Major Gene with Polygene Inheritance Analysis of Prostrate Growth Habit in Hybrids of Chrysanthemum yantaiense × C. indicum
Abstract
1. Introduction
2. Results
2.1. Frequency Distributions and Statistical Analysis
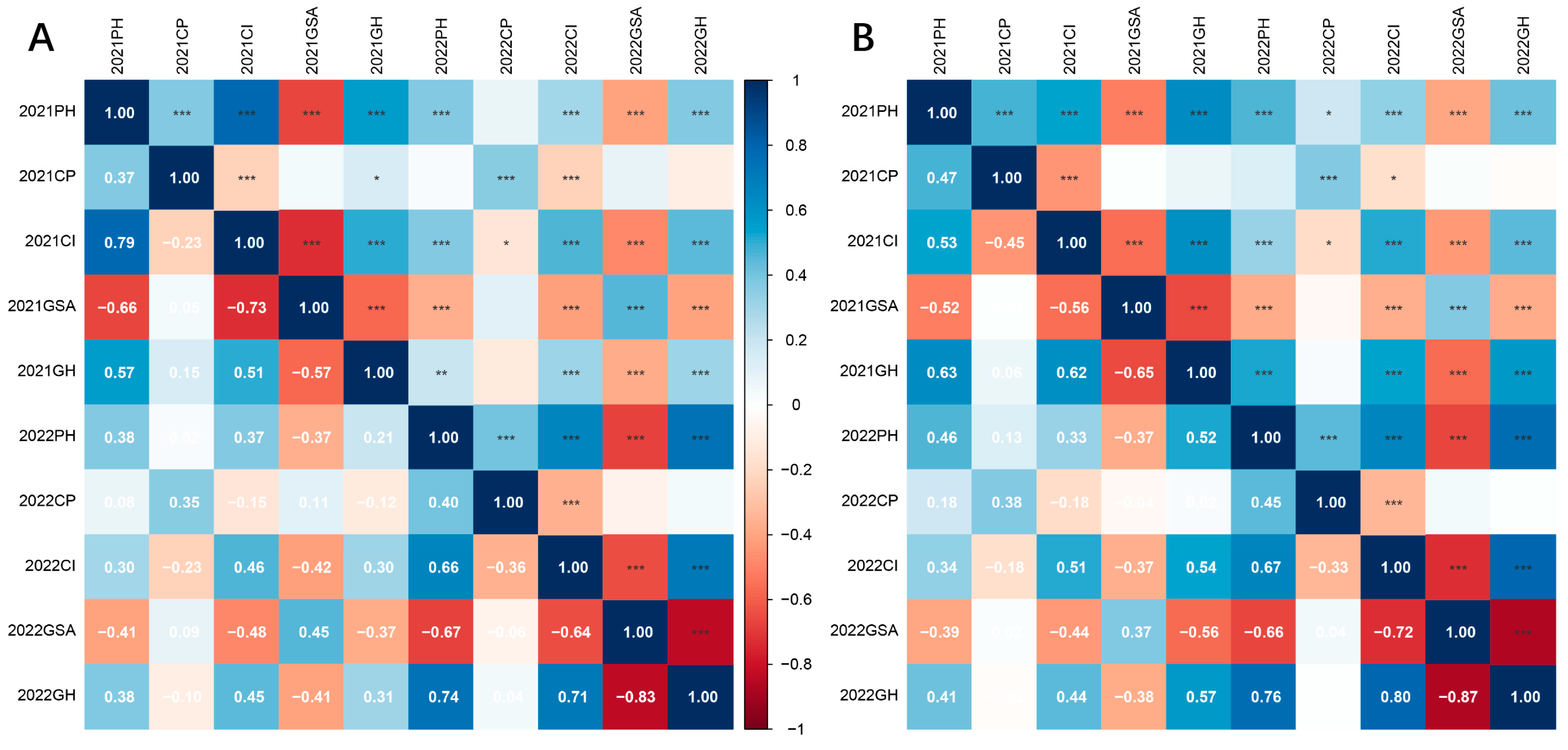
2.2. Correlation Between Plant Architecture Traits
2.3. Evaluation of Optimal Genetic Models
2.4. Genetic Parameter Estimation of Plant Architecture Traits Under the Optimal Genetic Model
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Trait Investigation and Phenotyping
4.3. Statistical and Joint Segregation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Teixeira da Silva, J.A.; Shinoyama, H.; Aida, R.; Matsushita, Y.; Raj, S.K.; Chen, F. Chrysanthemum biotechnology: Quo vadis? Crit. Rev. Plant Sci. 2013, 32, 21–52. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kourmpetli, S.; Davey, M.R. Practical Applications of Manipulating Plant Architecture by Regulating Gibberellin Metabolism. J. Plant Growth Regul. 2010, 29, 249–256. [Google Scholar] [CrossRef]
- Chen, J.; Wang, S.; Wang, X.; Wang, P. Thirty Years’ Studies on Breeding Ground-Cover Chrysanthemum New Cultivars; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1995; pp. 30–36. [Google Scholar]
- McSteen, P.; Leyser, O. Shoot branching. Annu. Rev. Plant Biol. 2005, 56, 353–374. [Google Scholar] [CrossRef] [PubMed]
- Kucukoglu, M.; Nilsson, J.; Zheng, B.; Chaabouni, S.; Nilsson, O. Wuschel-Related Homeobox 4 (WOX 4)-like genes regulate cambial cell division activity and secondary growth in Populus trees. New Phytol. 2017, 215, 642–657. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Zhang, C.; Ma, Q.; Joo, S.-H.; Kim, S.-K.; Xu, Z.; Chong, K. OsLIC, a novel CCCH-type zinc finger protein with transcription activation, mediates rice architecture via brassinosteroids signaling. PLoS ONE 2008, 3, e3521. [Google Scholar] [CrossRef]
- Ye, Y.-J.; Wu, J.-Y.; Feng, L.; Ju, Y.-Q.; Cai, M.; Cheng, T.-R.; Pan, H.-T.; Zhang, Q.-X. Heritability and gene effects for plant architecture traits of crape myrtle using major gene plus polygene inheritance analysis. Sci. Hortic. 2017, 225, 335–342. [Google Scholar] [CrossRef]
- Du, X.; Wang, H.; Liu, J.; Zhu, X.; Mu, J.; Feng, X.; Liu, H. Major gene with polygene inheritance analysis of shoot architecture traits in Viola cornuta. Sci. Hortic. 2022, 303, 111204. [Google Scholar] [CrossRef]
- Dougherty, L.; Singh, R.; Brown, S.; Dardick, C.; Xu, K. Exploring DNA variant segregation types in pooled genome sequencing enables effective mapping of weeping trait in Malus. J. Exp. Bot. 2018, 69, 1499–1516. [Google Scholar] [CrossRef]
- Fan, M.; Gao, Y.; Wu, Z.; Zhang, Q. Linkage Map Development by EST-SSR Markers and QTL Analysis for Inflorescence and Leaf Traits in Chrysanthemum (Chrysanthemum morifolium Ramat.). Plants 2020, 9, 1342. [Google Scholar] [CrossRef]
- Song, X.; Xu, Y.; Gao, K.; Fan, G.; Zhang, F.; Deng, C.; Dai, S.; Huang, H.; Xin, H.; Li, Y. High-density genetic map construction and identification of loci controlling flower-type traits in Chrysanthemum (Chrysanthemum × morifolium Ramat.). Hortic. Res. 2020, 7, 108. [Google Scholar] [CrossRef]
