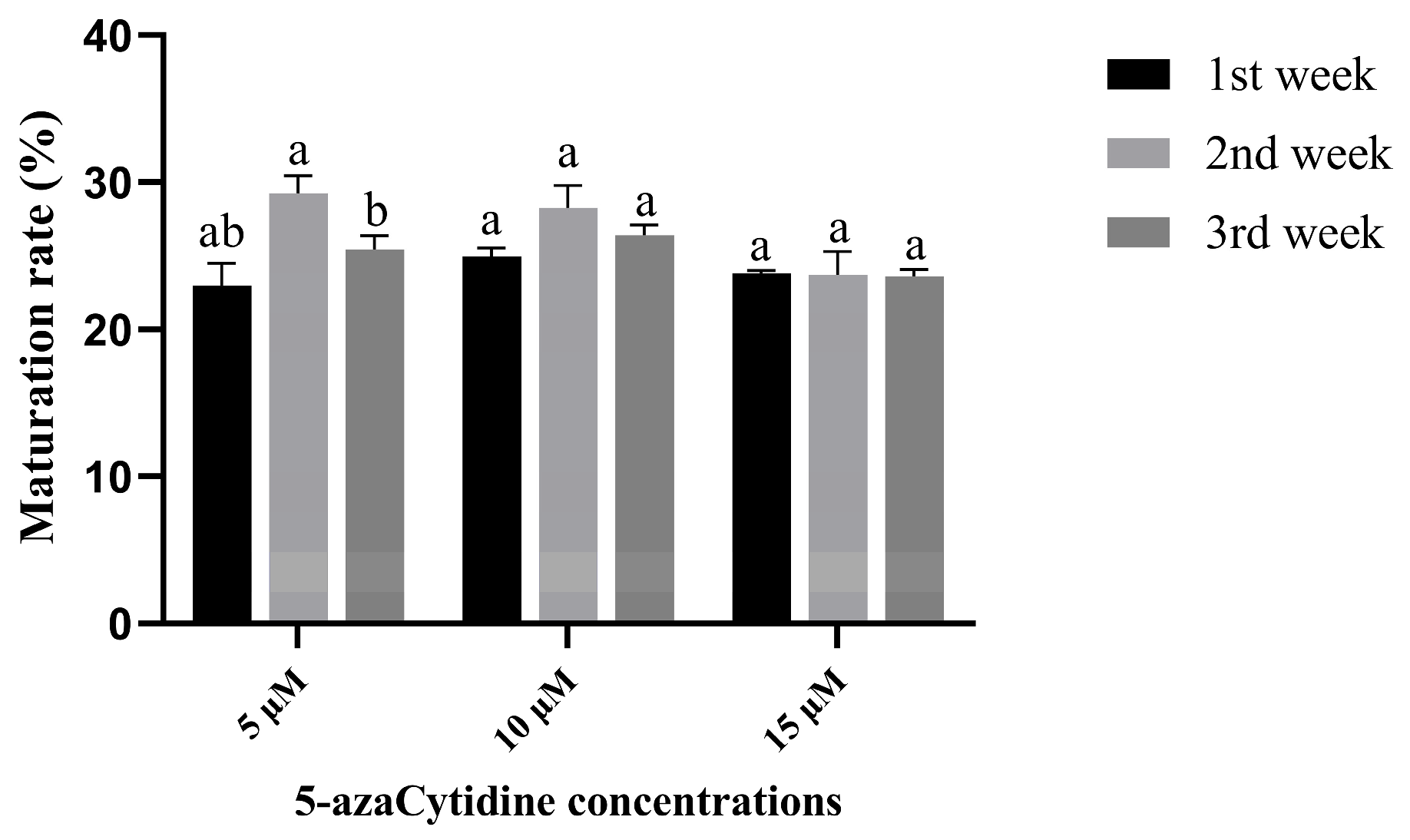
2.3. The Best Time for Promoting Somatic Embryogenesis of 5-azaC Was in the Second Week
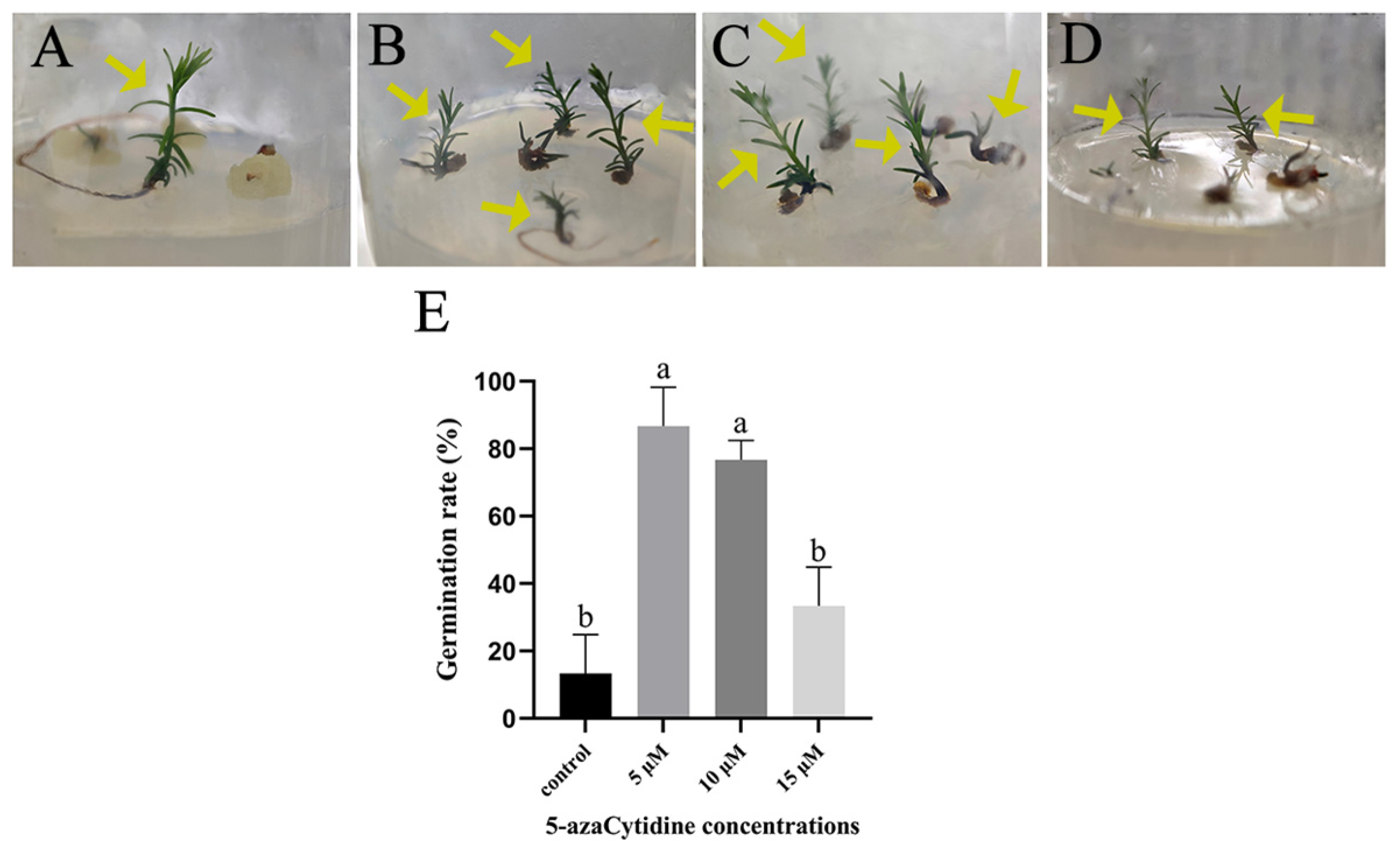
2.4. SE with 5-azaC Treatments Increased Plantlet Germination Rate
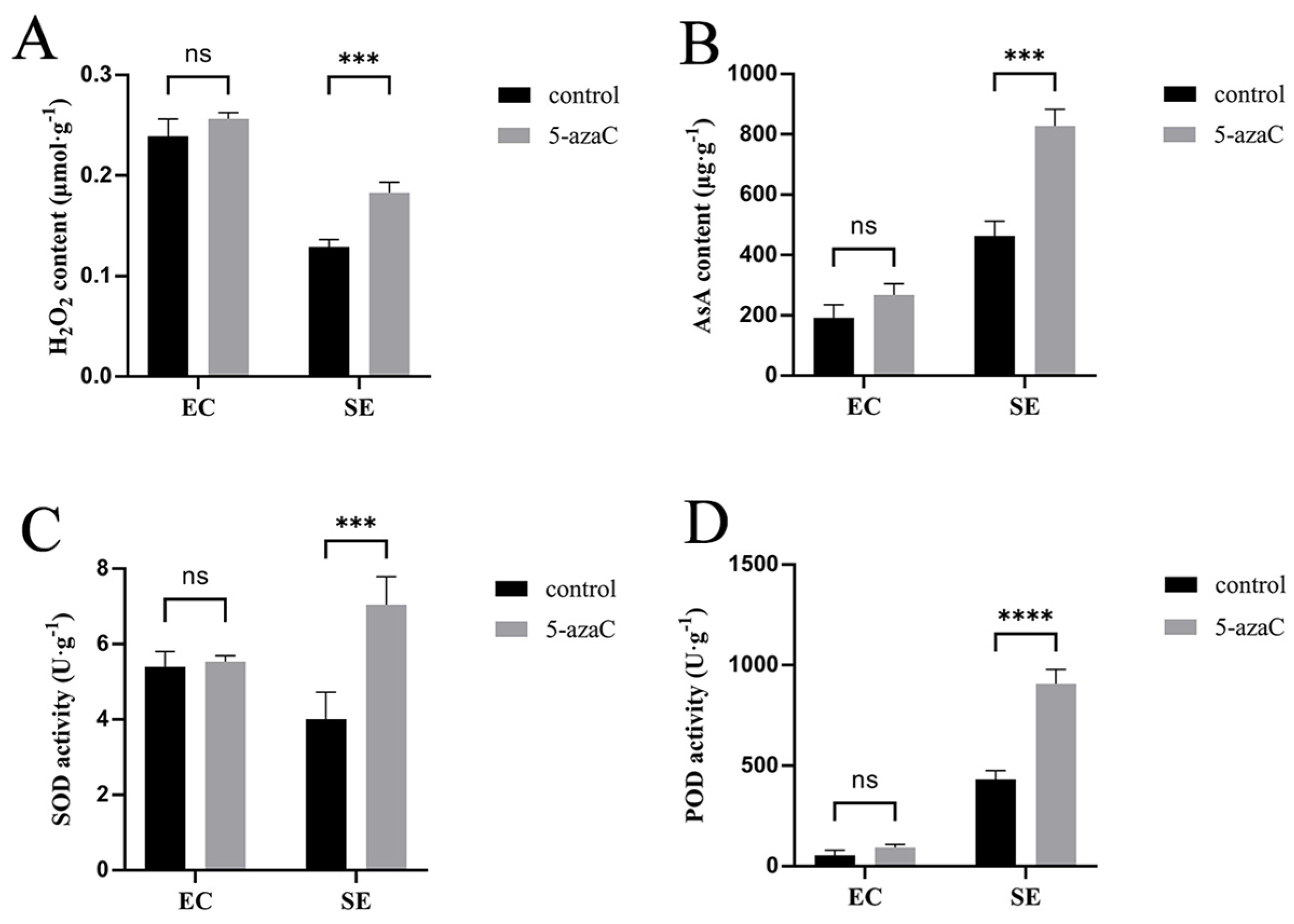
2.5. 5-azaC Affected Embryo Induction by Regulating Redox Homeostasis
3. Discussion
3.1. The Effect of 5-azaC on Somatic Embryogenesis Depends on Concentration and Action Time
3.2. 5-azaC Promotes Somatic Embryogenesis by Regulating Redox Homeostasis
4. Materials and Methods
4.1. Plant Materials
4.2. The Impact of Different 5-azaC Concentrations on EC Proliferation
4.3. The Impact of Different 5-azaC Concentrations on Embryo Development
4.4. Experiment on the Action Time of 5-azaC
4.5. The Impact of 5-azaC Treatments on the Growth of Taxodium Hybrid ‘Zhongshanshan’ Plantlets
4.6. Histochemical Analysis of ROS and Determination of ROS
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-azaC | 5-azaCytidine |
| EC | Embryogenic cells |
| SE | Somatic embryo |
| WPM | Woody plant medium |
| ABA | Abscisic acid |
| GA | Gibberellins |
| ROS | Reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| AsA | Ascorbic acid |
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| DAB | 3,3′-Diaminobenzidine |
References
- Duan, H.; Guo, J.; Xuan, L.; Wang, Z.; Li, M.; Yin, Y.; Yang, Y. Comparative chloroplast genomics of the genus Taxodium. BMC Genom. 2020, 21, 114. [Google Scholar] [CrossRef]
- Lu, Y.; Xiang, P.; Zhang, S.; Lu, Z.; Zhou, Z.; Yin, Y.; Hua, J.; Shi, Q.; Yu, W.; Yu, C. Physiological and transcriptional regulation in Taxodium hybrid ‘Zhongshanshan’ leaves in acclimation to prolonged partial submergence. Planta 2023, 258, 66. [Google Scholar] [CrossRef] [PubMed]
- Yeung, E.C. Structural and Developmental Patterns in Somatic Embryogenesis. In In Vitro Embryogenesis in Plants; Thorpe, T.A., Ed.; Springer: Dordrecht, The Netherlands, 1995; pp. 205–247. [Google Scholar]
