Photosynthetic Responses of Pontederia cordata to Cadmium Stress: Anatomical Structure, Ultrastructure, Physiology, and Gene Expression
Abstract
:1. Introduction
2. Results
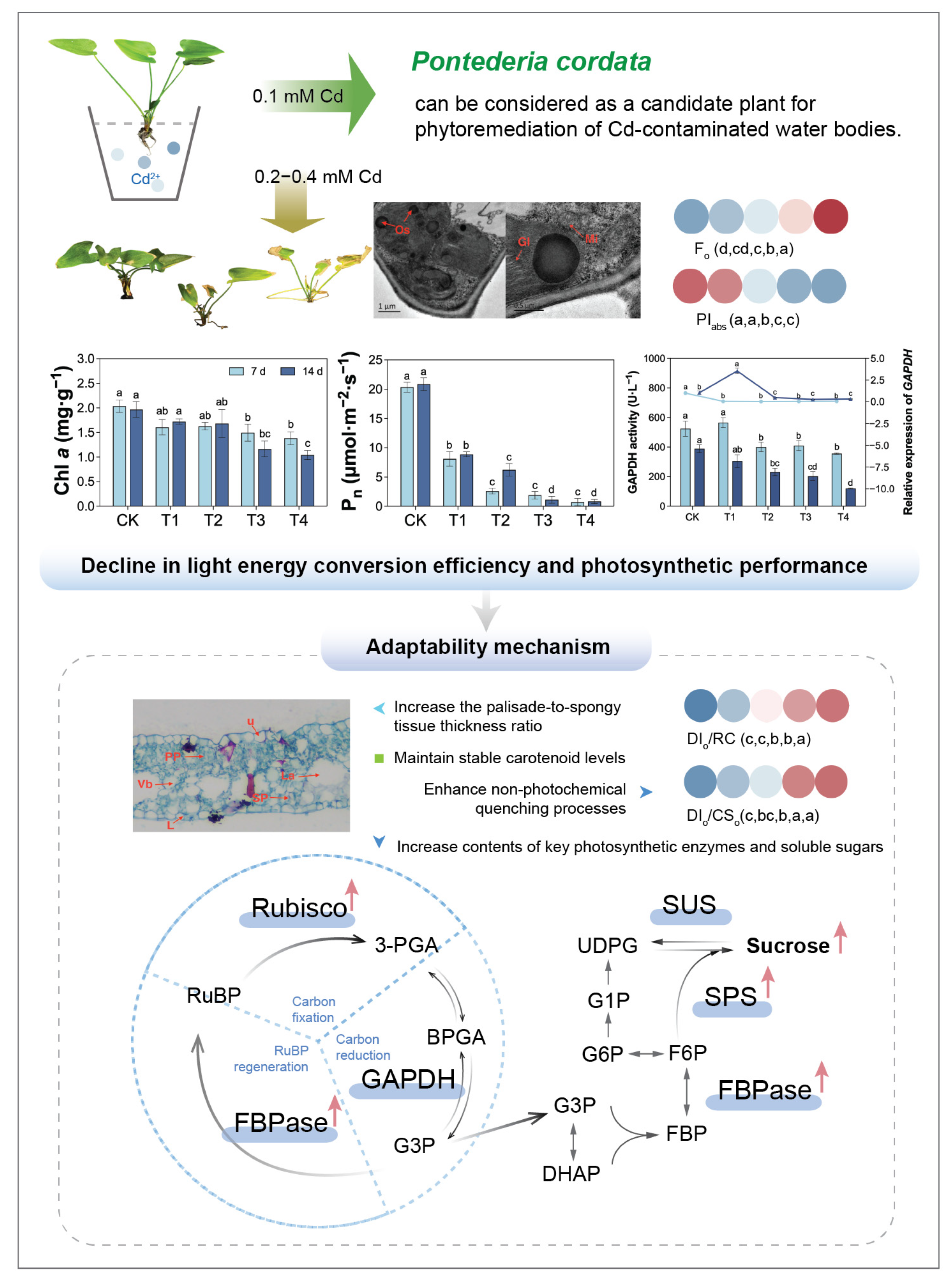
2.1. Morphological Changes in Pontederia cordata Under Cd Treatment
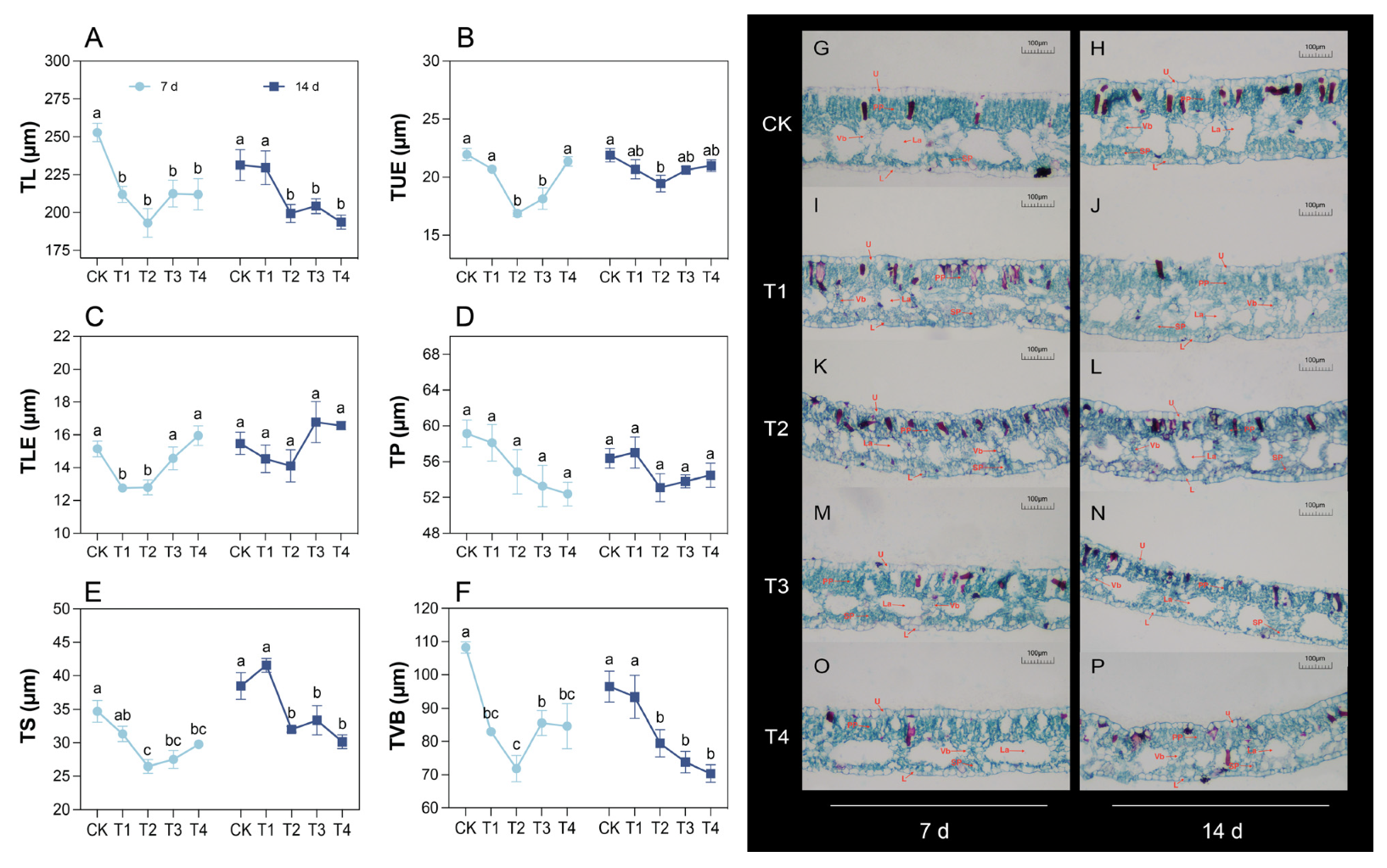
2.2. Changes in Leaf Anatomical Structure Under Cd Treatment
2.3. Changes in Chloroplast Ultrastructure Under Cd Treatment
2.4. Changes in Photosynthetic Pigment Content Under Cd Treatment
2.5. Changes in Photosynthetic Gas Exchange Under Cd Treatment
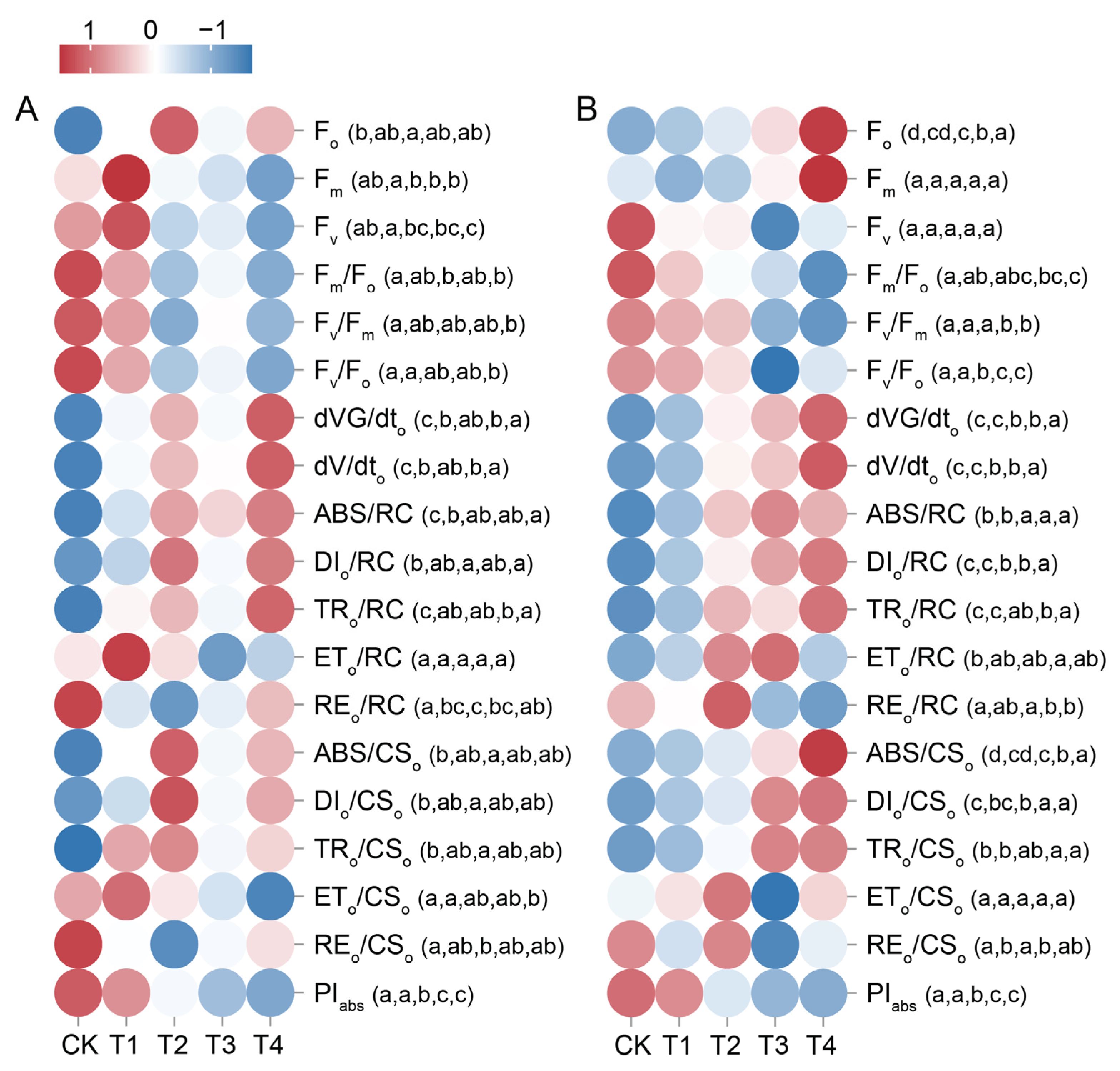
2.6. Changes in Leaf Chlorophyll a Fluorescence Parameter Under Cd Treatment
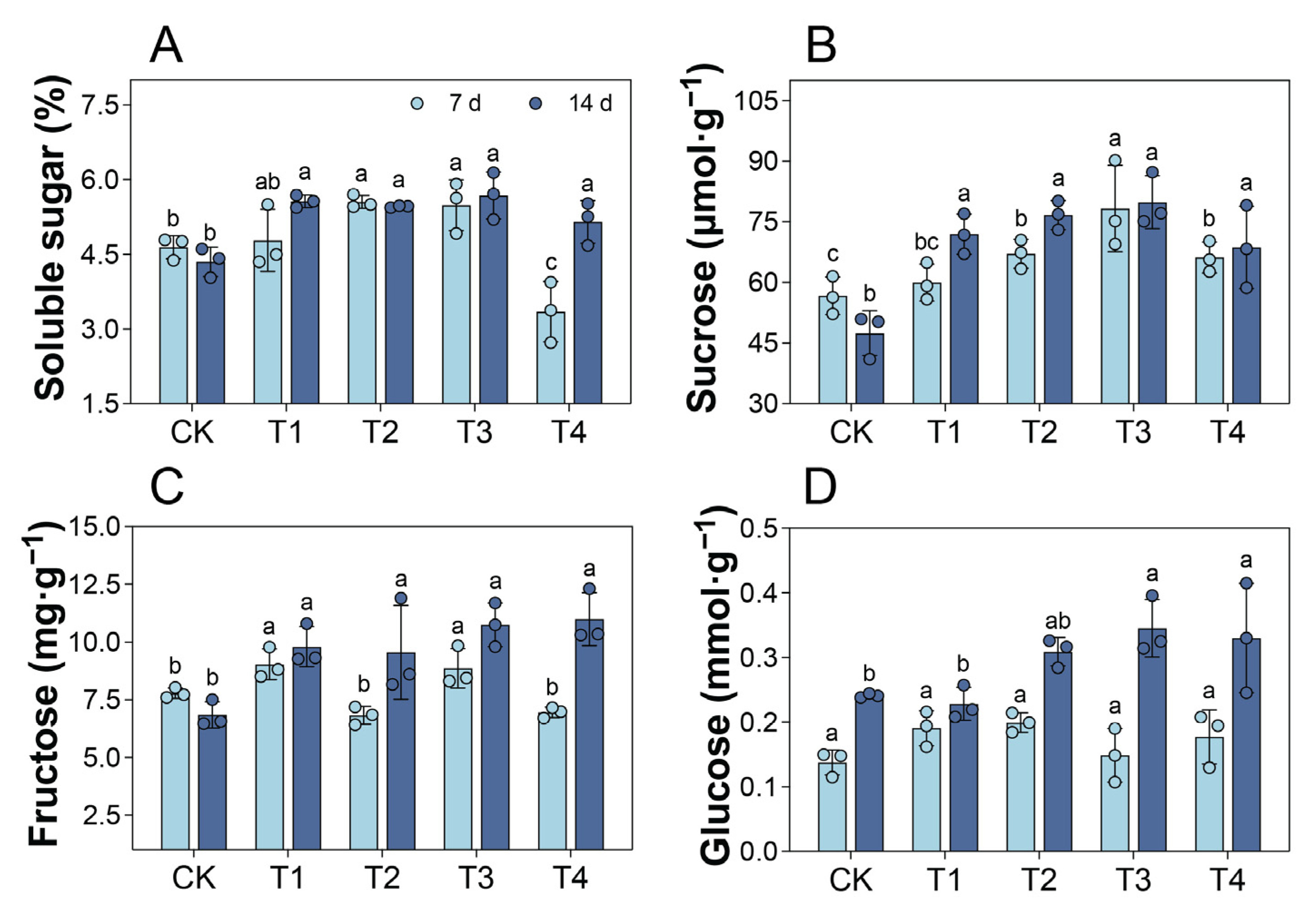
2.7. Changes in Leaf Soluble Sugar Content Under Cd Treatment
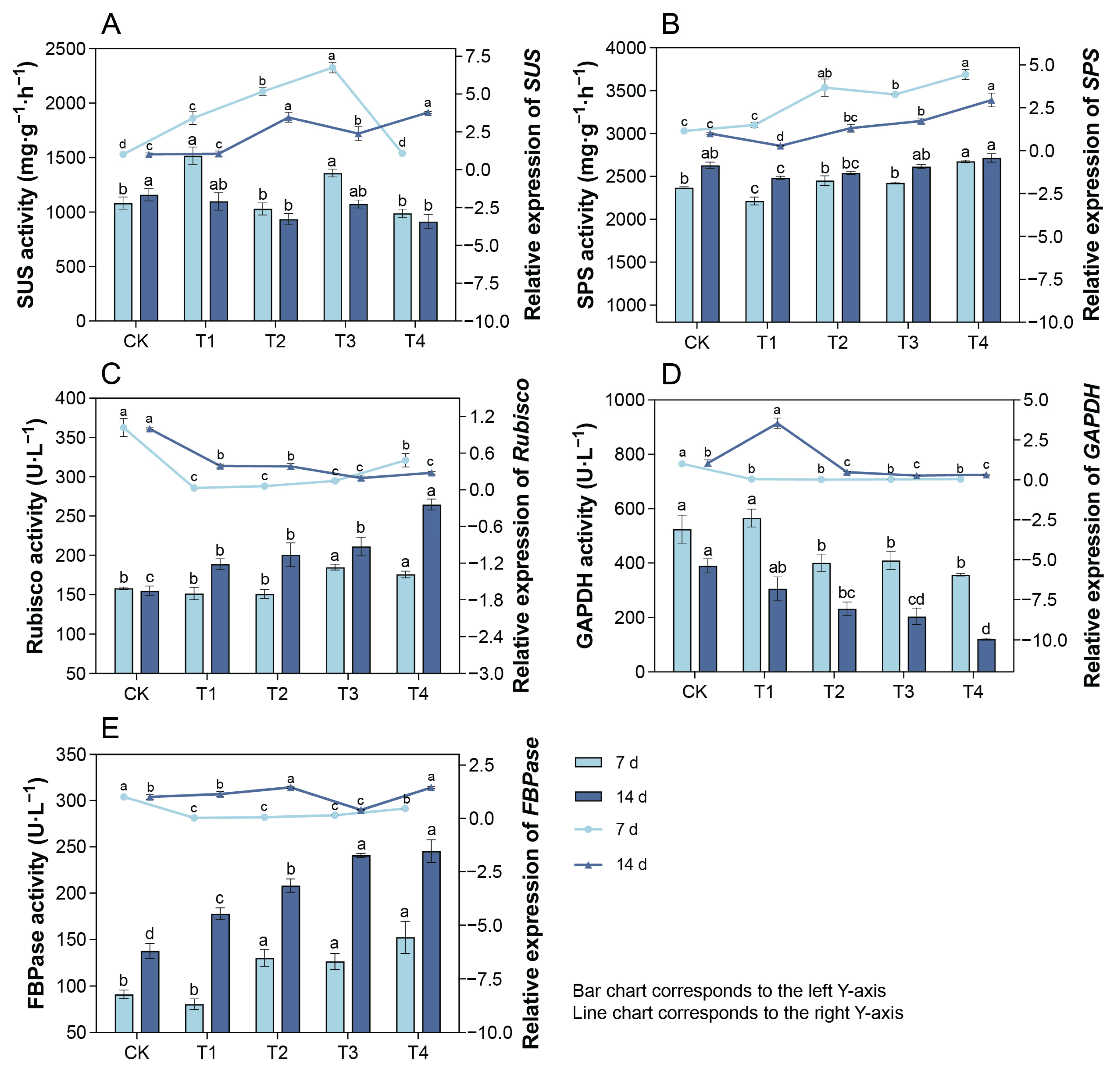
2.8. Changes in Leaf SUS and SPS Activities and Their Gene Expression Under Cd Treatment
2.9. Changes in Leaf RuBisCO, GAPDH, and FBPase Activities and Their Gene Expression Under Cd Treatment
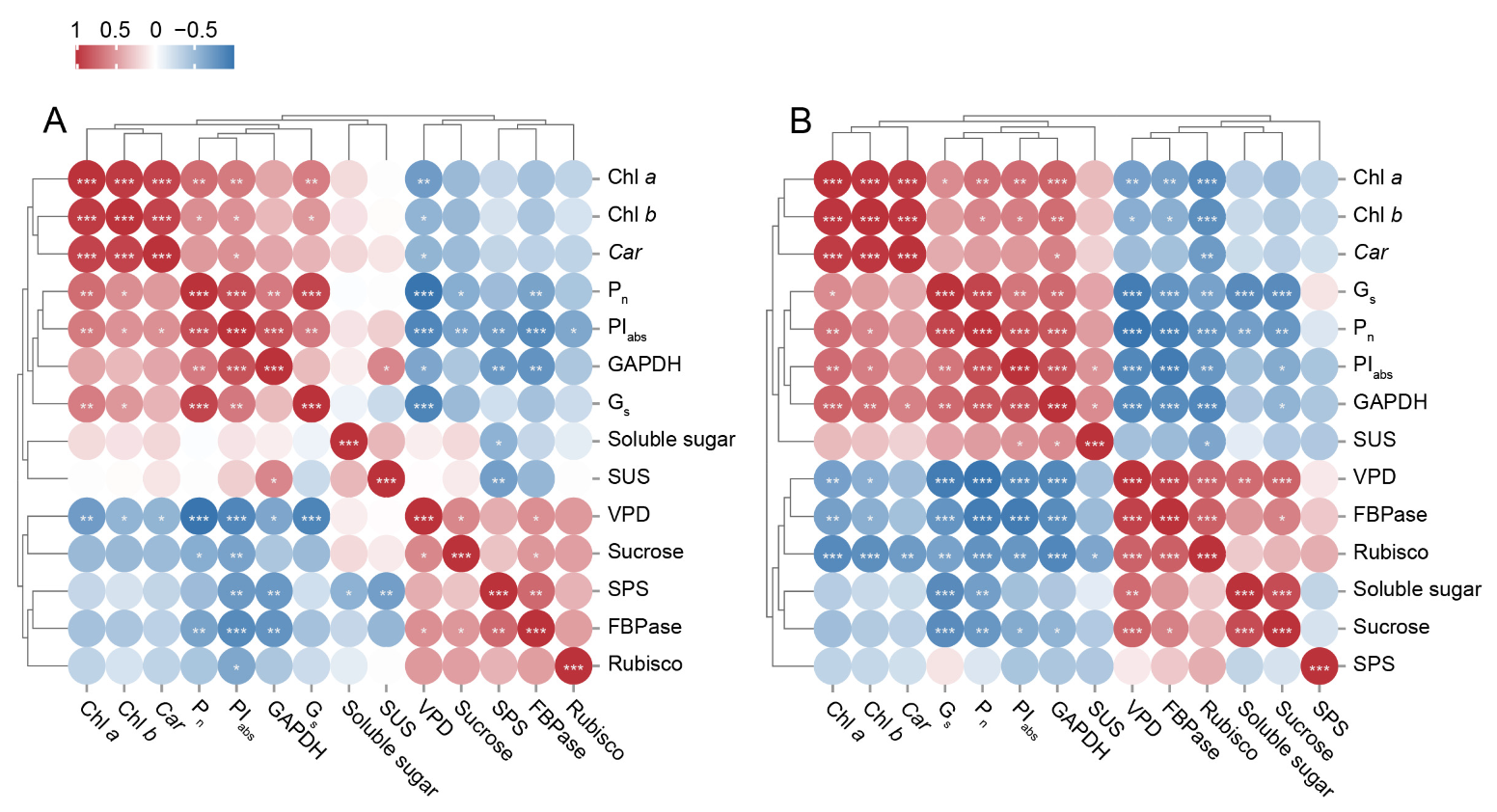
2.10. Correlation Analysis of Leaf Photosynthetic Physiological Indices Under Cd Treatment
3. Discussion
4. Materials and Methods
4.1. Experimental Materials
4.2. Experimental Design
4.3. Observation of Leaf Structure Characters in P. cordata
4.3.1. Leaf Anatomical Structure
4.3.2. Chloroplast Ultrastructure
4.4. Measurement of Photosynthetic Physiological Parameters
4.4.1. Photosynthetic Pigment Content
4.4.2. Photosynthetic Gas Exchange Parameter
4.4.3. Chlorophyll a Fluorescence Parameter
4.5. Measurement of Photosynthetic Carbon Metabolism
4.5.1. Soluble Sugar Content
4.5.2. Sucrose, Fructose, and Glucose Contents
4.5.3. SUS and SPS Activities
4.5.4. RuBisCO, GAPDH, and FBPase Activities
4.5.5. Gene Expression