- van Geest, G.; Bourke, P.M.; Voorrips, R.E.; Marasek-Ciolakowska, A.; Liao, Y.; Post, A.; van Meeteren, U.; Visser, R.G.; Maliepaard, C.; Arens, P. An ultra-dense integrated linkage map for hexaploid chrysanthemum enables multi-allelic QTL analysis. Theor. Appl. Genet. 2017, 130, 2527–2541. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Jiang, J.; Zhang, F.; Liu, Y.; Ding, L.; Chen, S.; Chen, F. Current achievements and future prospects in the genetic breeding of chrysanthemum: A review. Hortic. Res. 2019, 6, 109. [Google Scholar] [CrossRef]
- Tang, F.; Chen, F.; Chen, S.; Teng, N.; Fang, W. Intergeneric hybridization and relationship of genera within the tribe Anthemideae Cass.(I. Dendranthema crassum (kitam.) kitam. × Crossostephium chinense (L.) Makino). Euphytica 2009, 169, 133–140. [Google Scholar] [CrossRef]
- Boase, M.; Miller, R.; Deroles, S. Chrysanthemum systematics, genetics, and breeding. Plant Breed. Rev. 1997, 14, 321–361. [Google Scholar]
- Fukai, S.; Nagira, T.; Goi, M. Cross compatibility between chrysanthemum and Dendranthema species native to Japan. Acta Hortic. 2000, 508, 337–340. [Google Scholar] [CrossRef]
- Sun, C.-Q.; Chen, F.-D.; Teng, N.-J.; Liu, Z.-L.; Fang, W.-M.; Hou, X.-L. Interspecific hybrids between Chrysanthemum grandiflorum (Ramat.) Kitamura and C. indicum (L.) Des Moul. and their drought tolerance evaluation. Euphytica 2010, 174, 51–60. [Google Scholar] [CrossRef]
- Gai, J.-Y. Segregation analysis of genetic system of quantitative traits in plants. Yi Chuan = Hered. 2005, 27, 130–136. [Google Scholar]
- Gai, J.-Y.; Wang, J.-K. Identification and estimation of a QTL model and its effects. Theor. Appl. Genet. 1998, 97, 1162–1168. [Google Scholar] [CrossRef]
- Gai, J.; Wang, Y.; Wu, X.; Chen, S. A comparative study on segregation analysis and QTL mapping of quantitative traits in plants—With a case in soybean. Front. Agric. China 2007, 1, 1–7. [Google Scholar] [CrossRef]
- Sun, X.-R.; Liu, L.; Zhi, X.-N.; Bai, J.-R.; Cui, Y.-N.; Shu, J.-S.; Li, J.-M. Genetic analysis of tomato internode length via mixed major gene plus polygene inheritance model. Sci. Hortic. 2019, 246, 759–764. [Google Scholar] [CrossRef]
- Dong, R.; Yu, B.; Yan, S.; Qiu, Z.; Lei, J.; Chen, C.; Li, Y.; Cao, B. Analysis of Vitamin P Content and Inheritance Models in Eggplant. Hortic. Plant J. 2020, 6, 240–246. [Google Scholar] [CrossRef]
- Fan, Z.; Gao, Y.; Liu, R.; Wang, X.; Guo, Y.; Zhang, Q. The major gene and polygene effects of ornamental traits in bearded iris (Iris germanica) using joint segregation analysis. Sci. Hortic. 2020, 260, 108882. [Google Scholar] [CrossRef]
- Gao, J.; Cai, W.; Xiao, Y.; Yu, F.; Zheng, Y.; Bi, X. Variation and inheritance of the degree of style branching in hybrids of Iris dichotoma × I. domestica. Sci. Hortic. 2021, 288, 110303. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, F.; Jiang, J.; Chen, S.; Fang, W.; Guan, Z.; Chen, F. Identification of quantitative trait loci for branching traits of spray cut chrysanthemum. Euphytica 2015, 202, 385–392. [Google Scholar] [CrossRef]
- Zhang, F.; Jiang, J.; Chen, S.; Chen, F.; Fang, W. Mapping single-locus and epistatic quantitative trait loci for plant architectural traits in chrysanthemum. Mol. Breed. 2012, 30, 1027–1036. [Google Scholar] [CrossRef]
- Uchino, H.; Uozumi, S.; Touno, E.; Kawamoto, H.; Deguchi, S. Soybean growth traits suitable for forage production in an Italian ryegrass living mulch system. Field Crops Res. 2016, 193, 143–153. [Google Scholar] [CrossRef]