- Méndez-Hernández, H.A.; Ledezma-Rodríguez, M.; Avilez-Montalvo, R.N.; Juárez-Gómez, Y.L.; Skeete, A.; Avilez-Montalvo, J.; De-la-Peña, C.; Loyola-Vargas, V.M. Signaling Overview of Plant Somatic Embryogenesis. Front. Plant Sci. 2019, 10, 77. [Google Scholar] [CrossRef]
- Ashapkin, V.V.; Kutueva, L.I.; Aleksandrushkina, N.I.; Vanyushin, B.F. Epigenetic Mechanisms of Plant Adaptation to Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2020, 21, 7457. [Google Scholar] [CrossRef] [PubMed]
- Nowak, K.; Gaj, M.D. Transcription Factors in the Regulation of Somatic Embryogenesis. In Somatic Embryogenesis: Fundamental Aspects and Applications; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 53–79. [Google Scholar]
- Smulders, M.J.M.; de Klerk, G.J. Epigenetics in plant tissue culture. Plant Growth Regul. 2011, 63, 137–146. [Google Scholar] [CrossRef]
- Piccolo, F.M.; Fisher, A.G. Getting rid of DNA methylation. Trends Cell Biol. 2014, 24, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Miguel, C.; Marum, L. An epigenetic view of plant cells cultured in vitro: Somaclonal variation and beyond. J. Exp. Bot. 2011, 62, 3713–3725. [Google Scholar] [CrossRef]
- Friedman, S. The inhibition of DNA(cytosine-5)methylases by 5-azacytidine. The effect of azacytosine-containing DNA. Mol. Pharmacol. 1981, 19, 314–320. [Google Scholar] [CrossRef]
- Grzybkowska, D.; Morończyk, J.; Wójcikowska, B.; Gaj, M.D. Azacitidine (5-AzaC)-treatment and mutations in DNA methylase genes affect embryogenic response and expression of the genes that are involved in somatic embryogenesis in Arabidopsis. Plant Growth Regul. 2018, 85, 243–256. [Google Scholar] [CrossRef]
- Zhong, L.; Xu, Y.H.; Wang, J.B. The effect of 5-azacytidine on wheat seedlings responses to NaCl stress. Biol. Plant. 2010, 54, 753–756. [Google Scholar] [CrossRef]
