Phytophthora inundata: A New Root Pathogen of Citrus in Europe and the Mediterranean Region
Abstract
1. Introduction
2. Results
2.1. Isolation and Identification of the Phytophthora Species
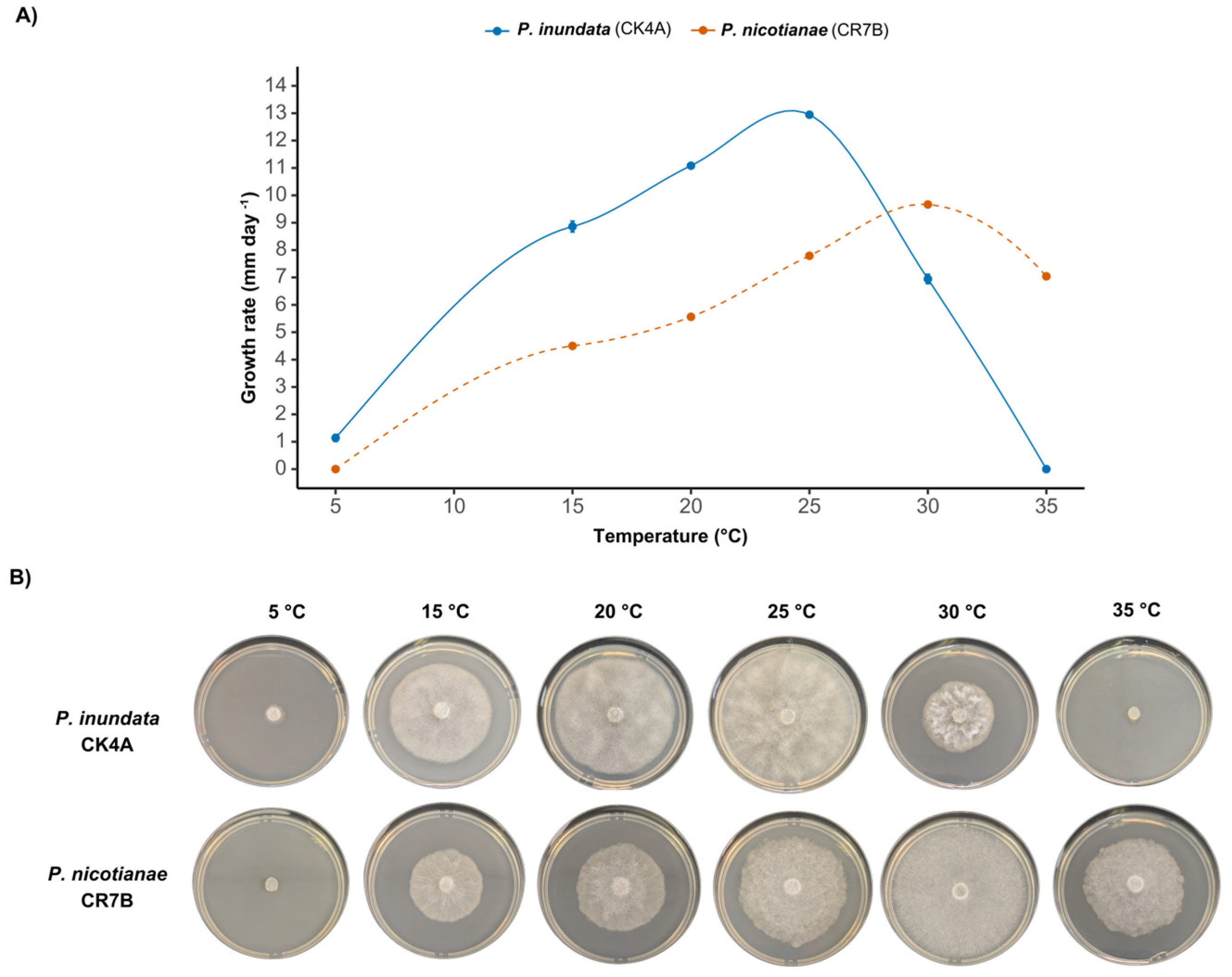
2.2. Radial Growth of Phytophthora spp. at Different Temperatures
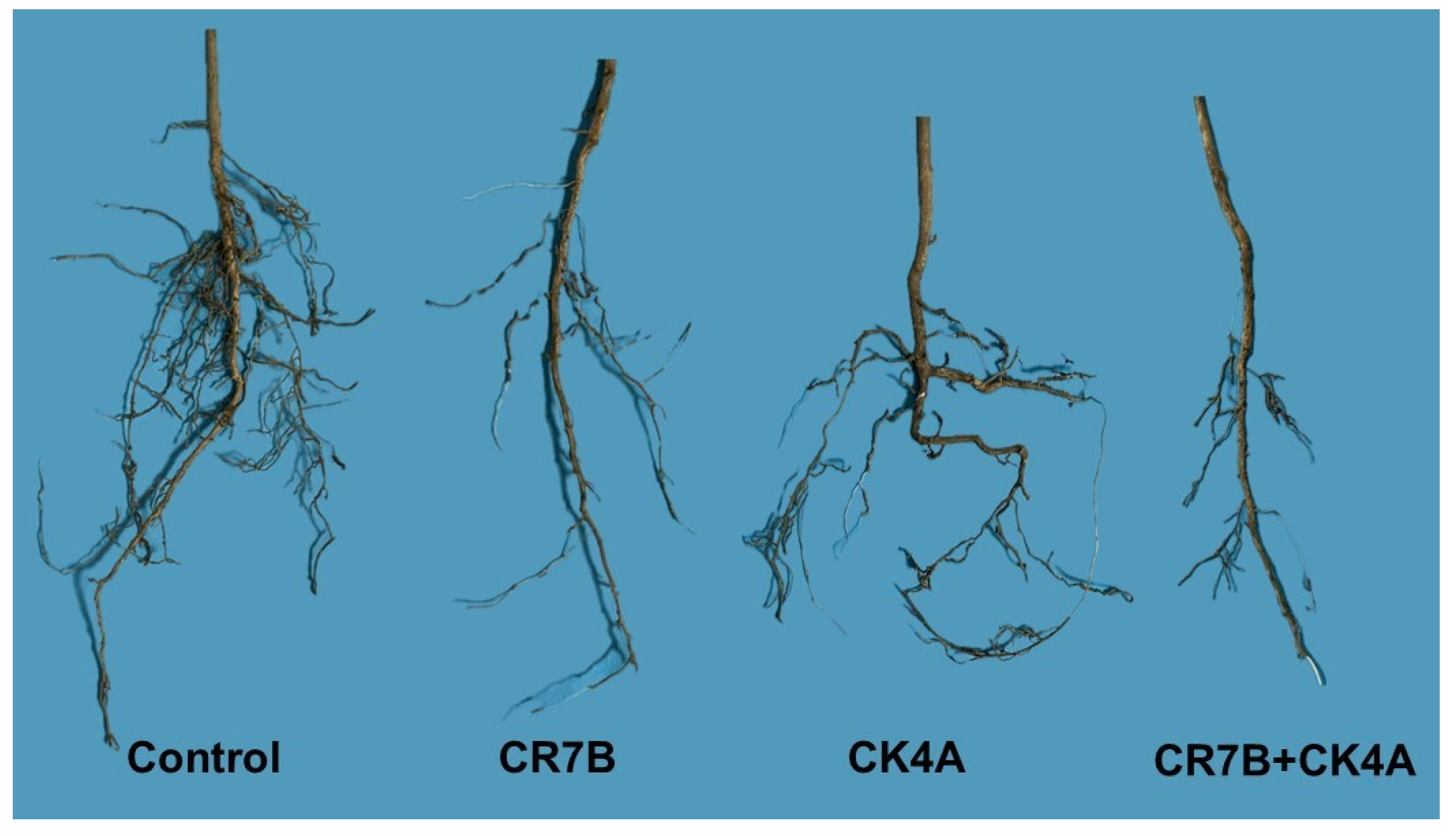
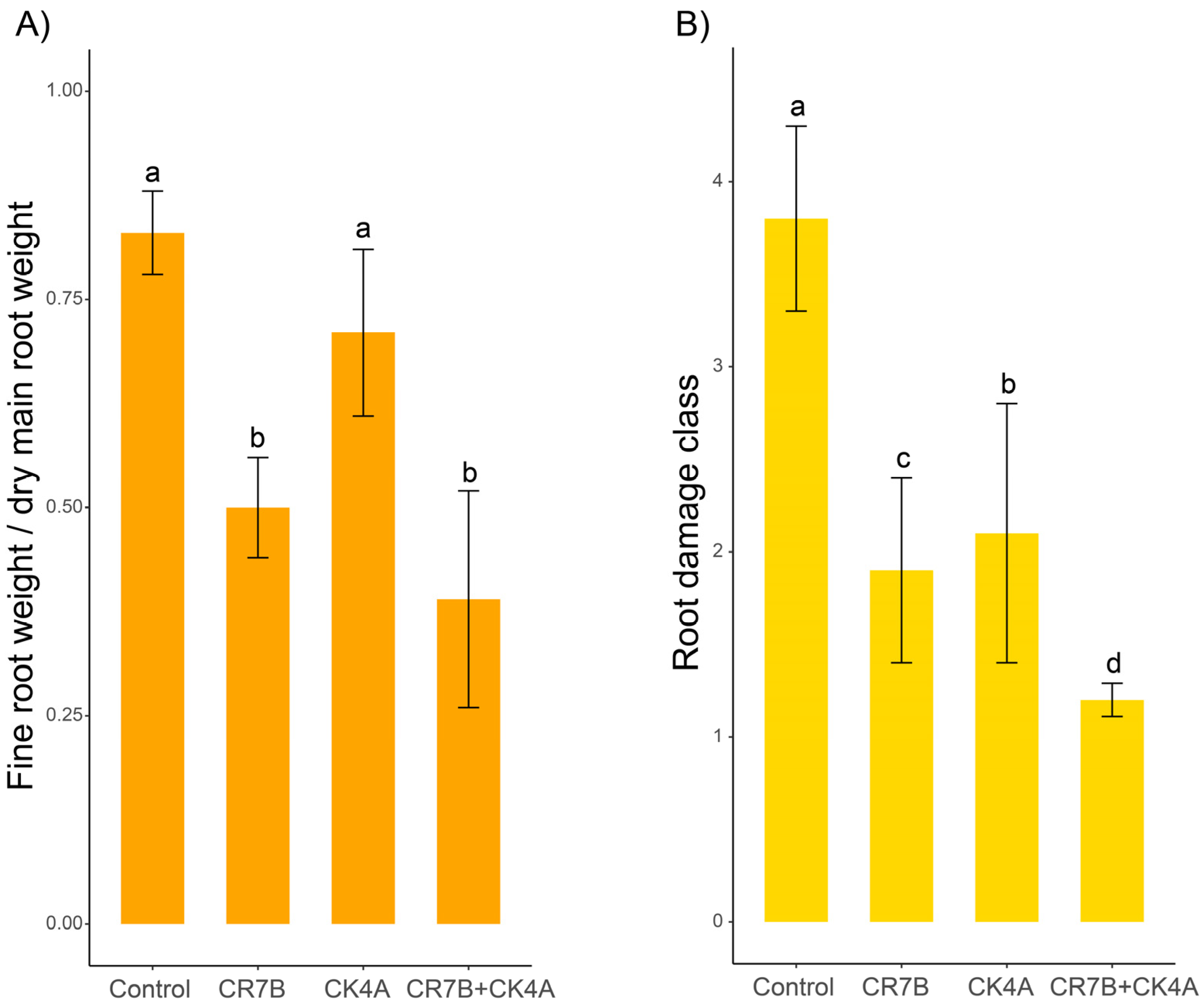
2.3. Pathogenicity Test
3. Discussion
4. Material and Methods
4.1. Isolation and Morphological Identification of Isolates
4.2. Assessment of the Effect of Temperature-On Mycelial Growth
4.3. Molecular Identification
4.4. Pathogenicity Tests
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Citrus Fruit Statistical Compendium Rome 2021. 2020. Available online: https://www.fao.org/publications/card/fr/c/cB6492en/ (accessed on 28 October 2023).
- Rovetto, E.I.; Luz, C.; La Spada, F.; Meca, G.; Riolo, M.; Cacciola, S.O. Diversity of mycotoxins and other secondary metabolites recovered from blood oranges infected by Colletotrichum, Alternaria, and Penicillium species. Toxins 2023, 15, 407. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, H.B.; Fernandes, L.S.; Pontes, J.G.d.M.; Pereira, A.K.; Fill, T.P. An overview of the most threating diseases that affect worldwide citriculture: Main features, diagnose, and current control strategies. Front. Nat. Prod. 2023, 2, 1045364. [Google Scholar] [CrossRef]
- Rovetto, E.I.; La Spada, F.; Aloi, F.; Riolo, M.; Pane, A.; Garbelotto, M.; Cacciola, S.O. Green solutions and new technologies for sustainable management of fungus and oomycete diseases in the citrus fruit supply chain. J. Plant Pathol. 2024, 106, 411–437. [Google Scholar] [CrossRef]
- Ezrari, S.; Radouane, N.; Tahiri, A.; El Housni, Z.; Mokrini, F.; Özer, G.; Lazraq, A.; Belabess, Z.; Amiri, S.; Lahlali, R. Dry Root rot disease, an emerging threat to citrus industry worldwide under climate change: A review. Physiol. Mol. Plant Pathol. 2022, 117, 101753. [Google Scholar] [CrossRef]
- Alvarez, L.A.; Vicent, A.; De La Roca, E.; Bascón, J.; Abad-Campos, P.; Armengol, J.; García-Jiménez, J. Branch cankers on citrus trees in Spain caused by Phytophthora citrophthora. Plant Pathol. 2008, 57, 84–91. [Google Scholar] [CrossRef]