- Li, L.; Yang, X.; Cui, S.; Meng, X.; Mu, G.; Hou, M.; He, M.; Zhang, H.; Liu, L.; Chen, C.Y. Construction of high-density genetic map and mapping quantitative trait loci for growth habit-related traits of peanut (Arachis hypogaea L.). Front. Plant Sci. 2019, 10, 745. [Google Scholar] [CrossRef]
- Marone, D.; Rodriguez, M.; Saia, S.; Papa, R.; Rau, D.; Pecorella, I.; Laidò, G.; Pecchioni, N.; Lafferty, J.; Rapp, M.; et al. Genome-wide association mapping of prostrate/erect growth habit in winter durum wheat. Int. J. Mol. Sci. 2020, 21, 394. [Google Scholar] [CrossRef]
- Werner, D.J.; Chaparro, J.X. Genetic interactions of pillar and weeping peach genotypes. HortScience 2005, 40, 18–20. [Google Scholar] [CrossRef]
- Roychoudhry, S.; Del Bianco, M.; Kieffer, M.; Kepinski, S. Auxin controls gravitropic setpoint angle in higher plant lateral branches. Curr. Biol. 2013, 23, 1497–1504. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Qian, Q.; Fu, Z.; Wang, M.; Zeng, D.; Li, B.; Wang, X.; Li, J. LAZY1 controls rice shoot gravitropism through regulating polar auxin transport. Cell Res. 2007, 17, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Su, J.; Jia, F.; He, Y.; Liao, Y.; Wang, Z.; Jiang, J.; Guan, Z.; Fang, W.; Chen, F.; et al. Genetic architecture and genomic prediction of plant height-related traits in chrysanthemum. Hortic. Res. 2024, 11, uhad236. [Google Scholar] [CrossRef]
- Song, X.; Zhao, X.; Fan, G.; Gao, K.; Dai, S.; Zhang, M.; Ma, C.; Wu, X. Genetic analysis of the corolla tube merged degree and the relative number of ray florets in chrysanthemum (Chrysanthemum × morifolium Ramat.). Sci. Hortic. 2018, 242, 214–224. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.; Chen, F.; Fang, W.; Deng, Y.; Chang, Q.; Liu, P. Genetic analysis and associated SRAP markers for flowering traits of chrysanthemum (Chrysanthemum morifolium). Euphytica 2011, 177, 15–24. [Google Scholar] [CrossRef]
- Gao, K.; Song, X.; Kong, D.; Dai, S. Genetic analysis of leaf traits in small-flower chrysanthemum (Chrysanthemum× morifolium Ramat.). Agronomy 2020, 10, 697. [Google Scholar] [CrossRef]
- Jiang, C.; Zeng, Z.B. Multiple trait analysis of genetic mapping for quantitative trait loci. Genetics 1995, 140, 1111–1127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ye, Y.; Feng, L.; Zhang, Y.; Lin, Q.; Liu, J.; Cai, M.; Wang, J.; Cheng, T.; Zhang, Q.; et al. A genetic linkage map of BC2 population reveals QTL associated with plant architecture traits in Lagerstroemia. Forests 2021, 12, 322. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.; Liu, F.; Sun, X.; Li, C.; Zhu, Z.; Fu, Y.; Cai, H.; Wang, X.; Xie, D.; et al. Control of a key transition from prostrate to erect growth in rice domestication. Nat. Genet. 2008, 40, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Zhou, X.; Ahmad, N.; Zhang, K.; Tang, R.; Zhao, H.; Jiang, J.; Tian, M.; Li, C.; Li, A.; et al. BSA-seq and genetic mapping identified candidate genes for branching habit in peanut. Theor. Appl. Genet. 2022, 135, 4457–4468. [Google Scholar] [CrossRef]
- Ravelombola, W.; Qin, J.; Shi, A.; Weng, Y.; Bhattarai, G.; Dong, L.; Morris, J.B. A SNP-based association analysis for plant growth habit in worldwide cowpea (Vigna unguiculata (L.) Walp) Germplasm. Euphytica 2017, 213, 284. [Google Scholar] [CrossRef]