- Li, Z.; Luo, D.; Cao, S.; Mubeen, S.; Rehman, M.; Wang, C.; Jin, G.; Li, R.; Chen, T.; Chen, P. DNA Methylome Provide New Insights into the Physiological-Molecular Regulation of Salt Stress in Kenaf Using 5-azaC Pretreatment. J. Soil Sci. Plant Nutr. 2024, 24, 3889–3907. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, X.; Liu, C.; Zhao, H.; Dai, F.; Zhao, J.; Zhang, J.; Kong, L. Involvement of 5mC DNA demethylation via 5-aza-2′-deoxycytidine in regulating gene expression during early somatic embryo development in white spruce (Picea glauca). For. Res. 2023, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, K.; Noceda, C.; Pelletier, G.; Label, P.; Rodriguez, R.; Lelu-Walter, M.A. Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.). In Vitr. Cell. Dev. Biol. Plant 2009, 45, 20–33. [Google Scholar] [CrossRef]
- Pecinka, A.; Liu, C.-H. Drugs for Plant Chromosome and Chromatin Research. Cytogenet. Genome Res. 2014, 143, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, Y.; Wang, G. Salt stress-induced programmed cell death in tobacco protoplasts is mediated by reactive oxygen species and mitochondrial permeability transition pore status. J. Plant Physiol. 2006, 163, 731–739. [Google Scholar] [CrossRef]
- Steiner, N.; Santa-Catarina, C.; Silveira, V.; Floh, E.I.S.; Guerra, M.P. Polyamine effects on growth and endogenous hormones levels in Araucaria angustifolia embryogenic cultures. Plant Cell Tissue Organ Cult. 2007, 89, 55–62. [Google Scholar] [CrossRef]
- Martin, F.; Abati, V.; Burel, A.; Clément-Vidal, A.; Sanier, C.; Fabre, D.; Woraathasin, N.; Rio, M.; Besret, P.; Farinas, B.; et al. Overexpression of EcGSH1 induces glutathione production and alters somatic embryogenesis and plant development in Hevea brasiliensis. Ind. Crops Prod. 2018, 112, 803–814. [Google Scholar] [CrossRef]
- Pasternak, T.; Potters, G.; Caubergs, R.; Jansen, M.A.K. Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J. Exp. Bot. 2005, 56, 1991–2001. [Google Scholar] [CrossRef]
- Zhou, T.; Yang, X.; Guo, K.; Deng, J.; Xu, J.; Gao, W.; Lindsey, K.; Zhang, X. ROS Homeostasis Regulates Somatic Embryogenesis via the Regulation of Auxin Signaling in Cotton. Mol. Cell. Proteom. 2016, 15, 2108–2124. [Google Scholar] [CrossRef]
