A Preliminary Study on the Qualitative and Quantitative Changes of Amino Acids in Whole Apple, Apple Peel, and Flesh Samples Grown in Lithuania
Abstract
:1. Introduction
2. Results
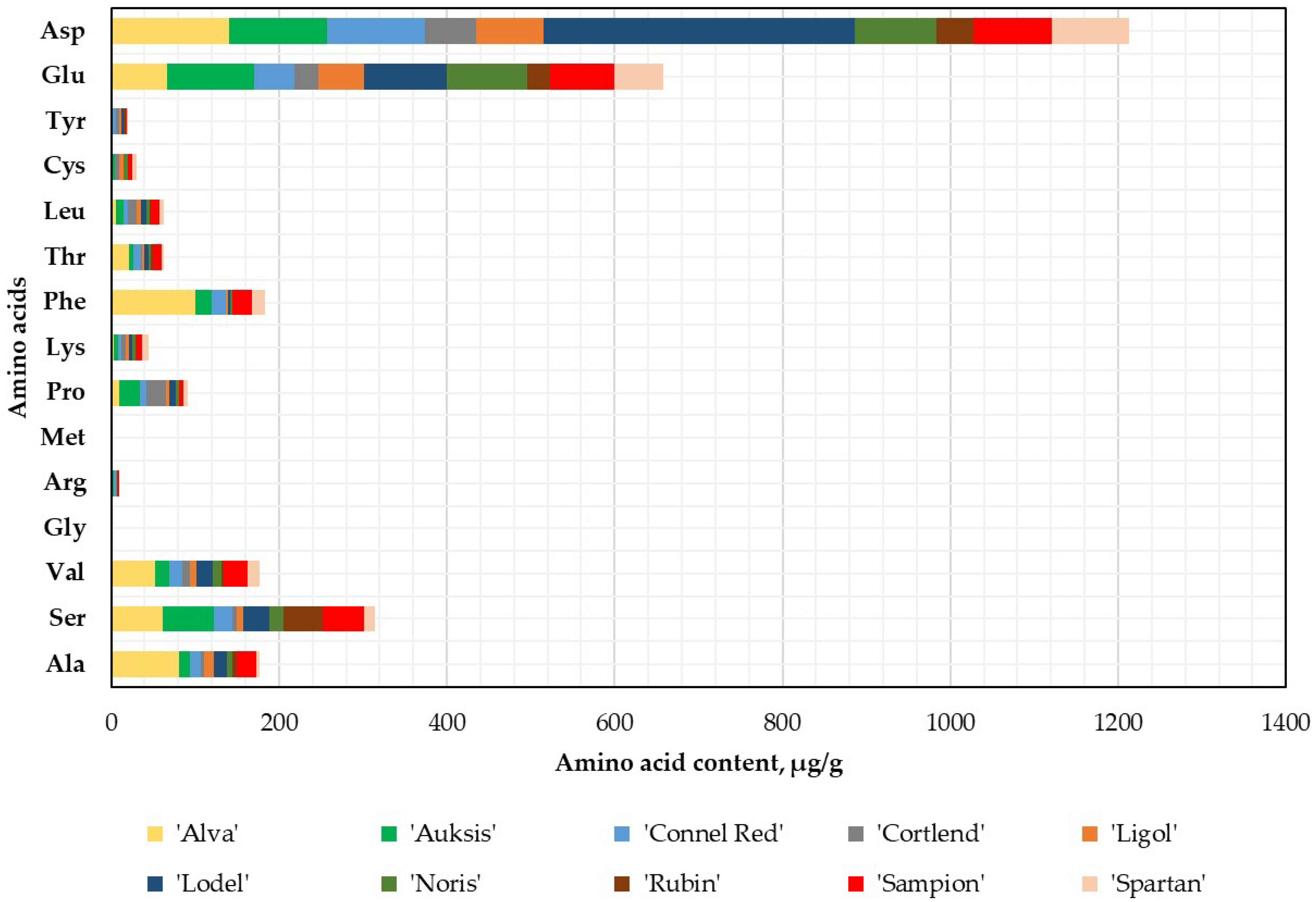
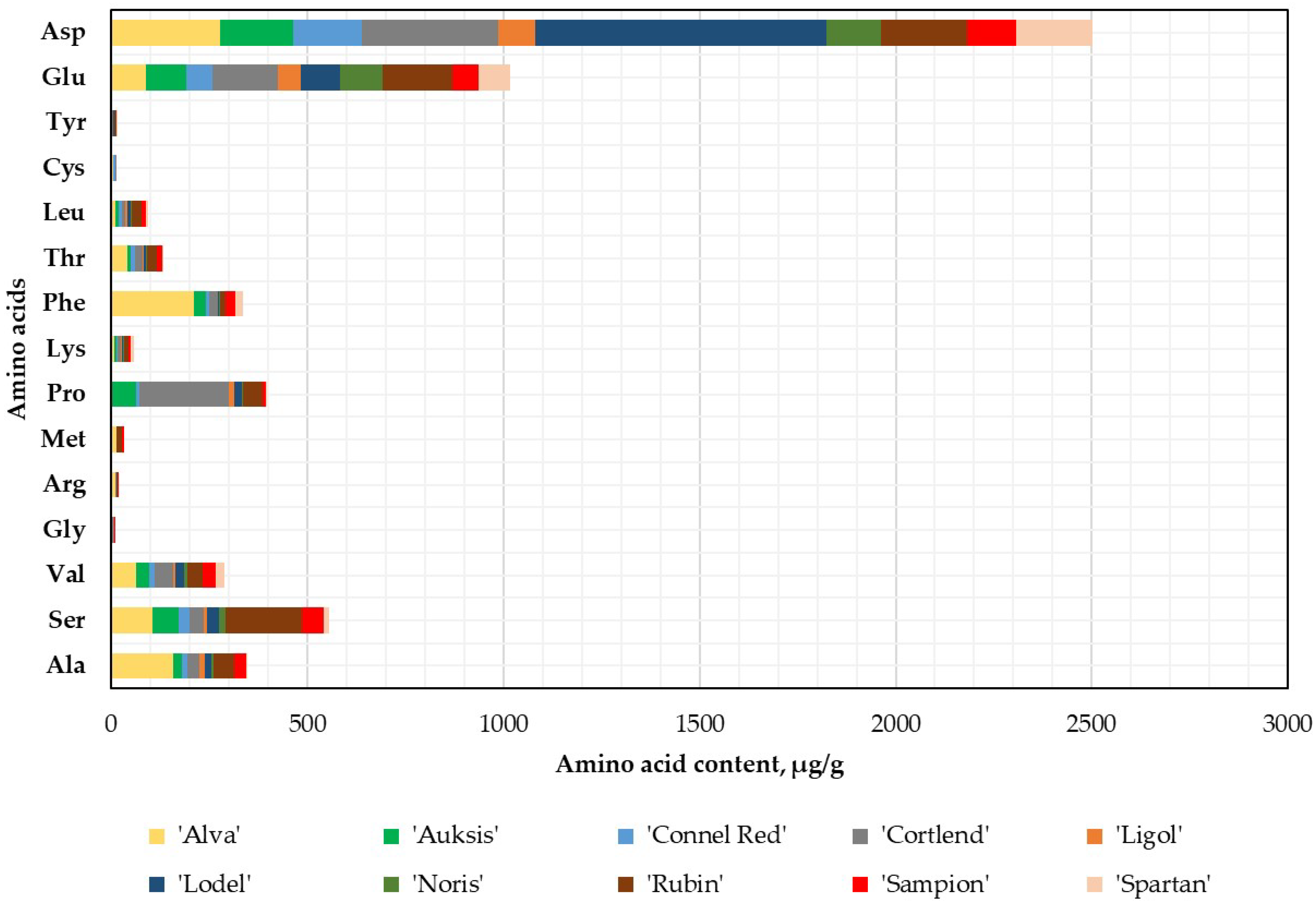
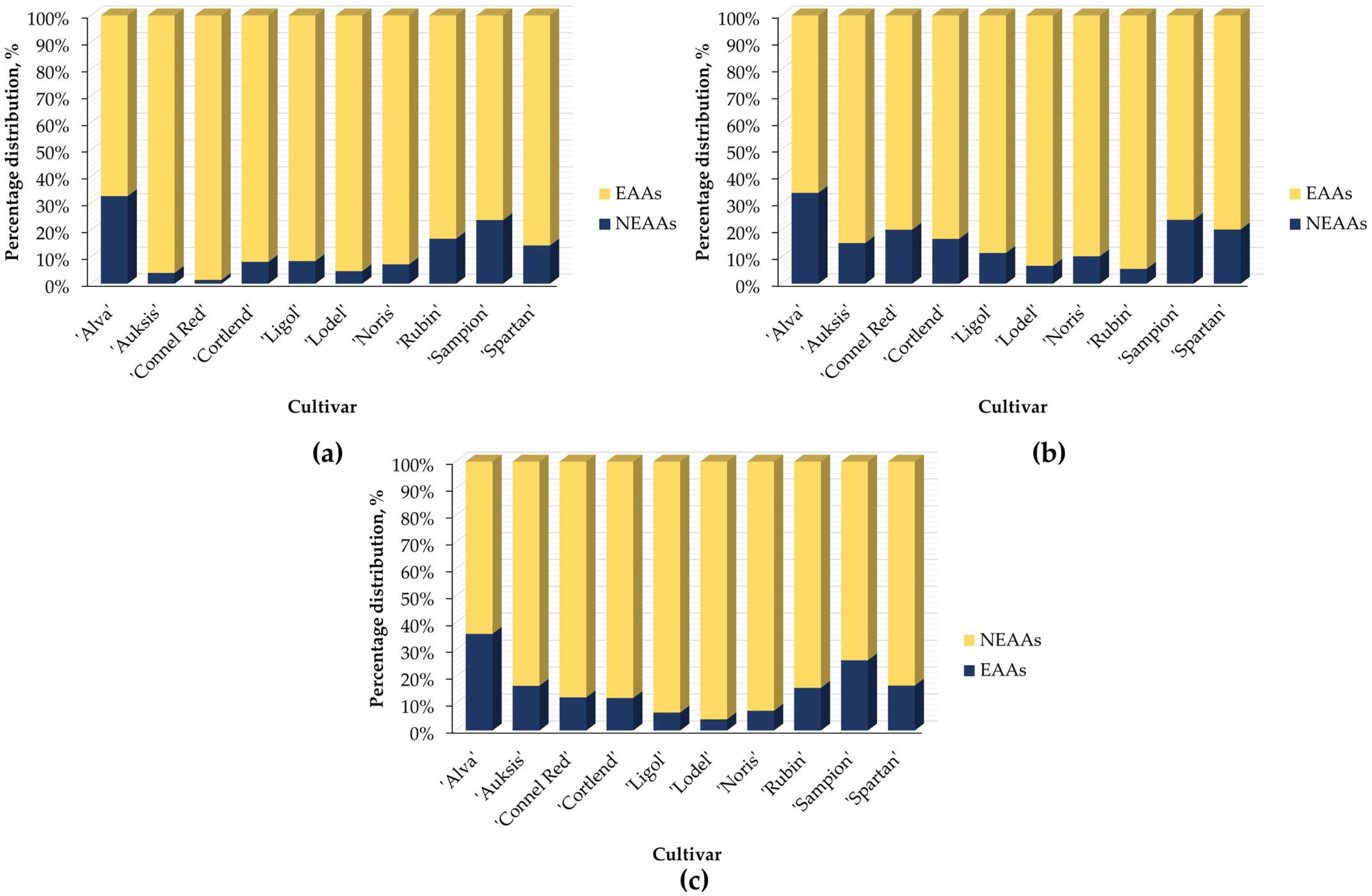
2.1. Variation in Amino Acids Content in Whole Apple, Apple Peel, and Flesh Samples
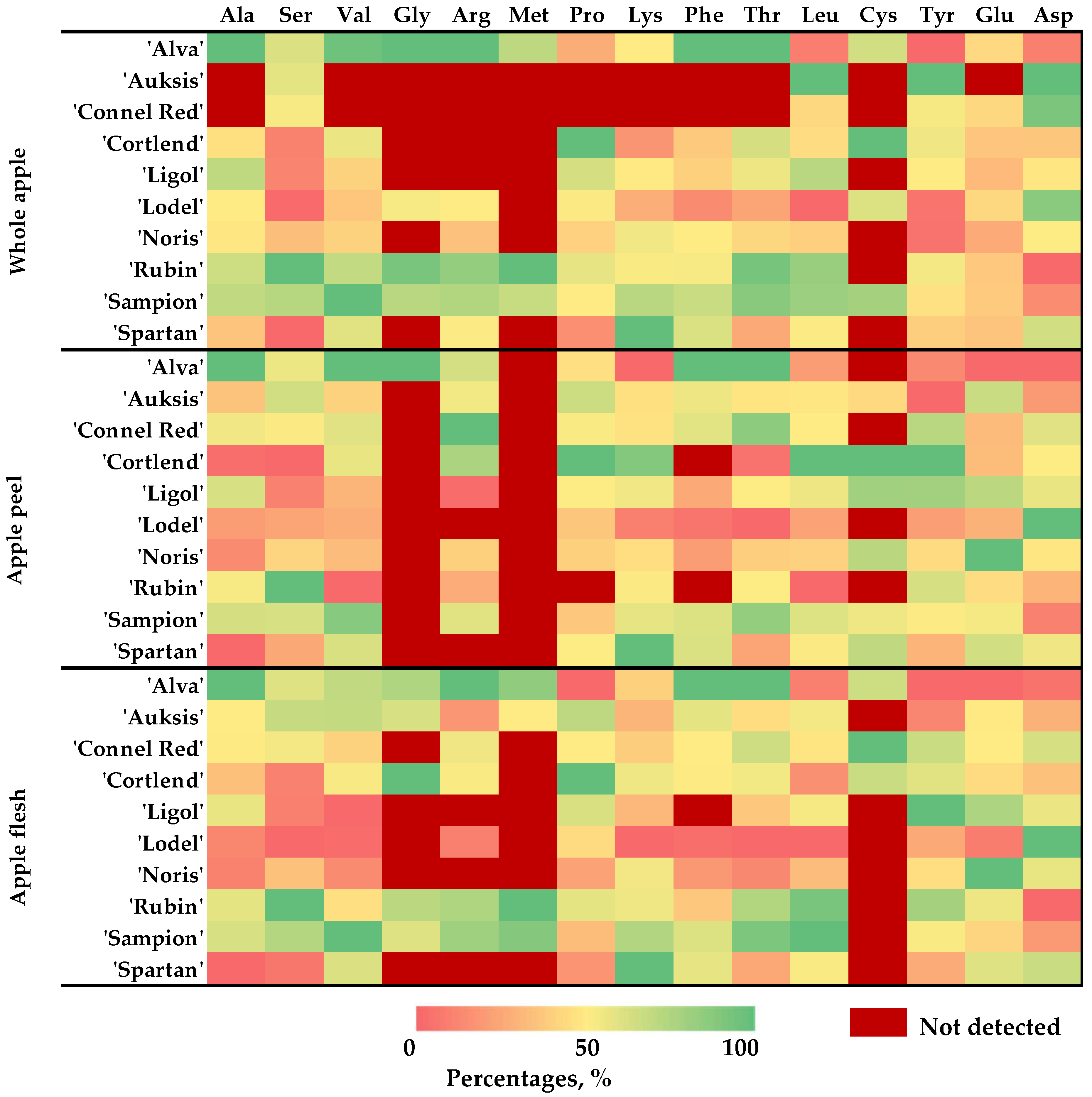
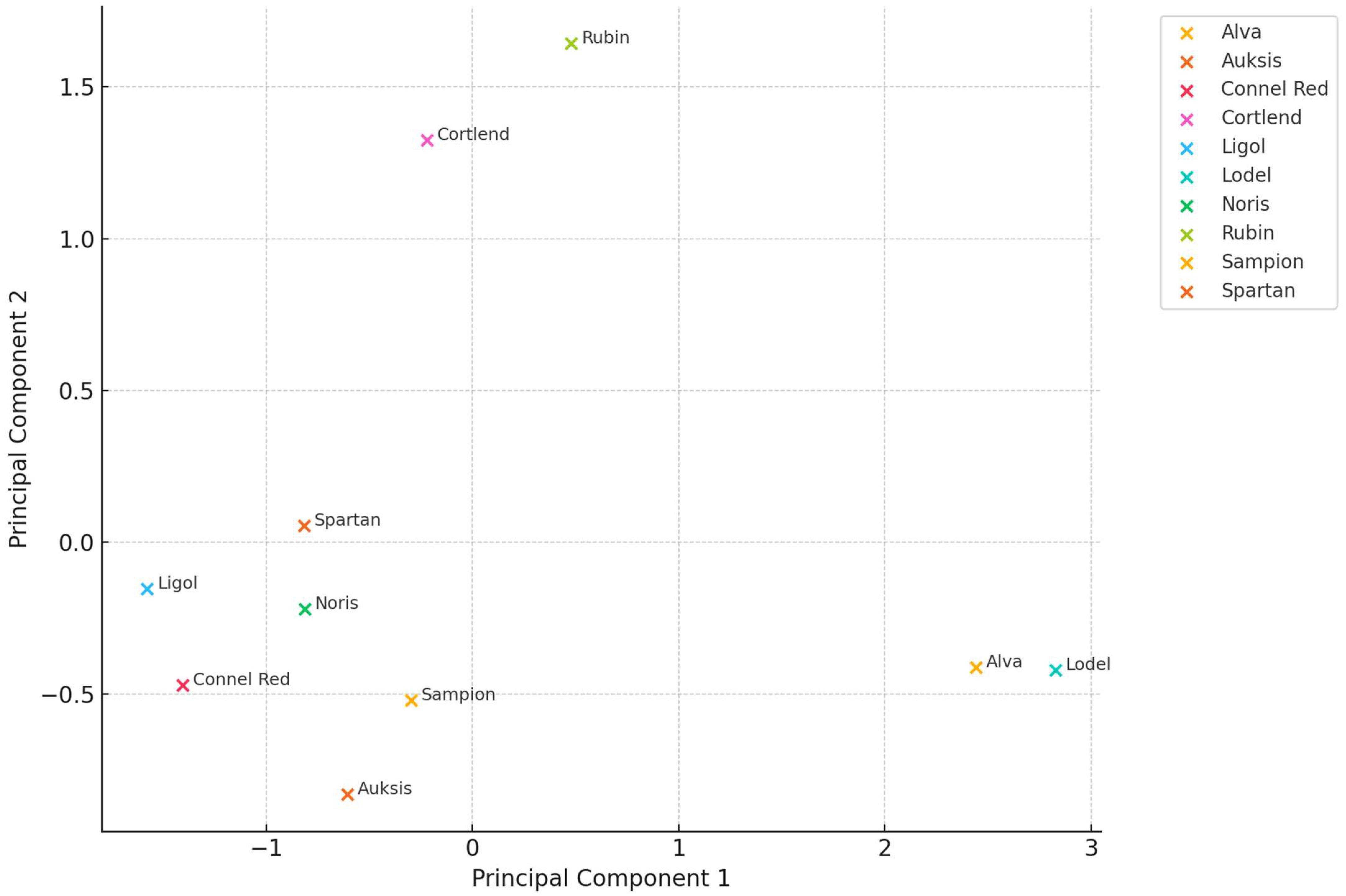