- Graham, J.; Feichtenberger, E. Citrus Phytophthora Diseases: Management Challenges and Successes. J. Citrus Pathol. 2015, 2, 1. [Google Scholar] [CrossRef]
- La Spada, F.; Bua, C.; Pane, A.; Tuccitto, N.; Riolo, M.; Cacciola, S.O. Exploring eco-friendly solutions for Phytophthora disease management: Harnessing the anti-oomycete potential of a fermented lemon waste formulation. J. Agric. Food Res. 2024, 17, 101227. [Google Scholar] [CrossRef]
- Gaikwad, P.N.; Sharma, V.; Singh, J.; Singh Sidhu, G.; ìSingh, H.; Omar, A.A. Biotechnological advancements in Phytophthora disease diagnosis, interaction and management in citrus. Sci. Hortic. 2023, 310, 111739. [Google Scholar] [CrossRef]
- Cacciola, S.O.; Magnano di San Lio, G. Management of citrus diseases caused by Phytophthora spp. In Integrated Management of Diseases Caused by Fungi, Phytoplasma and Bacteria; Integrated management of Plant Pests and Diseases; Ciancio, A., Mukerji, K.G., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 3, pp. 61–82. [Google Scholar] [CrossRef]
- Graham, J.H.; Menge, J. Root Diseases. In Citrus Health Management; Timmer, L.W., Duncan, L.W., Eds.; American Phytopathological Society: St. Paul, MN, USA, 1999; pp. 126–135. [Google Scholar]
- Graham, J.H.; Menge, J.A. Phytophthora-Induced Diseases. In Compendium of Citrus Diseases; Timmer, L.W., Garnsey, S.M., Graham, J.H., Eds.; American Phytopathological Society: St. Paul, MN, USA, 2000; pp. 12–15. [Google Scholar]
- Aparicio-Durán, L.; Arjona-López, J.M.; Hervalejo, A.; Calero-Velázquez, R.; Arenas-Arenas, F.J. Preliminary findings of new citrus rootstocks potentially tolerant to foot rot caused by Phytophthora. Horticulturae 2021, 7, 389. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; La Spada, F.; Sciandrello, S.; Moricca, S.; Santilli, E.; Pane, A.; Cacciola, S.O. Diversity of Phytophthora communities across different types of Mediterranean vegetation in a nature reserve area. Forests 2020, 11, 853. [Google Scholar] [CrossRef]
- Riolo, M.; Aloi, F.; Conti Taguali, S.; Pane, A.; Franco, M.; Cacciola, S.O. Phytophthora × cambivora as a major factor inciting the decline of European beech in a stand within the southernmost limit of its natural range in Europe. J. Fungi 2022, 8, 973. [Google Scholar] [CrossRef] [PubMed]