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- El Rasafi, T.; Oukarroum, A.; Haddioui, A.; Song, H.; Kwon, E.E.; Bolan, N.; Tack, F.M.; Sebastian, A.; Prasad, M.N.V.; Rinklebe, J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022, 52, 675–726. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Adrees, M.; Ibrahim, M.; Tsang, D.C.; Zia-Ur-Rehman, M.; Zahir, Z.A.; Rinklebe, J.; Tack, F.M.; Ok, Y.S. A critical review on effects, tolerance mechanisms and management of cadmium in vegetables. Chemosphere 2017, 182, 90–105. [Google Scholar] [CrossRef]
- Dobrikova, A.; Apostolova, E.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.-D.S.; Moustakas, M. Cadmium toxicity in Salvia sclarea L.: An integrative response of element uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol. Environ. Saf. 2021, 209, 11851. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Y.; Li, Z.; Tan, X.-T.; Xin, G.-R.; He, C.-T. Defense guard: Strategies of plants in the fight against cadmium stress. Adv. Biotechnol. 2024, 2, 44. [Google Scholar] [CrossRef]
- Liang, A.; Ma, C.; Xiao, J.; Hao, Y.; Li, H.; Guo, Y.; Cao, Y.; Jia, W.; Han, L.; Chen, G.; et al. Micro/nanoscale bone char alleviates cadmium toxicity and boosts rice growth via positively altering the rhizosphere and endophytic microbial community. J. Hazard. Mater. 2023, 454, 131491. [Google Scholar] [CrossRef]
- Lysenko, E.; Klaus, A.; Kartashov, A.; Kusnetsov, V.V. Specificity of Cd, Cu, and Fe effects on barley growth, metal contents in leaves and chloroplasts, and activities of photosystem I and photosystem II. Plant Physiol. Biochem. 2020, 147, 191–204. [Google Scholar] [CrossRef]