- Chen, J.-T.; Zhong, J.; Shi, X.-J.; Zhang, Q.-X.; Sun, M. Chrysanthemum yantaiense, a rare new species of Asteraceae from China. Phytotaxa 2018, 374, 92–96. [Google Scholar] [CrossRef]
- Wang, J.-T.; Zhang, Y.-W.; Du, Y.-W.; Ren, W.-L.; Li, H.-F.; Sun, W.-X.; Ge, C.; Zhang, Y.-M. SEA v2.0: An R software package for mixed major genes plus polygenes inheritance analysis of quantitative traits. Acta Agron. Sin. 2022, 48, 1416–1424. [Google Scholar] [CrossRef]


| Trait | Year | Generation | Min | Max | Mean | SD | Skewness | Kurtosis | CV (%) |
|---|---|---|---|---|---|---|---|---|---|
| PH/cm | 2021 | P1 | 12.60 | 16.60 | 14.51 | 1.70 | −0.02 | −1.96 | 11.72% |
| P2 | 30.70 | 58.90 | 42.63 | 10.16 | 0.21 | −1.59 | 23.84% | ||
| F1 | 8.10 | 64.20 | 29.43 | 12.58 | 0.66 | −0.20 | 42.75% | ||
| F2 | 6.30 | 65.30 | 34.79 | 11.56 | 0.19 | −0.11 | 33.24% | ||
| 2022 | P1 | 9.20 | 19.20 | 11.65 | 3.56 | 1.16 | −0.31 | 30.57% | |
| P2 | 51.70 | 69.80 | 59.51 | 5.51 | 0.43 | −0.90 | 9.26% | ||
| F1 | 10.20 | 79.30 | 30.28 | 11.16 | 1.10 | 1.61 | 36.84% | ||
| F2 | 13.70 | 72.50 | 36.85 | 12.47 | 0.56 | −0.38 | 33.84% | ||
| CP/cm | 2021 | P1 | 100.95 | 155.30 | 132.63 | 22.70 | −0.20 | −1.94 | 17.12% |
| P2 | 39.90 | 79.20 | 55.18 | 15.39 | 0.55 | −1.59 | 27.89% | ||
| F1 | 61.20 | 196.90 | 108.48 | 27.92 | 0.42 | −0.25 | 25.74% | ||
| F2 | 40.80 | 152.60 | 85.38 | 28.84 | 0.43 | −0.87 | 33.78% | ||
| 2022 | P1 | 174.20 | 257.90 | 213.85 | 33.16 | 0.28 | −1.83 | 15.51% | |
| P2 | 77.40 | 120.80 | 89.54 | 14.09 | 1.19 | 0.16 | 15.74% | ||
| F1 | 60.20 | 231.80 | 135.30 | 34.67 | 0.12 | −0.33 | 25.62% | ||
| F2 | 61.10 | 175.40 | 105.34 | 26.05 | 0.37 | −0.54 | 24.72% | ||
| CI | 2021 | P1 | 0.10 | 0.12 | 0.11 | 0.01 | 0.00 | −1.98 | 8.42% |
| P2 | 0.62 | 1.08 | 0.79 | 0.15 | 0.67 | −0.74 | 18.51% | ||
| F1 | 0.08 | 0.63 | 0.28 | 0.11 | 0.71 | 0.10 | 41.31% | ||
| F2 | 0.12 | 0.88 | 0.43 | 0.15 | 0.55 | 0.31 | 34.75% | ||
| 2022 | P1 | 0.04 | 0.08 | 0.06 | 0.02 | 0.33 | −1.58 | 27.49% | |
| P2 | 0.58 | 0.78 | 0.67 | 0.08 | 0.25 | −1.89 | 11.71% | ||
| F1 | 0.08 | 0.59 | 0.23 | 0.08 | 0.93 | 1.46 | 35.34% | ||
| F2 | 0.11 | 0.73 | 0.36 | 0.11 | 0.49 | 0.35 | 31.70% | ||
| GSA/° | 2021 | P1 | 78.90 | 82.73 | 80.73 | 1.27 | −0.08 | −1.35 | 1.58% |
| P2 | 18.03 | 41.97 | 32.93 | 7.62 | −0.63 | −0.81 | 23.13% | ||
| F1 | 28.10 | 89.00 | 68.83 | 11.46 | −0.75 | 0.33 | 16.64% | ||
| F2 | 29.30 | 88.30 | 58.11 | 12.85 | 0.10 | −0.43 | 22.12% | ||
| 2022 | P1 | 80.90 | 88.33 | 84.20 | 2.66 | 0.33 | −1.67 | 3.16% | |
| P2 | 17.83 | 29.33 | 25.57 | 3.91 | −0.78 | −0.79 | 15.30% | ||
| F1 | 29.30 | 87.90 | 59.75 | 10.91 | −0.13 | −0.33 | 18.27% | ||
| F2 | 14.07 | 73.13 | 46.52 | 12.41 | −0.04 | −0.40 | 26.68% | ||
| GH\Grade | 2021 | P1 | 1.00 | 1.00 | 1.00 | 0.00 | NA | NA | 0.00% |
| P2 | 6.00 | 6.00 | 6.00 | 0.00 | NA | NA | 0.00% | ||
| F1 | 1.00 | 6.00 | 3.08 | 1.08 | 0.44 | −0.25 | 35.17% | ||
| F2 | 1.00 | 6.00 | 4.18 | 1.01 | −0.67 | 0.24 | 24.22% | ||
| 2022 | P1 | 1.00 | 1.00 | 1.00 | 0.00 | NA | NA | 0.00% | |
| P2 | 6.00 | 6.00 | 6.00 | 0.00 | NA | NA | 0.00% | ||
| F1 | 1.00 | 6.00 | 2.92 | 0.87 | 0.51 | 0.12 | 29.88% | ||
| F2 | 2.00 | 6.00 | 3.91 | 1.00 | 0.06 | −0.46 | 25.54% |