- Abad, Z.G.; Burgess, T.I.; Bourret, T.; Bensch, K.; Cacciola, S.O.; Scanu, B.; Mathew, R.; Kasiborski, B.; Srivastava, S.; Kageyama, K.; et al. Phytophthora: Taxonomic and phylogenetic revision of the genus. Stud. Mycol. 2023, 106, 259–348. [Google Scholar] [CrossRef]
- Jung, T.; Milenković, I.; Balci, Y.; Janoušek, J.; Kudláček, T.; Nagy, Z.; Baharuddin, B.; Bakonyi, J.; Broders, K.D.; Cacciola, S.O.; et al. Worldwide forest surveys reveal forty-three new species in Phytophthora major clade 2 with fundamental implications for the evolution and biogeography of the genus and global plant biosecurity. Stud. Mycol. 2024, 107, 251–388. [Google Scholar] [CrossRef] [PubMed]
- Conti Taguali, S.; Bua, C.; Rovetto, E.I.; Pane, A.; La Spada, F.; Cacciola, S.O. Bleeding stem cankers and root rot caused by Phytophthora multivora in Morus alba, Pistacia atlantica and Sterculia diversifolia trees in Eastern Sicily. J. Plant Pathol. 2024, 106, 291. [Google Scholar] [CrossRef]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society: St. Paul, MN, USA, 1996; p. 562. ISBN 0-89054-212-0. [Google Scholar]
- Naqvi, S.A.M.H. Diagnosis and management of pre and post-harvest diseases of citrus fruit. In Diagnosis and Management. Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume 1, pp. 339–359. [Google Scholar] [CrossRef]
- Vial, A.; Latorre, B.A.; Ortúzar, J. Characterization of Phytophthora citrophthora and P. inundata associated to foot and root rot of citrus trees in Chile. Cienc. Investig. Agrar. 2006, 33, 173–184. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.E.S.J.; Barber, P.A.; Alvarado, P.; Barnes, C.W.; Buchanan, P.K.; Heykoop, M.; Moreno, G.; et al. Fungal planet description sheets: 558–624. Persoonia Mol. Phylogeny Evol. Fungi 2017, 38, 240–384. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Nerkar, S.; Thakre, N.; Kumar, A. First report of an atypical strain of Phytophthora inundata causing Kinnow mandarin decline in India. Can. J. Plant Pathol. 2017, 39, 365–372. [Google Scholar] [CrossRef]
- Khanchouch, K.; Pane, A.; Chriki, A.; Cacciola, S.O. Major and emerging fungal diseases of citrus in the Mediterranean region. In Citrus Pathology; Gill, H., Ed.; IntechOpen, 2017. [Google Scholar] [CrossRef]