- Waheed, A.; Zhang, Q.; Xu, H.; Dou, H.; Muhammad, M.; Aili, A.; O Alshaharni, M. Mitigation of cadmium stress by salicylic acid: Physiological and biochemical responses in NM-2006, NM-92, and Mash-88 mung bean varieties. J. Hazard. Mater. 2025, 485, 136878. [Google Scholar] [CrossRef]
- Carmosilva, E.; Scales, J.; Madgwick, P.; Parry, M.A.J. Optimizing rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 2015, 38, 1817. [Google Scholar] [CrossRef]
- Sarabi, B.; Fresneau, C.; Ghaderi, N.; Bolandnazar, S.; Streb, P.; Badeck, F.-W.; Citerne, S.; Tangama, M.; David, A.; Ghashghaie, J. Stomatal and non-stomatal limitations are responsible in down-regulation of photosynthesis in melon plants grown under the saline condition: Application of carbon isotope discrimination as a reliable proxy. Plant Physiol. Biochem. 2019, 141, 1–19. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, X.; Xiang, J.; Qin, X.; Xiang, L.; Zou, X.; Zhu, F. PSI-mediated NADPH production and enzyme regulation play critical roles in carbon assimilation of pepper seedlings under cadmium stress. Hortic. Plant J. 2025, in press. [Google Scholar]
- Croce, R.; Carmo-Silva, E.; Cho, Y.B.; Ermakova, M.; Harbinson, J.; Lawson, T.; McCormick, A.J.; Niyogi, K.K.; Ort, D.R.; Patel-Tupper, D.; et al. Perspectives on improving photosynthesis to increase crop yield. Plant Cell 2024, 36, 3944–3973. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Mukherjee, S.; Kumar, R.; Alansi, S.; Shah, A.A.; Kalaji, H.M.; Javed, T.; Raza, A. Potassium and melatonin–mediated regulation of fructose-1,6-bisphosphatase (FBPase) and sedoheptulose-1,7-bisphosphatase (SBPase) activity improve photosynthetic efficiency, carbon assimilation and modulate glyoxalase system accompanying tolerance to cadmium stress in tomato seedlings. Plant Physiol. Biochem. 2022, 171, 49–65. [Google Scholar]
