Re-Expression of the Lorenz Asymmetry Coefficient on the Rotated and Right-Shifted Lorenz Curve of Leaf Area Distributions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Leaf Sampling
2.2. Data Acquisition
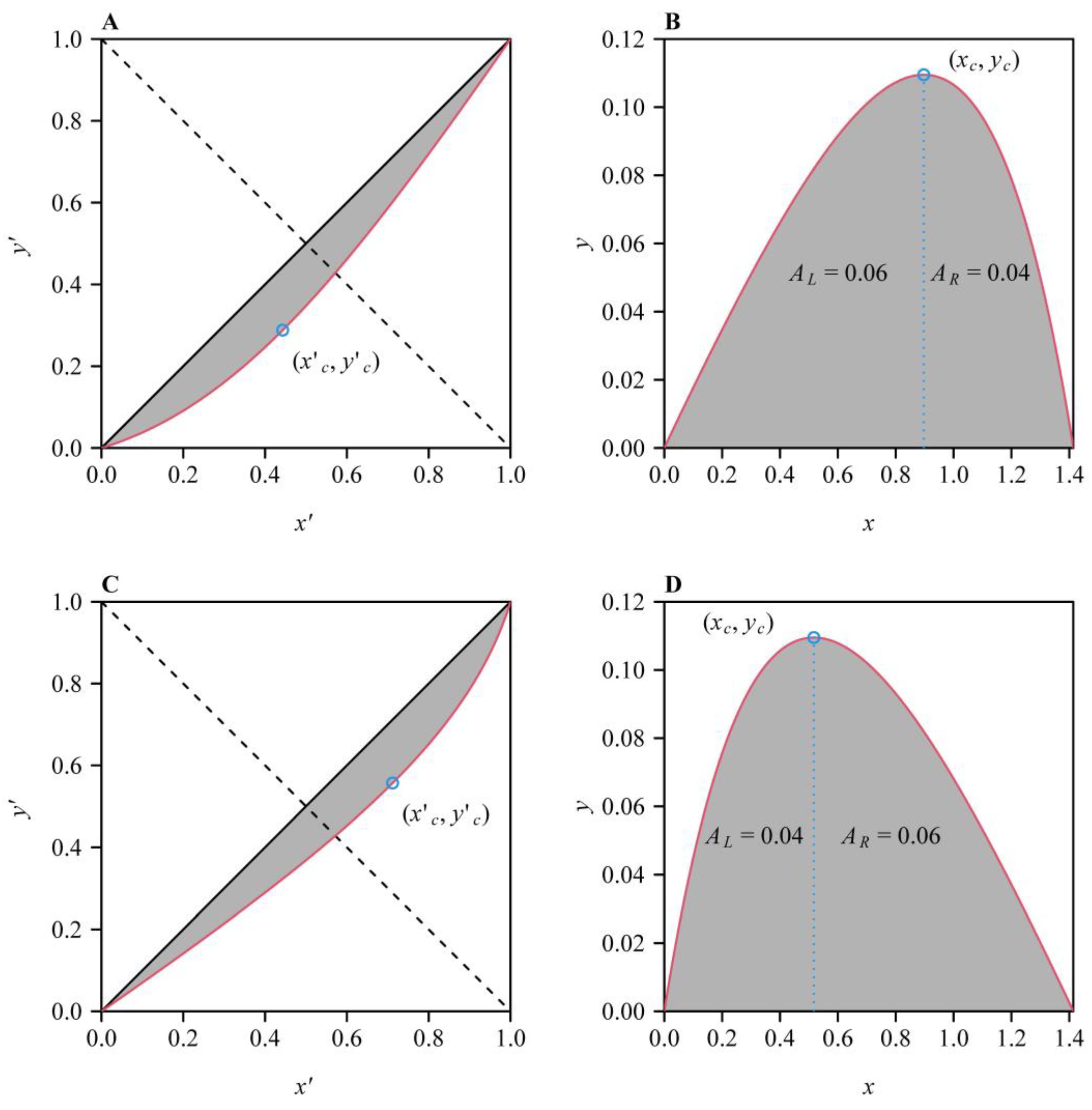
2.3. Three Indicators for Measuring the Asymmetry of the Lorenz Curve
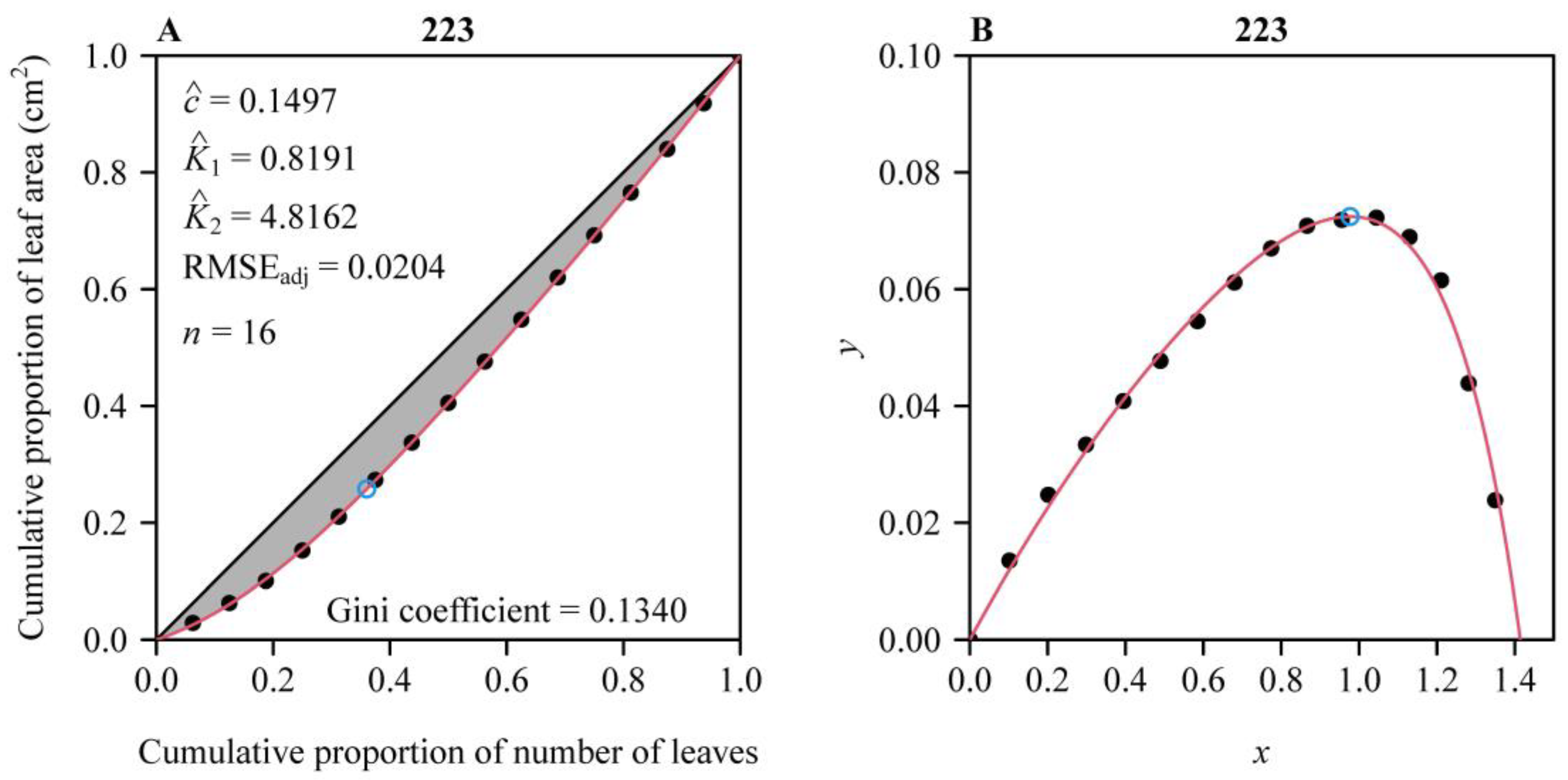
2.4. Parametric Estimation of the Performance Equation
2.5. Calculation of the Gini Coefficient for the Leaf Area Distribution per Shoot
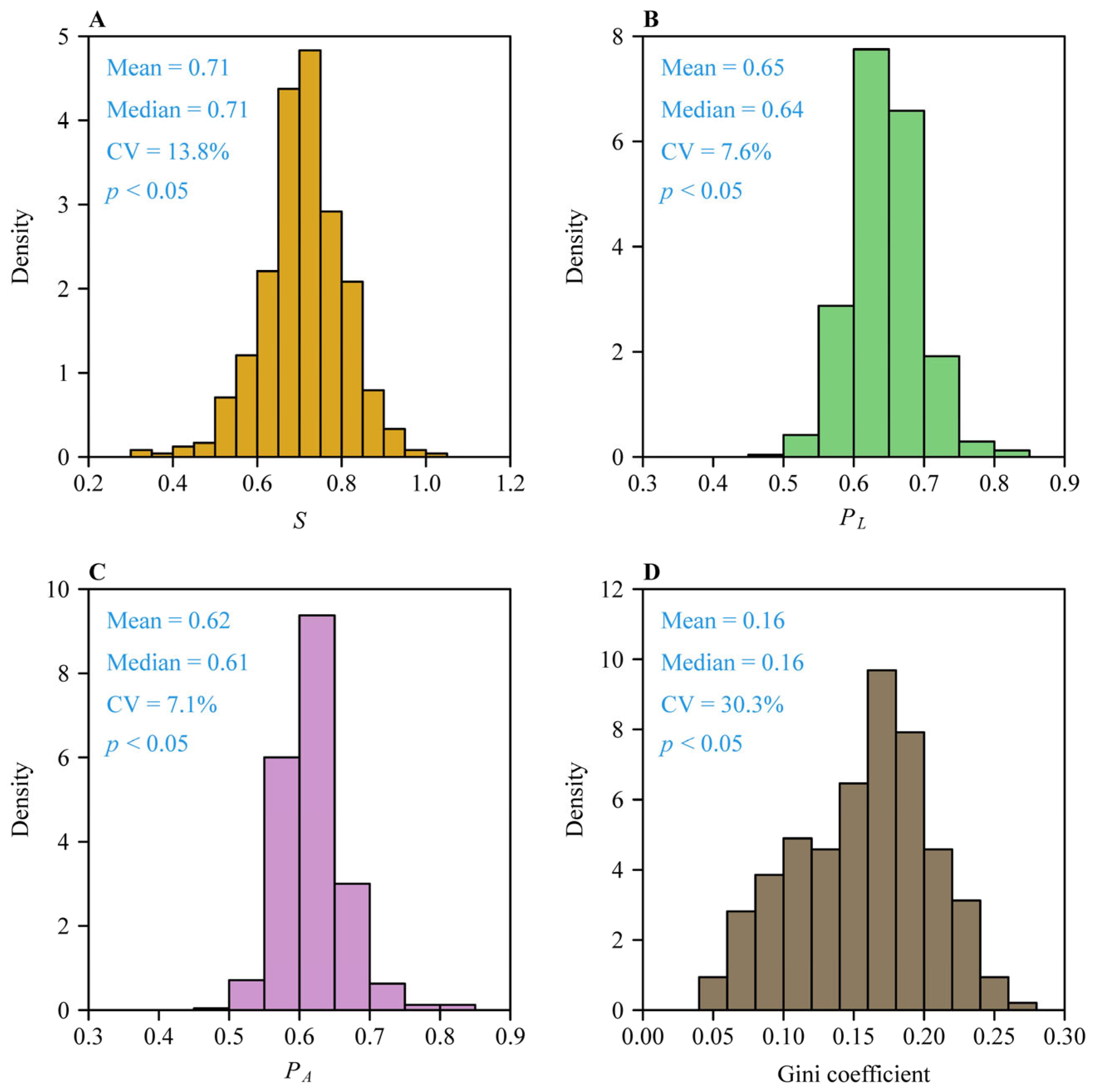
3. Results
4. Discussion
4.1. Ecological Implications of Asymmetric Leaf Area Distributions and Resource Allocation Strategies
4.2. Methodological Advancements: From the Gini Coefficient to Multidimensional Asymmetry Metrics
4.3. Practical Applications and Future Directions: Bridging Theory and Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Relationship Between Two Asymmetry Measures
References
- Evans, J.R. Leaf anatomy enables more equal access to light and CO2 between chloroplasts. New Phytol. 1999, 143, 93–104. [Google Scholar] [CrossRef]
- Taiz, L.; Møller, I.M.; Murphy, A.; Zeiger, E. Plant Physiology and Development, 7th ed.; Sinauer Associates: Sunderland, MA, USA, 2022. [Google Scholar]