| Model Code | Model Implication | AIC Value (2021) | AIC Value (2022) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | CP | CI | GSA | GH | PH | CP | CI | GSA | GH | ||
| A-1 | 1MG-AD | 3784.31 | 4600.48 | −519.46 | 3776.10 | 1499.49 | 3624.61 | 4651.82 | −725.75 | 3644.90 | 1321.48 |
| A-2 | 1MG-A | 3786.59 | 4607.12 | −520.15 | 3783.51 | 1472.06 | 3634.58 | 4637.40 | −779.41 | 3605.72 | 1287.71 |
| A-3 | 1MG-EAD | 3782.91 | 4597.52 | −516.33 | 3774.10 | 1496.05 | 3626.42 | 4649.36 | −728.11 | 3642.28 | 1319.62 |
| A-4 | 1MG-AEND | 3803.68 | 4651.12 | −388.44 | 3860.11 | 1572.92 | 3699.10 | 4690.91 | −581.22 | 3749.48 | 1429.92 |
| B-1 | 2MG-ADI | 3779.53 | 4580.37 | −583.68 | 3746.94 | 1425.41 | 3613.99 | 4574.68 | −860.98 | 3573.78 | 1251.91 |
| B-2 | 2MG-AD | 3781.62 | 4592.15 | −556.42 | 3753.61 | 1466.93 | 3615.64 | 4637.49 | −828.84 | 3605.00 | 1277.94 |
| B-3 | 2MG-A | 3783.62 | 4609.07 | −532.30 | 3780.51 | 1463.89 | 3623.65 | 4630.79 | −819.51 | 3596.82 | 1274.22 |
| B-4 | 2MG-EA | 3781.62 | 4610.08 | −534.30 | 3778.51 | 1461.89 | 3621.65 | 4628.79 | −821.51 | 3594.82 | 1272.23 |
| B-5 | 2MG-AED | 4091.38 | 4771.75 | −178.96 | 3993.56 | 2023.33 | 4075.73 | 5305.54 | −110.04 | 4218.27 | 1888.19 |
| B-6 | 2MG-EEAD | 3777.55 | 4585.46 | −561.96 | 3748.20 | 1464.37 | 3619.24 | 4634.18 | −794.29 | 3611.66 | 1285.14 |
| C-0 | PG-ADI | 3763.74 | 4570.02 | −597.10 | 3731.59 | 1402.93 | 3602.50 | 4550.74 | −874.19 | 3556.06 | 1232.15 |
| C-1 | PG-AD | 3771.96 | 4582.32 | −584.91 | 3736.88 | 1433.12 | 3605.75 | 4609.24 | −847.52 | 3593.81 | 1257.06 |
| D-0 | MX1-AD-ADI | 3767.74 | 4571.50 | −603.22 | 3734.93 | 1406.93 | 3599.48 | 4554.74 | −875.17 | 3559.70 | 1235.54 |
| D-1 | MX1-AD-AD | 3774.75 | 4580.62 | −565.46 | 3738.55 | 1433.60 | 3619.28 | 4607.55 | −841.23 | 3590.81 | 1255.24 |
| D-2 | MX1-A-AD | 3773.96 | 4583.31 | −583.01 | 3737.44 | 1435.12 | 3607.24 | 4611.24 | −845.65 | 3589.61 | 1254.40 |
| D-3 | MX1-EAD-AD | 3773.96 | 4584.31 | −582.92 | 3738.76 | 1435.12 | 3607.75 | 4611.24 | −845.53 | 3593.98 | 1258.97 |
| D-4 | MX1-AEND-AD | 3773.96 | 4579.83 | −582.92 | 3737.55 | 1435.12 | 3607.75 | 4611.24 | −845.53 | 3593.80 | 1258.97 |
| E-0 | MX2-ADI-ADI | 3775.74 | 4579.50 | −596.59 | 3742.76 | 1412.36 | 3607.48 | 4562.74 | −869.43 | 3567.50 | 1243.30 |
| E-1 | MX2-ADI-AD | 3771.59 | 4574.10 | −602.12 | 3736.96 | 1408.19 | 3601.38 | 4556.98 | −875.15 | 3562.28 | 1237.58 |
| E-2 | MX2-AD-AD | 3771.96 | 4577.83 | −591.76 | 3735.32 | 1433.12 | 3598.70 | 4609.24 | −856.12 | 3587.62 | 1252.28 |
| E-3 | MX2-A-AD | 3767.96 | 4577.34 | −589.05 | 3731.50 | 1429.12 | 3601.21 | 4605.24 | −851.71 | 3583.65 | 1248.44 |
| E-4 | MX2-EA-AD | 3765.96 | 4575.55 | −591.05 | 3729.69 | 1427.12 | 3599.02 | 4603.24 | −853.71 | 3581.81 | 1246.57 |
| E-5 | MX2-AED-AD | 3771.73 | 4583.16 | −593.71 | 3734.06 | 1439.16 | 3595.99 | 4623.43 | −858.56 | 3586.99 | 1251.78 |
| E-6 | MX2-EEAD-AD | 3765.96 | 4576.30 | −597.21 | 3730.85 | 1427.12 | 3595.46 | 4603.24 | −862.12 | 3585.14 | 1246.71 |
| Trait | Year | Model Code | Generation | U12 | P(U12) | U22 | P(U22) | U32 | P(U32) | nW2 | P(nW2) | Dn | P(Dn) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PH | 2021 | C-0 | P1 | 0.0000 | 1.0000 | 0.5843 | 0.4446 | 9.3504 | 0.0022 | 0.4550 | 0.0533 | 0.4312 | 0.0723 |
| P2 | 0.0027 | 0.9584 | 0.0287 | 0.8655 | 0.2257 | 0.6348 | 0.0332 | 0.9644 | 0.0884 | 1.0000 | |||