- Song, X.; Liu, Y.; Liu, S.; Li, J.; Wang, Y.; Zhang, Y.; Song, W. Proteomic and physiological analyses reveal the mechanisms through which melatonin ameliorates heat stress-induced photoinhibition in Nicotiana tabacum. Plant Physiol. Biochem. 2025, 221, 109665. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Mukherjee, A.; Das, P.; Bandyopadhyay, S.; Chattopadhyay, D.; Chatterjee, J.; Majumder, A.L. A salt-tolerant chloroplastic FBPase from Oryza coarctata confers improved photosynthesis with higher yield and multistress tolerance to indica rice. Plant Cell Tissue Organ Cult. 2021, 145, 561–578. [Google Scholar] [CrossRef]
- Formela-Luboińska, M.; Chadzinikolau, T.; Drzewiecka, K.; Jeleń, H.; Bocianowski, J.; Kęsy, J.; Labudda, M.; Jeandet, P.; Morkunas, I. The role of sugars in the regulation of the level of endogenous signaling molecules during defense response of yellow lupine to Fusarium oxysporum. Int. J. Mol. Sci. 2020, 21, 4133. [Google Scholar] [CrossRef]
- Tang, W.; Liang, L.; Yang, H.; Yu, X.; Ye, X.; Xie, Y.; Li, R.; Lin, L.; Huang, Z.; Sun, B.; et al. Exogenous salicylic acid reduces cadmium content in spinach (Spinacia oleracea L.) shoots under cadmium stress. BMC Plant Biol. 2024, 24, 1226. [Google Scholar] [CrossRef]
- Hu, J.; Duan, Y.; Hu, J.; Zhang, S.; Li, G. Phylogenetic and expression analysis of the sucrose synthase and sucrose phosphate synthase gene family in potatoes. Metabolites 2024, 14, 70. [Google Scholar] [CrossRef]
- Li, X.; Du, J.; Guo, J.; Wang, H.; Ma, S.; Lü, J.; Sui, X.; Zhang, Z. The functions of cucumber sucrose phosphate synthases 4 (CsSPS4) in carbon metabolism and transport in sucrose- and stachyose-transporting plants—ScienceDirect. J. Plant Physiol. 2018, 228, 150–157. [Google Scholar] [CrossRef]