- Küppers, M. Ecological significance of above-ground architectural patterns in woody plants: A question of cost-benefit relationships. Trends Ecol. Evol. 1989, 4, 375–379. [Google Scholar] [CrossRef]
- de Casas, R.R.; Vargas, P.; Pérez-Corona, E.; Manrique, E.; García-Verdugo, C.; Balaguer, L. Sun and shade leaves of Olea europaea respond differently to plant size, light availability and genetic variation. Funct. Ecol. 2011, 25, 802–812. [Google Scholar] [CrossRef]
- Niinemets, Ü. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Terashima, I.; Hanba, Y.T.; Tazoe, Y.; Vyas, P.; Yano, S. Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 2006, 57, 343–354. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef]
- Dörken, V.M.; Lepetit, B. Morpho-anatomical and physiological differences between sun and shade leaves in Abies alba Mill. (Pinaceae, Coniferales): A combined approach. Plant Cell Environ. 2018, 41, 1683–1697. [Google Scholar] [CrossRef]
- Hirose, T.; Werger, M.J.A. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 1987, 72, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Kikuzawa, K. Phenological and morphological adaptations to the light environment in two woody and two herbaceous plant species. Funct. Ecol. 2003, 17, 29–38. [Google Scholar] [CrossRef]
- Lian, M.; Shi, P.; Zhang, L.; Yao, W.; Gielis, J.; Niklas, K.J. A generalized performance equation and its application in measuring the Gini index of leaf size inequality. Trees Struct. Funct. 2023, 37, 1555–1565. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to sun and shade: A whole-plant perspective. Aust. J. Plant Physiol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Lorenz, M.O. Methods of measuring the concentration of wealth. Am. Statist. Assoc. 1905, 9, 209–219. [Google Scholar] [CrossRef]
- Gastwirth, J.L. A general definition of the Lorenz curve. Econometrica 1971, 39, 1037–1039. [Google Scholar] [CrossRef]
- Sarabia, J.-M. A hierarchy of Lorenz curves based on the generalized Tukey’s lambda distribution. Econom. Rev. 1997, 16, 305–320. [Google Scholar] [CrossRef]
- Gini, C. Variability and Mutability: Contribution to the Study of Distributions and Statistical Relationships; Paolo Cuppini Typography: Bologna, Italy, 1912. [Google Scholar]
- Weiner, J.; Solbrig, O.T. The meaning and measurement of size hierarchies in plant populations. Oecologia 1984, 61, 334–336. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Deng, L.; Niklas, K.J. Rotated Lorenz curves of biological size distributions follow two performance equations. Symmetry 2024, 16, 565. [Google Scholar] [CrossRef]
- Damgaard, C.; Weiner, J. Describing inequality in plant size or fecundity. Ecology 2000, 81, 1139–1142. [Google Scholar] [CrossRef]