| F1 | 1.6360 | 0.2009 | 0.8573 | 0.3545 | 1.5631 | 0.2112 | 0.5920 | 0.0236 | 0.0020 | 1.0000 | |||
| F2 | 0.0268 | 0.8700 | 0.1287 | 0.7198 | 0.6413 | 0.4232 | 0.0758 | 0.7258 | 0.0057 | 1.0000 | |||
| E-4 | P1 | 5.7686 | 0.0163 | 6.3987 | 0.0114 | 0.6662 | 0.4144 | 0.9754 | 0.0028 | 0.6892 | 0.0002 | ||
| P2 | 3.7668 | 0.0523 | 3.1170 | 0.0775 | 0.2068 | 0.6493 | 0.3860 | 0.0822 | 0.2510 | 0.6098 | |||
| F1 | 3.4167 | 0.0645 | 2.1815 | 0.1397 | 1.5649 | 0.2109 | 0.8163 | 0.0067 | 0.0024 | 1.0000 | |||
| F2 | 2.1691 | 0.1408 | 1.4704 | 0.2253 | 0.7287 | 0.3933 | 0.2896 | 0.1514 | 0.0043 | 1.0000 | |||
| 2022 | E-6 | P1 | 2.8986 | 0.0887 | 4.0887 | 0.0432 | 2.2332 | 0.1351 | 0.5777 | 0.0257 | 0.4046 | 0.1074 | |
| P2 | 2.6103 | 0.1062 | 3.3445 | 0.0674 | 1.1188 | 0.2902 | 0.3802 | 0.0853 | 0.3092 | 0.3532 | |||
| F1 | 3.9417 | 0.0471 | 4.0172 | 0.0450 | 0.1075 | 0.7430 | 0.7946 | 0.0076 | 0.0035 | 1.0000 | |||
| F2 | 0.1593 | 0.6898 | 0.1190 | 0.7301 | 0.0275 | 0.8682 | 0.1733 | 0.3267 | 0.0032 | 1.0000 | |||
| E-5 | P1 | 2.7050 | 0.1000 | 3.9180 | 0.0478 | 2.3952 | 0.1217 | 0.5564 | 0.0290 | 0.3931 | 0.1264 | ||
| P2 | 2.4277 | 0.1192 | 3.1685 | 0.0751 | 1.1786 | 0.2776 | 0.3584 | 0.0979 | 0.2972 | 0.4005 | |||
| F1 | 4.0294 | 0.0447 | 3.7292 | 0.0535 | 0.0025 | 0.9602 | 0.7937 | 0.0076 | 0.0035 | 1.0000 | |||
| F2 | 0.7713 | 0.3798 | 0.3329 | 0.5640 | 1.1956 | 0.2742 | 0.1749 | 0.3228 | 0.0035 | 1.0000 | |||
| CP | 2021 | C-0 | P1 | 0.0031 | 0.9558 | 0.0012 | 0.9719 | 0.1266 | 0.7220 | 0.0634 | 0.7965 | 0.2045 | 0.8295 |
| P2 | 0.0031 | 0.9558 | 0.2404 | 0.6239 | 3.0502 | 0.0807 | 0.1296 | 0.4624 | 0.1908 | 0.8834 | |||
| F1 | 0.3812 | 0.5369 | 0.1135 | 0.7362 | 1.0890 | 0.2967 | 0.1379 | 0.4316 | 0.0028 | 1.0000 | |||
| F2 | 0.4414 | 0.5064 | 0.0462 | 0.8298 | 2.9363 | 0.0866 | 0.3852 | 0.0825 | 0.0097 | 1.0000 | |||
| D-0 | P1 | 0.0037 | 0.9516 | 0.0001 | 0.9914 | 0.0368 | 0.8479 | 0.0642 | 0.7918 | 0.1957 | 0.8651 | ||
| P2 | 0.0038 | 0.9510 | 0.2237 | 0.6362 | 2.7361 | 0.0981 | 0.1222 | 0.4922 | 0.1818 | 0.9134 | |||
| F1 | 0.4204 | 0.5167 | 0.0244 | 0.8758 | 3.5561 | 0.0593 | 0.1924 | 0.2841 | 0.0030 | 1.0000 | |||
| F2 | 0.2143 | 0.6434 | 0.0968 | 0.7557 | 0.3007 | 0.5834 | 0.2472 | 0.1974 | 0.0165 | 1.0000 | |||
| 2022 | C-0 | P1 | 0.0218 | 0.8827 | 0.0005 | 0.9822 | 0.4370 | 0.5086 | 0.0780 | 0.7138 | 0.0811 | 1.0000 | |
| P2 | 0.0035 | 0.9528 | 0.4037 | 0.5252 | 5.3472 | 0.0208 | 0.2407 | 0.2057 | 0.1606 | 0.9654 | |||
| F1 | 0.0278 | 0.8676 | 0.1944 | 0.6593 | 5.8057 | 0.0160 | 0.1358 | 0.4391 | 0.0024 | 1.0000 | |||
| F2 | 0.1120 | 0.7379 | 0.7945 | 0.3727 | 5.1504 | 0.0232 | 0.1744 | 0.3239 | 0.0131 | 1.0000 | |||
| D-0 | P1 | 0.0218 | 0.8827 | 0.0005 | 0.9822 | 0.4370 | 0.5086 | 0.0780 | 0.7138 | 0.0811 | 1.0000 | ||
| P2 | 0.0035 | 0.9528 | 0.4037 | 0.5252 | 5.3472 | 0.0208 | 0.2407 | 0.2057 | 0.1606 | 0.9654 | |||
| F1 | 0.0278 | 0.8676 | 0.1944 | 0.6593 | 5.8057 | 0.0160 | 0.1358 | 0.4391 | 0.0024 | 1.0000 | |||
| F2 | 0.1120 | 0.7379 | 0.7945 | 0.3727 | 5.1504 | 0.0232 | 0.1744 | 0.3239 | 0.0131 | 1.0000 | |||
| CI | 2021 | D-0 | P1 | 0.0019 | 0.9652 | 0.3744 | 0.5406 | 6.8470 | 0.0089 | 0.3719 | 0.0898 | 0.4099 | 0.0995 |
| P2 | 0.1303 | 0.7181 | 0.0560 | 0.8130 | 0.2038 | 0.6516 | 0.0525 | 0.8616 | 0.1199 | 0.9986 | |||