- Xin, J.; Zhao, C.; Li, Y.; Ma, S.; Tian, R. Transcriptional, secondary metabolic, and antioxidative investigations elucidate the rapid response mechanism of Pontederia cordata to cadmium. Ecotoxicol. Environ. Saf. 2022, 232, 113236. [Google Scholar] [CrossRef]
- Borsuk, A.; Roddy, A.; Guillaume Théroux-Rancourt, G.; Brodersen, C.R. Structural organization of the spongy mesophyll. New Phytologist. 2022, 234, 946–960. [Google Scholar] [CrossRef]
- Borsuk, A.; Brodersen, C. The spatial distribution of chlorophyll in leaves. Plant Physiol. 2019, 180, 1406–1417. [Google Scholar] [CrossRef] [PubMed]
- Théroux-Rancourt, G.; Roddy, A.; Earles, J.; Gilbert, M.E.; Zwieniecki, M.A.; Boyce, C.K.; Tholen, D.; McElrone, A.J.; Simonin, K.A.; Brodersen, C.R. Maximum CO2 diffusion inside leaves is limited by the scaling of cell size and genome size. Proc. R. Soc. B Biol. Sci. 2021, 288, 20203145. [Google Scholar]
- Guo, Z.; Zeng, P.; Xiao, X.; Peng, C. Physiological, anatomical, and transcriptional responses of mulberry (Morus alba L.) to Cd stress in contaminated soil. Environ. Pollut. 2021, 284, 117387. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Zang, J.; Shao, T.; Jiang, Q.; Li, Y.; Zhang, W.; Liu, M. Cadmium distribution and transformation in leaf cells involved in detoxification and tolerance in barley. Ecotoxicol. Environ. Saf. 2023, 249, 114391. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Z.; Guo, K.; Huo, Y.; He, G.; Sun, H.; Guan, Y.; Xu, N.; Yang, W.; Sun, G. Toxic effects of heavy metal Cd and Zn on chlorophyll, carotenoid metabolism and photosynthetic function in tobacco leaves revealed by physiological and proteomics analysis. Ecotoxicol. Environ. Saf. 2020, 202, 110856. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhao, C.; Jiang, X.; Gao, D. Responses of non-structural carbohydrates and biomass in plant to heavy metal treatment. Sci. Total Environ. 2024, 909, 168559. [Google Scholar] [CrossRef]