- Prudente, D.d.O.; de Souza, L.B.; Paiva, R. Plant Somatic Embryogenesis: Modulatory Role of Oxidative Stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 483–487. [Google Scholar] [CrossRef]
- Poborilova, Z.; Ohlsson, A.B.; Berglund, T.; Vildova, A.; Provaznik, I.; Babula, P. DNA hypomethylation concomitant with the overproduction of ROS induced by naphthoquinone juglone on tobacco BY-2 suspension cells. Environ. Exp. Bot. 2015, 113, 28–39. [Google Scholar] [CrossRef]
- Chen, Y.; Li, D.; Zhang, X.; Ma, Q.; Xu, Y.; Luo, Z. Azacytidine-induced hypomethylation delays senescence and coloration in harvested strawberries by stimulating antioxidant enzymes and modulating abscisate metabolism to minimize anthocyanin overproduction. Food Chem. 2023, 407, 135189. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Chang, M.; Kashif, M.H.; Tang, M.; Luo, D.; Cao, S.; Lu, H.; Zhang, W.; Huang, Z.; et al. 5-azacytidine pre-treatment alters DNA methylation levels and induces genes responsive to salt stress in kenaf (Hibiscus cannabinus L.). Chemosphere 2021, 271, 129562. [Google Scholar] [CrossRef] [PubMed]
- Pila Quinga, L.A.; Pacheco de Freitas Fraga, H.; do Nascimento Vieira, L.; Guerra, M.P. Epigenetics of long-term somatic embryogenesis in Theobroma cacao L.: DNA methylation and recovery of embryogenic potential. Plant Cell Tissue Organ Cult. 2017, 131, 295–305. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kobayashi, H.; Togashi, T.; Mori, Y.; Kikuchi, K.; Kuriyama, K.; Tokuji, Y. Formation of embryogenic cell clumps from carrot epidermal cells is suppressed by 5-azacytidine, a DNA methylation inhibitor. J. Plant Physiol. 2005, 162, 47–54. [Google Scholar] [CrossRef]
- Teyssier, C.; Maury, S.; Beaufour, M.; Grondin, C.; Delaunay, A.; Le Metté, C.; Ader, K.; Cadene, M.; Label, P.; Lelu-Walter, M.-A. In search of markers for somatic embryo maturation in hybrid larch (Larix × eurolepis): Global DNA methylation and proteomic analyses. Physiol. Plant. 2014, 150, 271–291. [Google Scholar] [CrossRef]