| F1 | 0.9557 | 0.3283 | 0.9172 | 0.3382 | 0.0020 | 0.9645 | 0.3842 | 0.0831 | 0.0023 | 1.0000 | |||
| F2 | 0.0017 | 0.9666 | 0.1242 | 0.7245 | 1.5571 | 0.2121 | 0.1428 | 0.4146 | 0.0030 | 1.0000 | |||
| E-1 | P1 | 0.0100 | 0.9205 | 0.5695 | 0.4505 | 6.9290 | 0.0085 | 0.3745 | 0.0884 | 0.4254 | 0.0790 | ||
| P2 | 0.2081 | 0.6482 | 0.1035 | 0.7477 | 0.2306 | 0.6311 | 0.0619 | 0.8051 | 0.1191 | 0.9987 | |||
| F1 | 1.0046 | 0.3162 | 1.0223 | 0.3120 | 0.0264 | 0.8710 | 0.3930 | 0.0786 | 0.0023 | 1.0000 | |||
| F2 | 0.0017 | 0.9669 | 0.0255 | 0.8731 | 0.6395 | 0.4239 | 0.1034 | 0.5785 | 0.0053 | 1.0000 | |||
| 2022 | D-0 | P1 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | |
| P2 | 0.0118 | 0.9133 | 0.0020 | 0.9647 | 0.0596 | 0.8071 | 0.0782 | 0.7127 | 0.0961 | 1.0000 | |||
| F1 | 0.6038 | 0.4371 | 1.3091 | 0.2526 | 2.4560 | 0.1171 | 0.3166 | 0.1278 | 0.0035 | 1.0000 | |||
| F2 | 0.0078 | 0.9297 | 0.0522 | 0.8192 | 0.3277 | 0.5670 | 0.0258 | 0.9878 | 0.0053 | 1.0000 | |||
| E-1 | P1 | 0.0287 | 0.8656 | 0.9057 | 0.3413 | 9.9285 | 0.0016 | 0.6691 | 0.0152 | 0.5173 | 0.0164 | ||
| P2 | 0.0516 | 0.8202 | 0.0206 | 0.8860 | 0.0939 | 0.7592 | 0.0840 | 0.6807 | 0.1027 | 0.9999 | |||
| F1 | 0.6791 | 0.4099 | 1.3596 | 0.2436 | 2.1680 | 0.1409 | 0.3196 | 0.1254 | 0.0035 | 1.0000 | |||
| F2 | 0.0051 | 0.9429 | 0.0002 | 0.9896 | 0.0506 | 0.8220 | 0.0125 | 0.9999 | 0.0043 | 1.0000 | |||
| GSA | 2021 | E-4 | P1 | 4.0049 | 0.0454 | 2.4196 | 0.1198 | 2.3369 | 0.1263 | 0.8483 | 0.0056 | 0.2364 | 0.6810 |
| P2 | 3.1866 | 0.0742 | 2.7994 | 0.0943 | 0.0489 | 0.8250 | 0.3960 | 0.0771 | 0.0899 | 1.0000 | |||
| F1 | 2.8705 | 0.0902 | 3.0203 | 0.0822 | 0.1520 | 0.6967 | 0.6285 | 0.0192 | 0.0345 | 0.8652 | |||
| F2 | 1.4888 | 0.2224 | 1.2374 | 0.2660 | 0.0762 | 0.7825 | 0.1655 | 0.3465 | 0.0122 | 1.0000 | |||
| E-6 | P1 | 4.0217 | 0.0449 | 2.4372 | 0.1185 | 2.3175 | 0.1279 | 0.8500 | 0.0056 | 0.2362 | 0.6824 | ||
| P2 | 3.2014 | 0.0736 | 2.8127 | 0.0935 | 0.0490 | 0.8249 | 0.3977 | 0.0763 | 0.0900 | 1.0000 | |||
| F1 | 2.8635 | 0.0906 | 2.9772 | 0.0844 | 0.1211 | 0.7279 | 0.6275 | 0.0193 | 0.0347 | 0.8593 | |||
| F2 | 2.4481 | 0.1177 | 2.0042 | 0.1569 | 0.1576 | 0.6914 | 0.2606 | 0.1815 | 0.0130 | 1.0000 | |||
| 2022 | C-0 | P1 | 0.0000 | 0.9974 | 0.5088 | 0.4757 | 8.0682 | 0.0045 | 0.3226 | 0.1230 | 0.3488 | 0.2249 | |
| P2 | 0.0005 | 0.9815 | 0.3689 | 0.5436 | 6.3474 | 0.0118 | 0.2402 | 0.2063 | 0.3616 | 0.1917 | |||
| F1 | 0.0147 | 0.9034 | 0.1706 | 0.6796 | 1.3976 | 0.2371 | 0.1044 | 0.5735 | 0.0040 | 1.0000 | |||
| F2 | 0.0005 | 0.9826 | 0.0047 | 0.9453 | 0.1287 | 0.7198 | 0.0288 | 0.9799 | 0.0157 | 1.0000 | |||
| D-0 | P1 | 0.0000 | 0.9974 | 0.5088 | 0.4757 | 8.0682 | 0.0045 | 0.3226 | 0.1230 | 0.3488 | 0.2249 | ||
| P2 | 0.0005 | 0.9815 | 0.3689 | 0.5436 | 6.3474 | 0.0118 | 0.2402 | 0.2063 | 0.3616 | 0.1917 | |||
| F1 | 0.0147 | 0.9034 | 0.1706 | 0.6796 | 1.3976 | 0.2371 | 0.1044 | 0.5735 | 0.0040 | 1.0000 | |||
| F2 | 0.0005 | 0.9826 | 0.0047 | 0.9453 | 0.1287 | 0.7198 | 0.0288 | 0.9799 | 0.0157 | 1.0000 | |||
| GH | 2021 | C-0 | P1 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 |
| P2 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | |||
| F1 | 0.5813 | 0.4458 | 0.0862 | 0.7691 | 3.1638 | 0.0753 | 2.1808 | 0.0000 | 0.0024 | 1.0000 | |||