- Salmon, Y.; Lintunen, A.; Dayet, A.; Chan, T.; Dewar, R.; Vesala, T.; Hölttä, T. Leaf carbon and water status control stomatal and nonstomatal limitations of photosynthesis in trees. New Phytol. 2020, 226, 690–703. [Google Scholar] [CrossRef]
- Li, Y.; Xin, J.; Tian, R. Physiological defense and metabolic strategy of Pistia stratiotes in response to zinc-cadmium co-pollution. Plant Physiol. Biochem. 2022, 178, 1–11. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, K.; Zhang, J.; Wang, Z.; Sun, Z.; Zhang, H.; Hu, J. Response analysis of fluorescence parameters of tomato seedlings oriented to vertical light environment adaptation. Plant Sci. 2022, 314, 111118. [Google Scholar] [CrossRef]
- Gan, T.; Yin, G.; Zhao, N.; Tan, X.; Wang, Y.; Sheng, R.; Ye, Z. A new sensitive response index for detecting water toxicity based on microalgal fluorescence kinetics. J. Appl. Phycol. 2023, 35, 2219–2239. [Google Scholar] [CrossRef]
- Chen, J.; Chen, S.; He, N.; Wang, Q.-L.; Zhao, Y.; Gao, W.; Guo, F.-Q. Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat. Plants 2020, 6, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, L.; Xu, B.; Cao, R.; Charagh, S.; Hui, S.; Zhou, L.; Zhang, Y.; Chen, Y.; Sheng, Z.; et al. Chloroplast-localized transporter OsNTP1 mediates cadmium transport from root to shoot and sugar metabolism in rice. J. Hazard. Mater. 2025, 484, 136766. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Balal, R.; Khan, N.; Zotarelli, L.; Liu, G.D.; Sarkhosh, A.; Fernández-Zapata, J.C.; Nicolás, J.J.M.; Garcia-Sanchez, F. Selenium impedes cadmium and arsenic toxicity in potato by modulating carbohydrate and nitrogen metabolism. Ecotoxicol. Environ. Saf. 2019, 180, 588–599. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, H.; Wu, M.; Zhou, Y.; Yu, C.; Yang, Q.; Rolland, F.; Van de Poel, B.; Bouzayen, M.; Hu, N.; et al. A vacuolar invertase gene SlVI modulates sugar metabolism and postharvest fruit quality and stress resistance in tomato. Hortic. Res. 2025, 12, uhae283. [Google Scholar] [CrossRef]
- Lakhneko, O.; Fialová, I.; Fiala, R.; Kopáčová, M.; Kováč, A.; Danchenko, M. Silicon might mitigate nickel toxicity in maize roots via chelation, detoxification, and membrane transport. Ecotoxicol. Environ. Saf. 2024, 288, 117334. [Google Scholar] [CrossRef]
- Anur, R.; Mufithah, N.; Sawitri, W.; Sakakibara, H.; Sugiharto, B. Overexpression of sucrose phosphate synthase enhanced sucrose content and biomass production in transgenic sugarcane. Plants 2020, 9, 200. [Google Scholar] [CrossRef]
- Jiang, D.; Cope, A.; Zhang, J.; Pennell, M. On the decoupling of evolutionary changes in mRNA and protein levels. Mol. Biol. Evol. 2023, 40, msad169. [Google Scholar] [CrossRef]
- Li, X.; Guo, X.; Zhou, Y.; Shi, K.; Zhou, J.; Yu, J.-Q.; Xia, X.-J. Overexpression of a brassinosteroid biosynthetic gene Dwarf enhances photosynthetic capacity through activation of Calvin cycle enzymes in tomato. BMC Plant Biol. 2016, 16, 33. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X. Plant Anatomy; Higher Education Press: Beijing, China, 1983. [Google Scholar]
- Wang, Y.; Qin, Y.; Zeng, T.; Liao, P.; Zhang, W.; Zhou, Y.; Zhou, C.Y. Effects of citrus yellow vein clearing virus on photosynthetic characteristics and chloroplast ultrastructure of lemon. Acta Hortic. Sin. 2022, 49, 861–867. [Google Scholar]
- Qiu, N.; Wang, X.; Yang, F.; Yang, X.; Yang, W.; Diao, R.; Wang, X.; Cui, J.; Zhou, F. Fast extraction and precise determination of chlorophyll. Bull. Bot. 2016, 51, 667–678. [Google Scholar]