- Puglisi, I.; De Patrizio, A.; Schena, L.; Jung, T.; Evoli, M.; Pane, A.; Van Hoa, N.; Van Tri, M.; Wright, S.; Ramstedt, M.; et al. Two previously unknown Phytophthora species associated with brown rot of pomelo (Citrus grandis) fruits in Vietnam. PLoS ONE 2017, 12, e0172085. [Google Scholar] [CrossRef]
- Chi, N.M.; Thu, P.Q.; Nam, H.B.; Quang, D.Q.; Phong, L.V.; Van, N.D.; Trang, T.T.; Kien, T.T.; Tam, T.T.T.; Dell, B. Management of Phytophthora palmivora disease in Citrus reticulata with chemical fungicides. J. Gen. Plant Pathol. 2020, 86, 494–502. [Google Scholar] [CrossRef]
- La Spada, F.; Cock, P.J.A.; Randall, E.; Pane, A.; Cooke, D.E.L.; Cacciola, S.O. DNA Metabarcoding and isolation by baiting complement each other in revealing Phytophthora diversity in anthropized and natural ecosystems. J. Fungi 2022, 8, 330. [Google Scholar] [CrossRef]
- Madhu, G.S.; Rani, A.T.; Muralidhara, B.M.; Deepak, G.N.; Rajendiran, S.; Rakshith, V.; Venkataravanappa, V. Phylogenetic and pathogenic characterization of Phytophthora species associated with decline of horticultural crops in high humid tropic region of Western Ghats, India. Physiol. Mol. Plant Pathol. 2024, 133, 102355. [Google Scholar] [CrossRef]
- Aloi, F.; Parlascino, R.; Conti Taguali, S.; Faedda, R.; Pane, A.; Cacciola, S.O. Phytophthora pseudocryptogea, P. nicotianae and P. multivora associated to Cycas revoluta: First report worldwide. Plants 2023, 12, 1197. [Google Scholar] [CrossRef] [PubMed]
- Scanu, B.; Jung, T.; Masigol, H.; Linaldeddu, B.T.; Jung, M.H.; Brandano, A.; Mostowfizadeh-Ghalamfarsa, R.; Janoušek, J.; Riolo, M.; Cacciola, S.O. Phytophthora heterospora sp. nov., a new pseudoconidia-producing sister species of P. palmivora. J. Fungi 2021, 7, 870. [Google Scholar] [CrossRef]
- Safaiefarahani, B.; Mostowfizadeh-Ghalamfarsa, R.; Cooke, D.E.L. Characterisation of Phytophthora inundata according to host range, morphological variation and multigene molecular phylogeny. Phytopathol. Mediterr. 2013, 52, 46–65. [Google Scholar]
- Aghighi, S.; Hardy, G.E.S.J.; Scott, J.K.; Burgess, T.I. Phytophthora bilorbang sp. nov., a new species associated with the decline of Rubus anglocandicans (European blackberry) in Western Australia. Eur. J. Plant Pathol. 2012, 133, 841–855. [Google Scholar] [CrossRef]
- Scanu, B.; Linaldeddu, B.T.; Deidda, A.; Jung, T. Diversity of Phytophthora species from declining Mediterranean maquis vegetation, including two new species, Phytophthora crassamura and P. ornamentata sp. nov. PLoS ONE 2015, 10, e0143234. [Google Scholar] [CrossRef]