- Chen, R.; Chen, X.; Huo, W.; Zheng, S.; Lin, Y.; Lai, Z. Transcriptome analysis of azacitidine (5-AzaC)-treatment affecting the development of early somatic embryogenesis in longan. J. Hortic. Sci. Biotechnol. 2021, 96, 311–323. [Google Scholar] [CrossRef]
- Fraga, H.P.; Vieira, L.N.; Caprestano, C.A.; Steinmacher, D.A.; Micke, G.A.; Spudeit, D.A.; Pescador, R.; Guerra, M.P. 5-Azacytidine combined with 2,4-D improves somatic embryogenesis of Acca sellowiana (O. Berg) Burret by means of changes in global DNA methylation levels. Plant Cell Rep. 2012, 31, 2165–2176. [Google Scholar] [CrossRef]
- Carneros, E.; Díaz-Luzza, E.M.; Pérez-Pérez, Y.; Solís, M.T.; Testillano, P.S. DNA demethylation by transitory 5-azacytidine treatment improves somatic embryogenesis yield for regeneration and breeding of cork oak. Physiol. Plant. 2024, 176, e14143. [Google Scholar] [CrossRef]
- Tokuji, Y.; Takano, S.; Tonomura, M.; Tanaka, S.; Igari, K.; Watanabe, T. Influence of 5′-azacitidine on promoting recovery of cell competence for shoot organogenesis in Arabidopsis. Plant Cell Tissue Organ Cult. 2011, 106, 289–297. [Google Scholar] [CrossRef]
- LoSchiavo, F.; Pitto, L.; Giuliano, G.; Torti, G.; Nuti-Ronchi, V.; Marazziti, D.; Vergara, R.; Orselli, S.; Terzi, M. DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor. Appl. Genet. 1989, 77, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Pedrali-Noy, G.; Bernacchia, G.; do Rosario Alvelos, M.; Cella, R. Daucus carota Cells Contain Specific DNA Methyltransferase Inhibitors that Interfere with Somatic Embryogenesis. Plant Biol. 2003, 5, 383–392. [Google Scholar] [CrossRef]
- Vyskot, B.; Koukalová, B.; Kovařík, A.; Sachambula, L.; Reynolds, D.; Bezděk, M. Meiotic transmission of a hypomethylated repetitive DNA family in tobacco. Theor. Appl. Genet. 1995, 91, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Li, S.F.; Zhang, G.J.; Yuan, J.H.; Deng, C.L.; Lu, L.D.; Gao, W.J. Effect of 5-azaC on the growth, flowering time and sexual phenotype of spinach. Russ. J. Plant Physiol. 2015, 62, 670–675. [Google Scholar] [CrossRef]