- Huey, R.B.; Stevenson, R.D. Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Amer. Zool. 1979, 19, 357–366. [Google Scholar] [CrossRef]
- Ratkowsky, D.A. Nonlinear Regression Modeling; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Ratkowsky, D.A. Handbook of Nonlinear Regression Models; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Bates, D.M.; Watts, D.G. Nonlinear Regression Analysis and Its Applications; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Lian, M.; Chen, L.; Hui, C.; Shi, P. On the relationship between the Gini coefficient and skewness. Ecol. Evol. 2024, 14, e70637. [Google Scholar] [CrossRef]
- Shi, P.; Ratkowsky, D.; Li, Y.; Zhang, L.; Lin, S.; Gielis, J. General leaf-area geometric formula exists for plants—Evidence from the simplified Gielis equation. Forests 2018, 9, 714–768. [Google Scholar] [CrossRef]
- Su, J.; Niklas, K.J.; Huang, W.; Yu, X.; Yang, Y.; Shi, P. Lamina shape does not correlate with lamina surface area: An analysis based on the simplified Gielis equation. Glob. Ecol. Conserv. 2019, 19, e00666. [Google Scholar] [CrossRef]
- Shi, P.; Gielis, J.; Quinn, B.K.; Niklas, K.J.; Ratkowsky, D.A.; Schrader, J.; Ruan, H.; Wang, L.; Niinemets, Ü. ‘biogeom’: An R package for simulating and fitting natural shapes. Ann. N. Y. Acad. Sci. 2022, 1516, 123–134. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 June 2022).