2.2. Variation in Correlation Coefficient Between Different Amino Acids in Whole Apple, Apple Peel, and Flesh Samples
3. Discussion
4. Materials and Methods
4.1. Fruit Materials
4.2. Chemicals and Solvents
4.3. Preparation of Whole Apple, Apple Peel, and Flesh Extracts
4.4. Assay of Amino Acids by UPLC-MS/MS
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, M.; Yue, T.; Yuan, Y. Changes in the Profile of Volatile Compounds and Amino Acids during Cider Fermentation Using Dessert Variety of Apples. Eur. Food Res. Technol. 2014, 239, 67–77. [Google Scholar] [CrossRef]
- Khan, N.; Ali, S.; Zandi, P.; Mehmood, A.; Ullah, S.; Ikram, M.; Ismail; Shahid, M.A.; Babar, M.A. Role of Sugars, Amino Acids and Organic Acids in Improving Plant Abiotic Stress Tolerance. Pak. J. Bot. 2020, 52, 355–363. [Google Scholar] [CrossRef]
- Sun, W.; Shahrajabian, M.H.; Kuang, Y.; Wang, N. Amino Acids Biostimulants and Protein Hydrolysates in Agricultural Sciences. Plants 2024, 13, 210. [Google Scholar] [CrossRef]
- Dai, Z.L.; Wu, G.; Zhu, W.Y. Amino Acid Metabolism in Intestinal Bacteria: Links between Gut Ecology and Host Health. Front. Biosci. 2011, 16, 1768–1786. [Google Scholar] [CrossRef]
- Dai, Z.L.; Li, X.L.; Xi, P.B.; Zhang, J.; Wu, G.; Zhu, W.Y. Regulatory Role for L-Arginine in the Utilization of Amino Acids by Pig Small-Intestinal Bacteria. Amino Acids 2012, 43, 233–244. [Google Scholar] [CrossRef]
- Wu, G. Functional Amino Acids in Nutrition and Health. Amino Acids 2013, 45, 407–411. [Google Scholar] [CrossRef]
- Turkiewicz, I.P.; Wojdyło, A.; Tkacz, K.; Nowicka, P. Carotenoids, Chlorophylls, Vitamin E and Amino Acid Profile in Fruits of Nineteen Chaenomeles Cultivars. J. Food Compos. Anal. 2020, 93, 103608. [Google Scholar] [CrossRef]
- Botoran, O.R.; Ionete, R.E.; Miricioiu, M.G.; Costinel, D.; Radu, G.L.; Popescu, R. Amino Acid Profile of Fruits as Potential Fingerprints of Varietal Origin. Molecules 2019, 24, 4500. [Google Scholar] [CrossRef]
- Lu, M.; An, H.; Wang, D. Characterization of Amino Acid Composition in Fruits of Three Rosa Roxburghii Genotypes. Hortic. Plant J. 2017, 3, 232–236. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). FAOSTAT Database. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 10 October 2024).