- Scanu, B.; Hunter, G.C.; Linaldeddu, B.T.; Franceschini, A.; Maddau, L.; Jung, T.; Denman, S. A Taxonomic re-evaluation reveals that Phytophthora cinnamomi and P. cinnamomi var. parvispora are separate species. For. Pathol. 2014, 44, 1–20. [Google Scholar] [CrossRef]
- Brasier, C.M.; Sanchez-Hernandez, E.; Kirk, S.A. Phytophthora inundata sp. nov., a part heterothallic pathogen of trees and shrubs in wet or flooded soils. Mycol. Res. 2003, 107, 477–484. [Google Scholar] [CrossRef]
- Cunnington, J.H.; Jones, R.H.; De Alwis, S.; Minchinton, E.J. Two new Phytophthora records for Australia. Australas. Plant Pathol. 2006, 35, 383–384. [Google Scholar] [CrossRef]
- Ho, H.H.; Hong, C.X.; Erwin, D.C. Phytophthora inundata isolated from diseased alfalfa roots in Southern California. Mycotaxon 2006, 97, 349–358. [Google Scholar]
- Stukely, M.J.C.; Webster, J.L.; Ciampini, J.A.; Brown, E.; Dunstan, W.A.; Hardy, G.E.S.J.; Woodman, G.J.; Davison, E.M.; Tay, F.C.S. Phytophthora inundata from native vegetation in Western Australia. Australas. Plant Pathol. 2007, 36, 606–608. [Google Scholar] [CrossRef]
- Parkunan, V.; Johnson, C.S.; Bowman, B.C.; Hong, C.X. First report of Phytophthora inundata associated with a latent infection of tobacco (Nicotiana tabacum) in Virginia. Plant Pathol. 2010, 59, 1164. [Google Scholar] [CrossRef]
- Kurbetli, İ.; Sülü, G.; Taştekin, E.; Polat, İ. First report of Phytophthora inundata causing olive tree decline in Turkey. Can. J. Plant Pathol. 2016, 38, 254–257. [Google Scholar] [CrossRef]
- Nawaz, H.H.; Ali, M.; Rehman, A.; Iqbal, M.A.; Nawaz, K.; Amjad, M. First report of Phytophthora inundata causing crown rot on Olea europaea (olive) in Pakistan. Plant Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, S.; Faeddda, R.; Pane, A.; Scarito, G. Root and crown rot of olive caused by Phytophthora spp. In Olive Diseases and Disorders; Schena, L., Agosteo, G.E., Cacciola, S.O., Eds.; Transworld Research Network: Trivandrum, Kerala, India, 2011; pp. 305–327. ISBN 9788178955391. [Google Scholar]
- Haegi, A.; Luongo, L.; Garaguso, I.; Petrucci, M.; Vitale, S. First report of Phytophthora inundata associated with decline and death of walnut (Juglans regia) in Italy. Plant Dis. 2023, 107, 2267. [Google Scholar] [CrossRef] [PubMed]