- Testillano, P.S.; Gómez-Garay, A.; Pintos, B.; Risueño, M.C. Somatic Embryogenesis of Quercus suber L. from Immature Zygotic Embryos. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Springer: New York, NY, USA, 2018; pp. 247–256. [Google Scholar]
- Testillano, P.S.; Pintos, B.; Gomez-Garay, A.; Risueño, M.C. Stress-Induced Microspore Embryogenesis by Anther Culture of Quercus suber L. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants: Volume I; Jain, S.M., Gupta, P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 93–105. [Google Scholar]
- Zhong, S.; Fei, Z.; Chen, Y.-R.; Zheng, Y.; Huang, M.; Vrebalov, J.; McQuinn, R.; Gapper, N.; Liu, B.; Xiang, J.; et al. Single-base resolution methylomes of tomato fruit development reveal epigenome modifications associated with ripening. Nat. Biotechnol. 2013, 31, 154–159. [Google Scholar] [CrossRef]
- Hazubska-Przybył, T.; Obarska, A.; Konecka, A.; Kijowska-Oberc, J.; Wawrzyniak, M.K.; Piotrowska-Niczyporuk, A.; Staszak, A.M.; Ratajczak, E. Modulating ascorbic acid levels to optimize somatic embryogenesis in Picea abies (L.) H. Karst. Insights into oxidative stress and endogenous phytohormones regulation. Front. Plant Sci. 2024, 15, 1372764. [Google Scholar] [CrossRef]
- Smýkal, P.; Valledor, L.; Rodríguez, R.; Griga, M. Assessment of genetic and epigenetic stability in long-term in vitro shoot culture of pea (Pisum sativum L.). Plant Cell Rep. 2007, 26, 1985–1998. [Google Scholar] [CrossRef]
- Manning, K.; Tör, M.; Poole, M.; Hong, Y.; Thompson, A.J.; King, G.J.; Giovannoni, J.J.; Seymour, G.B. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006, 38, 948–952. [Google Scholar] [CrossRef]
- Kaeppler, S.M.; Phillips, R.L. Tissue culture-induced DNA methylation variation in maize. Proc. Natl. Acad. Sci. USA 1993, 90, 8773–8776. [Google Scholar] [CrossRef]