| F2 | 0.6457 | 0.4217 | 0.4264 | 0.5137 | 0.2500 | 0.6171 | 1.5860 | 0.0001 | 0.0398 | 0.9410 | |||
| D-0 | P1 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | ||
| P2 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | |||
| F1 | 0.5814 | 0.4458 | 0.0860 | 0.7693 | 3.1676 | 0.0751 | 2.1810 | 0.0000 | 0.0024 | 1.0000 | |||
| F2 | 0.6455 | 0.4217 | 0.4258 | 0.5140 | 0.2513 | 0.6161 | 1.5860 | 0.0001 | 0.0398 | 0.9408 | |||
| 2022 | C-0 | P1 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | |
| P2 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | |||
| F1 | 0.6114 | 0.4342 | 0.3949 | 0.5298 | 0.2651 | 0.6066 | 3.2069 | 0.0002 | 0.0033 | 1.0000 | |||
| F2 | 0.0049 | 0.9443 | 0.0007 | 0.9792 | 0.1406 | 0.7077 | 1.2477 | 0.0007 | 0.0181 | 1.0000 | |||
| D-0 | P1 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | ||
| P2 | 0.0000 | 1.0000 | 0.6250 | 0.4292 | 10.0000 | 0.0016 | 0.6667 | 0.0154 | 0.5000 | 0.0227 | |||
| F1 | 0.6176 | 0.4319 | 0.3766 | 0.5394 | 0.3471 | 0.5558 | 3.2151 | 0.0002 | 0.0033 | 1.0000 | |||
| F2 | 0.0003 | 0.9863 | 0.0050 | 0.9439 | 0.0463 | 0.8297 | 1.2371 | 0.0007 | 0.0200 | 1.0000 |
| Trait | Year | Model | 1st Order Genetic Parameter | 2nd Order Genetic Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| da | db | ha | hb | [d] | [h] | σ2mg | h2mg (%) | σ2pg | h2pg | |||
| PH | 2021 | C-0 | – | – | – | – | – | – | – | – | 0.00 | 0.00% |
| 2022 | E-5 | −1.3315 | −9.1163 | – | – | −13.484 | 0.5898 | 45.6899 | 29.39% | 0.00 | 0.00% | |
| CP | 2021 | C-0 | – | – | – | – | – | – | – | – | 78.1598 | 9.40% |
| 2022 | C-0 | – | – | – | – | – | – | – | – | 0.00 | 0.00% | |
| CI | 2021 | D-0 | −0.1219 | – | −0.1217 | – | – | – | 0.0094 | 41.57% | 0.00 | 0.00% |
| 2022 | D-0 | −0.0839 | – | −0.0836 | – | – | – | 0.0051 | 39.48% | 0.0007 | 5.45% | |
| GSA | 2021 | E-4 | 6.3498 | – | – | – | 11.1966 | 17.7547 | 38.1995 | 23.12% | 0.00 | 0.00% |
| 2022 | C-0 | – | – | – | – | – | – | – | – | 41.1057 | 26.69% | |
| GH | 2021 | C-0 | – | – | – | – | – | – | – | – | 0.00 | 0.00% |
| 2022 | C-0 | – | – | – | – | – | – | – | – | 0.2815 | 28.19% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Xu, Y.; Tang, Y.; Zhou, T.; Li, H.; Guo, Z.; Liang, Y.; Wang, Y.; Chen, Y.; Sun, M. Major Gene with Polygene Inheritance Analysis of Prostrate Growth Habit in Hybrids of Chrysanthemum yantaiense × C. indicum. Plants 2025, 14, 1338. https://doi.org/10.3390/plants14091338
Li D, Xu Y, Tang Y, Zhou T, Li H, Guo Z, Liang Y, Wang Y, Chen Y, Sun M. Major Gene with Polygene Inheritance Analysis of Prostrate Growth Habit in Hybrids of Chrysanthemum yantaiense × C. indicum. Plants. 2025; 14(9):1338. https://doi.org/10.3390/plants14091338
Chicago/Turabian StyleLi, Dawei, Yuxian Xu, Yuchao Tang, Tongjun Zhou, Hai Li, Ziyu Guo, Yilin Liang, Yuxin Wang, Yuyuan Chen, and Ming Sun. 2025. "Major Gene with Polygene Inheritance Analysis of Prostrate Growth Habit in Hybrids of Chrysanthemum yantaiense × C. indicum" Plants 14, no. 9: 1338. https://doi.org/10.3390/plants14091338
APA StyleLi, D., Xu, Y., Tang, Y., Zhou, T., Li, H., Guo, Z., Liang, Y., Wang, Y., Chen, Y., & Sun, M. (2025). Major Gene with Polygene Inheritance Analysis of Prostrate Growth Habit in Hybrids of Chrysanthemum yantaiense × C. indicum. Plants, 14(9), 1338. https://doi.org/10.3390/plants14091338