- Linaldeddu, B.T.; Rossetto, G.; Maddau, L.; Vatrano, T.; Bregant, C. Diversity and pathogenicity of Botryosphaeriaceae and Phytophthora species associated with emerging olive diseases in Italy. Agriculture 2023, 13, 1575. [Google Scholar] [CrossRef]
- Deidda, A.; Satta, G.G.A.; Brandano, A.; Morittu, C.; Mureddu, D.; Scanu, B. Multiple Phytophthora species associated with declining wild olive trees in Sardinia, Italy. Plant Pathol. 2024, 74, 465–475. [Google Scholar] [CrossRef]
- Hyun, I.H.; Choi, W. Phytophthora species, new threats to the plant health in Korea. Plant Pathol. J. 2014, 30, 331–342. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Venturi, V. Synergisms between microbial pathogens in plant disease complexes: A growing trend. Front. Plant Sci. 2015, 6, 385. [Google Scholar] [CrossRef]
- Aloi, F.; Zamora-Ballesteros, C.; Martín-García, J.; Diez, J.J.; Cacciola, S.O.; Ospina-Giraldo, M.; Castillo, P. Co-Infections by Fusarium circinatum and Phytophthora spp. on Pinus radiata: Complex phenotypic and molecular interactions. Plants 2021, 10, 1976. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Guarnaccia, V.; Polizzi, G.; Crous, P.W. Symptomatic citrus trees reveal a new pathogenic lineage in Fusarium and two new Neocosmospora species. Persoonia Mol. Phylogeny Evol. Fungi 2018, 40, 1–25. [Google Scholar] [CrossRef]
- Jung, T.; Cooke, D.E.L.; Blaschke, H.; Duncan, J.M.; Oßwald, W. Phytophthora quercina sp. nov., causing root rot of european oaks. Mycol. Res. 1999, 103, 785–798. [Google Scholar] [CrossRef]
- Cooke, D.E.L.; Drenth, A.; Duncan, J.M.; Wagels, G.; Brasier, C.M. A Molecular phylogeny of Phytophthora and related oomycetes. Fungal Genet. Biol. 2000, 30, 17–32. [Google Scholar] [CrossRef]
- Kroon, L.P.N.M.; Bakker, F.T.; Van Den Bosch, G.B.M.; Bonants, P.J.M.; Flier, W.G. Phylogenetic analysis of Phytophthora species based on mitochondrial and nuclear DNA sequences. Fungal Genet. Biol. 2004, 41, 766–782. [Google Scholar] [CrossRef] [PubMed]
- FinchTV, v.1.4.0. Available online: https://digitalworldbiology.com/FinchTV (accessed on 18 May 2020).
- MEGA—Molecular Evolutionary Genetics Analysis. Available online: https://www.megasoftware.net/ (accessed on 10 March 2024).