- Zeng, F.-S.; Sun, F.-K.; Liang, N.-S.; Zhao, X.-T.; Luo, W.; Zhan, Y.-G. Dynamic change of DNA methylation and cell redox state at different micropropagation phases in birch. Trees 2015, 29, 917–930. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.-K. Epigenetic regulation of stress responses in plants. Curr. Opin. Plant Biol. 2009, 12, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Inzé, D.; Montagu, M.V. Oxidative stress in plants. Curr. Opin. Biotechnol. 1995, 6, 153–158. [Google Scholar] [CrossRef]
- Cheng, W.-H.; Wang, F.-L.; Cheng, X.-Q.; Zhu, Q.-H.; Sun, Y.-Q.; Zhu, H.-G.; Sun, J. Polyamine and Its Metabolite H2O2 Play a Key Role in the Conversion of Embryogenic Callus into Somatic Embryos in Upland Cotton (Gossypium hirsutum L.). Front. Plant Sci. 2015, 6, 1063. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.-m.; Fan, R.; Yin, G.-x.; Wang, K.; Du, L.-p.; Xiao, L.-l.; Ye, X.-g. Effects of inter-culture, arabinogalactan proteins, and hydrogen peroxide on the plant regeneration of wheat immature embryos. J. Integr. Agric. 2015, 14, 11–19. [Google Scholar] [CrossRef]
- Konieczny, R.; Libik, M.; Tuleja, M.; Niewiadomska, E.; Miszalski, Z. Oxidative events during in vitro regeneration of sunflower. Acta Physiol. Plant. 2008, 30, 71–79. [Google Scholar] [CrossRef]
- Gupta, S.D.; Datta, S. Antioxidant Enzyme Activities during in vitro Morphogenesis of Gladiolus and the Effect of Application of Antioxidants on Plant Regeneration. Biol. Plant. 2003, 47, 179–183. [Google Scholar] [CrossRef]
- Guo, H.; Kang, X.; Yuan, M.; Wu, R.; Du, L. Relationship between somatic embryogenesis and endogenous hormones of Cinnamomum camphora L. Plant Cell Tissue Organ Cult. 2024, 156, 53. [Google Scholar] [CrossRef]
- Chen, T.; Jia, X.; Yu, C.; Yin, Y.; Hua, J. A simple and efficient protocol for cryopreservation of Taxodium hybrid ‘zhongshanshan’ embryogenic callus. Plant Cell Tissue Organ Cult. 2023, 156, 44. [Google Scholar] [CrossRef]