- Montgomery, E.G. Correlation Studies in Corn; Annual Report No. 24; Nebraska Agricultural Experimental Station: Lincoln, NB, USA, 1911; pp. 108–159. [Google Scholar]
- Schrader, J.; Shi, P.; Royer, D.L.; Peppe, D.J.; Gallagher, R.V.; Li, Y.; Wang, R.; Wright, I.J. Leaf size estimation based on leaf length, width and shape. Ann. Bot. 2021, 128, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Heng, Y.; Xu, Q.; Zhou, Y.; Sun, X.; Wang, Y.; Yao, W.; Lian, M.; Li, Q.; Zhang, L.; et al. Scaling relationships between the total number of leaves and the total leaf area per culm of two dwarf bamboo species. Ecol. Evol. 2024, 14, e70002. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J. Plant Allometry: The Scaling of Form and Process; The University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar]
- Shi, P.; Ge, F.; Sun, Y.; Chen, C. A simple model for describing the effect of temperature on insect developmental rate. J. Asia-Pacific Entomol. 2011, 14, 15–20. [Google Scholar] [CrossRef]
- Byrd, R.H.; Lu, P.; Nocedal, J.; Zhu, C. A limited memory algorithm for bound constrained optimization. SIAM J. Sci. Comput. 1995, 16, 1190–1208. [Google Scholar] [CrossRef]
- Shi, P.; Chen, L.; Quinn, B.K.; Yu, K.; Miao, Q.; Guo, X.; Lian, M.; Gielis, J.; Niklas, K.J. A simple way to calculate the volume and surface area of avian eggs. Ann. N. Y. Acad. Sci. 2023, 1524, 118–131. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Jiang, F.; Damgaard, C.F.; Shi, P.; Weiner, J. Re-Expression of the Lorenz Asymmetry Coefficient on the Rotated and Right-Shifted Lorenz Curve of Leaf Area Distributions. Plants 2025, 14, 1345. https://doi.org/10.3390/plants14091345
Chen Y, Jiang F, Damgaard CF, Shi P, Weiner J. Re-Expression of the Lorenz Asymmetry Coefficient on the Rotated and Right-Shifted Lorenz Curve of Leaf Area Distributions. Plants. 2025; 14(9):1345. https://doi.org/10.3390/plants14091345
Chicago/Turabian StyleChen, Yongxia, Feixue Jiang, Christian Frølund Damgaard, Peijian Shi, and Jacob Weiner. 2025. "Re-Expression of the Lorenz Asymmetry Coefficient on the Rotated and Right-Shifted Lorenz Curve of Leaf Area Distributions" Plants 14, no. 9: 1345. https://doi.org/10.3390/plants14091345
APA StyleChen, Y., Jiang, F., Damgaard, C. F., Shi, P., & Weiner, J. (2025). Re-Expression of the Lorenz Asymmetry Coefficient on the Rotated and Right-Shifted Lorenz Curve of Leaf Area Distributions. Plants, 14(9), 1345. https://doi.org/10.3390/plants14091345