- Santilli, E.; Riolo, M.; La Spada, F.; Pane, A.; Cacciola, S.O. First report of root rot caused by Phytophthora bilorbang on Olea europaea in Italy. Plants 2020, 9, 826. [Google Scholar] [CrossRef] [PubMed]
- Jung, T.; Jung, M.H.; Cacciola, S.O.; Cech, T.; Bakonyi, J.; Seress, D.; Mosca, S.; Schena, L.; Seddaiu, S.; Pane, A.; et al. Multiple new cryptic pathogenic Phytophthora species from Fagaceae forests in Austria, Italy and Portugal. IMA Fungus 2017, 8, 219–244. [Google Scholar] [CrossRef]
- Maserti, B.; Michelozzi, M.; Cencetti, G.; Riolo, M.; La Spada, F.; Aloi, F.; Pane, A.; Bartolini, P.; Pacori, F.; de Andrade Silva, E.M.; et al. Leaf volatile organic compounds profile from two citrus genotypes differing in susceptibility to Phytophthora citrophthora infection. Physiol. Mol. Plant Pathol. 2024, 133, 192319. [Google Scholar] [CrossRef]
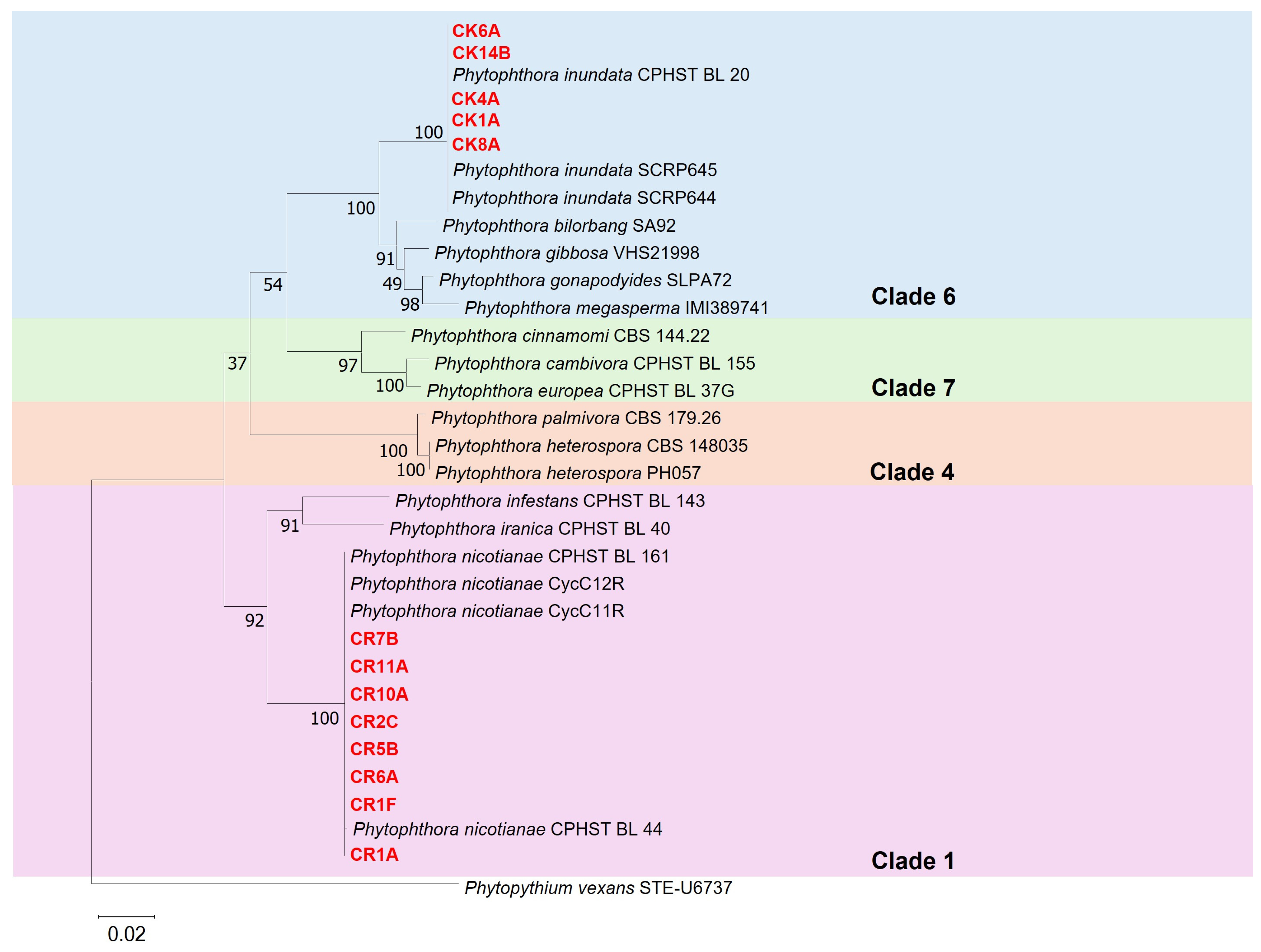
| Species | Isolate Code | Clade | Genbank Accession No. | Reference | ||
|---|---|---|---|---|---|---|
| ITS | COI | β-Tub | ||||
| Phytophthora iranica | CPHST BL40 | 1b | MG865519 | MH136913 | MH493961 | [28] |
| P. infestans | CPHST BL 143 | 1c | MG865513 | MH136907 | MH493955 | [28] |
| P. nicotianae | CPHST BL 44 | 1d | MG865550 | MH136943 | MH493985 | [28] |
| P. nicotianae | CPHST BL 161 | 1d | MG865551 | MH477751 | MH493987 | [28] |
| P. nicotianae | CycC12R | 1d | OP557998 | OP629938 | OP563139 | [29] |
| P. nicotianae | CycC11R | 1d | OP557997 OP563138 | OP629937 | OP629937 | [29] |
| P. heterospora | CBS 148035 | 4 | MT232394 | MZ782830 | MZ782809 | [30] |
| P. heterospora | PH057 | 4 | MZ927097 | MZ782829 | MZ782808 | [30] |
| P. palmivora | CBS 179.26 | 4 | MT232400 | MZ782817 | MZ782838 | [30] |
| P. inundata | SCRP645 | 6a | EF210201 | EF210207 | EF210203 | [31] |
| P. inundata | SCRP644 | 6a | EF210200 | EF210206 | EF210202 | [31] |
| P. inundata | CPHST BL 20 | 6a | MG865516 | MH136910 | MH493958 | [16] |
| P. gibbosa | VHS21998 | 6b | HQ012933 | HQ012846 | JN547596 | [32] |
| P. gonapodydes | SLPA72 | 6b | HQ012937 | HQ012850 | JN547598 | [32] |
| P. megasperma | IMI389741 | 6b | AF266794 | JN935959 | JN935977 | [33] |
| P. bilorbang | SA92 | 6c | JN547621 | JN547643 | JN547582 | [32] |
| P. cambivora | CPHST BL155 | 7a | MG783387 | MH136860 | MN207270 | [28] |