- Chen, T.; Jia, X.; Zhang, R.; Lu, Y.; Yu, C.; Yin, Y.; Hua, J.; Creech, D. Somatic Embryogenesis in Taxodium Hybrid ‘zhongshanshan’, an Opportunity to Propagate an Ecologically Friendly Species. J. Plant Growth Regul. 2024, 43, 1447–1457. [Google Scholar] [CrossRef]
- Gupta, P.K.; Durzan, D.J. Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep. 1985, 4, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, G.; Mccown, B.H. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propagators’ Soc. 1980, 30, 421–427. [Google Scholar]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—Powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]


| The First Time of Occurrence | Control | 5 μM | 10 μM | 15 μM | 30 μM | 50 μM |
|---|---|---|---|---|---|---|
| Columnar embryo | 30 d | 30 d | 30 d | 35 d | 35 d | 40 d |
| Mature cotyledonary embryo | 45 d | 40 d | 40 d | 45 d | 45 d | 50 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, G.; Wang, D.; Yu, C.; Hua, J.; Yin, Y.; Chen, T. 5-AzaCytidine Promotes Somatic Embryogenesis of Taxodium Hybrid ‘Zhongshanshan’ by Regulating Redox Homeostasis. Plants 2025, 14, 1354. https://doi.org/10.3390/plants14091354
Yuan G, Wang D, Yu C, Hua J, Yin Y, Chen T. 5-AzaCytidine Promotes Somatic Embryogenesis of Taxodium Hybrid ‘Zhongshanshan’ by Regulating Redox Homeostasis. Plants. 2025; 14(9):1354. https://doi.org/10.3390/plants14091354
Chicago/Turabian StyleYuan, Guoying, Dan Wang, Chaoguang Yu, Jianfeng Hua, Yunlong Yin, and Tingting Chen. 2025. "5-AzaCytidine Promotes Somatic Embryogenesis of Taxodium Hybrid ‘Zhongshanshan’ by Regulating Redox Homeostasis" Plants 14, no. 9: 1354. https://doi.org/10.3390/plants14091354
APA StyleYuan, G., Wang, D., Yu, C., Hua, J., Yin, Y., & Chen, T. (2025). 5-AzaCytidine Promotes Somatic Embryogenesis of Taxodium Hybrid ‘Zhongshanshan’ by Regulating Redox Homeostasis. Plants, 14(9), 1354. https://doi.org/10.3390/plants14091354