- Yang, H.; Gao, T.; Li, Q.; Sun, X.; Wang, D.; Cao, H. Effects of grape downy mildew on photosynthesis of ‘red globe’ grape leaves under high temperature stress. Int. J. Fruit Sci. 2022, 22, 581–594. [Google Scholar] [CrossRef]
- Wang, X. Principles and Techniques of Plant Physiology and Biochemistry Experiments; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Zhang, Z.; Qu, W.; Li, X. Guidelines for Plant Physiology Experiments; Higher Education Press: Beijing, China, 2009. [Google Scholar]
- Li, Y.; Xin, J.; Ge, W.; Tian, R. Tolerance mechanism and phytoremediation potential of Pistia stratiotes to zinc and cadmium co-contamination. Int. J. Phytoremediat. 2022, 24, 1259–1266. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhou, W.; Xiao, H.; Xin, J.; Zhao, C.; Tian, R. Photosynthetic Responses of Pontederia cordata to Cadmium Stress: Anatomical Structure, Ultrastructure, Physiology, and Gene Expression. Plants 2025, 14, 1344. https://doi.org/10.3390/plants14091344
Li Y, Zhou W, Xiao H, Xin J, Zhao C, Tian R. Photosynthetic Responses of Pontederia cordata to Cadmium Stress: Anatomical Structure, Ultrastructure, Physiology, and Gene Expression. Plants. 2025; 14(9):1344. https://doi.org/10.3390/plants14091344
Chicago/Turabian StyleLi, Yan, Wei Zhou, Hanwen Xiao, Jianpan Xin, Chu Zhao, and Runan Tian. 2025. "Photosynthetic Responses of Pontederia cordata to Cadmium Stress: Anatomical Structure, Ultrastructure, Physiology, and Gene Expression" Plants 14, no. 9: 1344. https://doi.org/10.3390/plants14091344
APA StyleLi, Y., Zhou, W., Xiao, H., Xin, J., Zhao, C., & Tian, R. (2025). Photosynthetic Responses of Pontederia cordata to Cadmium Stress: Anatomical Structure, Ultrastructure, Physiology, and Gene Expression. Plants, 14(9), 1344. https://doi.org/10.3390/plants14091344