| P. europaea | CPHST BL37G | 7a | MG865488 | MH136884 | MH493935 | [28] |
| P. cinnamomi | CBS 144.22 | 7c | KC478663 | KC609419 | KC609408 | [34] |
| Phytopythium vexans | STE-U6737 | - | GU133616 | GU133509 | GU133454 | [28] |
| Species | Isolate Code | Clade | Genbank Accession No. | ||
|---|---|---|---|---|---|
| ITS | COI | β-Tub | |||
| Phytophthora nicotianae | CR1A | 1b | PQ838813 | PQ855579 | PQ855571 |
| P. nicotianae | CR1F | 1b | PQ838814 | PQ855580 | PQ855572 |
| P. nicotianae | CR6A | 1b | PQ838815 | PQ855581 | PQ855573 |
| P. nicotianae | CR5B | 1b | PQ838816 | PQ855582 | PQ855574 |
| P. nicotianae | CR2C | 1b | PQ838817 | PQ855583 | PQ855575 |
| P. nicotianae | CR10A | 1b | PQ838818 | PQ855584 | PQ855576 |
| P. nicotianae | CR11A | 1b | PQ838819 | PQ855585 | PQ855577 |
| P. nicotianae | CR7B | 1b | PQ838820 | PQ855586 | PQ855578 |
| Phytophthora inundata | CK1A | 6a | PQ838648 | PQ855587 | PQ855566 |
| P. inundata | CK4A | 6a | PQ838649 | PQ855588 | PQ855567 |
| P. inundata | CK6A | 6a | PQ838650 | PQ855589 | PQ855568 |
| P. inundata | CK8A | 6a | PQ838651 | PQ855590 | PQ855569 |
| P. inundata | CK14B | 6a | PQ838652 | PQ855591 | PQ855570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bua, C.; Tambè, M.C.; Conti Taguali, S.; Riolo, M.; Vitale, A.; Pane, A.; Cacciola, S.O. Phytophthora inundata: A New Root Pathogen of Citrus in Europe and the Mediterranean Region. Plants 2025, 14, 1333. https://doi.org/10.3390/plants14091333
Bua C, Tambè MC, Conti Taguali S, Riolo M, Vitale A, Pane A, Cacciola SO. Phytophthora inundata: A New Root Pathogen of Citrus in Europe and the Mediterranean Region. Plants. 2025; 14(9):1333. https://doi.org/10.3390/plants14091333
Chicago/Turabian StyleBua, Cristian, Maria Catena Tambè, Sebastiano Conti Taguali, Mario Riolo, Alessandro Vitale, Antonella Pane, and Santa Olga Cacciola. 2025. "Phytophthora inundata: A New Root Pathogen of Citrus in Europe and the Mediterranean Region" Plants 14, no. 9: 1333. https://doi.org/10.3390/plants14091333
APA StyleBua, C., Tambè, M. C., Conti Taguali, S., Riolo, M., Vitale, A., Pane, A., & Cacciola, S. O. (2025). Phytophthora inundata: A New Root Pathogen of Citrus in Europe and the Mediterranean Region. Plants, 14(9), 1333. https://doi.org/10.3390/plants14091333