- Fărcaș, A.C.; Ancuț, S.; Socaci, S.; Chis, M.S.; Dulf, F.V.; Podea, P.; Tofană, M. Analysis of Fatty Acids, Amino Acids and Volatile Profile of Apple By-Products by Gas Chromatography-Mass Spectrometry. Molecules 2022, 27, 1987. [Google Scholar] [CrossRef]
- Colak, N.; Kolcuoglu, Y.; Tarkowski, P.; Benická, S.; Colak, A.; Hayirlioglu-Ayaz, S.; Ayaz, F.A. Nutritional Evaluation of an Outstanding Apple Cultivar, ‘Göbek’ (Malus communis L.). J. Food Nutr. Res. 2017, 5, 874–881. [Google Scholar] [CrossRef]
- dos Santos, C.M.E.; Pietrowski, G.A.M.; Braga, C.M.; Rossi, M.J.; Ninow, J.; dos Santos, T.P.M.; Wosiacki, G.; Jorge, R.M.M.; Nogueira, A. Apple Amino Acid Profile and Yeast Strains in the Formation of Fusel Alcohols and Esters in Cider Production. J. Food Sci. 2015, 80, C1170–C1177. [Google Scholar]
- Duralija, B.; Putnik, P.; Brdar, D.; Markovinović, A.B.; Zavadlav, S.; Pateiro, M.; Domínguez, R.; Lorenzo, J.M.; Kovačević, D.B. The Perspective of Croatian Old Apple Cultivars in Extensive Farming for the Production of Functional Foods. Foods 2021, 10, 708. [Google Scholar] [CrossRef]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef]
- Preti, R.; Tarola, A.M. Study of Polyphenols, Antioxidant Capacity and Minerals for the Valorisation of Ancient Apple Cultivars from Northeast Italy. Eur. Food Res. Technol. 2021, 247, 273–283. [Google Scholar] [CrossRef]
- Wandjou, J.G.N.; Sut, S.; Giuliani, C.; Fico, G.; Papa, F.; Ferraro, S.; Caprioli, G.; Maggi, F.; Dall’Acqua, S. Characterization of Nutrients, Polyphenols and Volatile Components of the Ancient Apple Cultivar ‘Mela Rosa Dei Monti Sibillini’ from Marche Region, Central Italy. Int. J. Food Sci. Nutr. 2019, 70, 796–812. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lachowicz, S.; Glawdel, E.; Cebulak, T.; Ochmian, I. Determination of Phytochemical Composition and Antioxidant Capacity of 22 Old Apple Cultivars Grown in Poland. Eur. Food Res. Technol. 2017, 244, 647–662. [Google Scholar] [CrossRef]
- Shehzadi, K.; Rubab, Q.; Asad, L.; Ishfaq, M.; Shafique, B.; Ali, M.M.; Mahmood, S.; Mueen-Ud-Din, G.; Javaid, T.; Sabtain, B. A Critical Review on Presence of Polyphenols in Commercial Varieties of Apple Peel, Their Extraction and Health Benefits. Open Access J. Bio Sci. Res. 2020, 6, 18. [Google Scholar]
- Jakobek, L.; Garcia-Villalba, R.; Tomas-Barberan, F.A. Polyphenolic Characterisation of Old Local Apple Varieties from Southeastern European Region. J. Food Compos. Anal. 2013, 31, 199–211. [Google Scholar] [CrossRef]
- Oszmianski, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical Analysis by Liquid Chromatography of Ten Old Apple Varieties Grown in Austria and Their Antioxidative Activity. Eur. Food Res. Technol. 2020, 246, 437–448. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Psomas, A.; Zovoili, A.; Siatis, V. Polyphenolic Profile and Antioxidant Activity of Five Apple Cultivars Grown Under Organic and Conventional Agricultural Practices. Int. J. Food Sci. Technol. 2009, 44, 1167–1175. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential Distribution of Amino Acids in Plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical Compositional Characterization of Some Apple Cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Lanauskas, J.; Kviklys, D.; Liaudanskas, M.; Janulis, V.; Uselis, N.; Viškelis, J.; Viškelis, P. Lower nitrogen nutrition determined higher phenolic content of organic apples. Hortic. Sci. 2017, 44, 113–119. [Google Scholar] [CrossRef]
- Samuoliene, G.; Ceidaite, A.; Sirtautas, R.; Duchovskis, P.; Kviklys, D. Effect of crop load on phytohormones, sugars, and biennial bearing in apple trees. Biol. Plant. 2016, 60, 394–400. [Google Scholar] [CrossRef]
- Uselis, N.; Viškelis, J.; Lanauskas, J.; Liaudanskas, M.; Janulis, V.; Kviklys, D. Planting distance affects apple tree growth, fruit yield and quality. Zemdirb. Agric. 2020, 107, 367–372. [Google Scholar] [CrossRef]
- Viškelis, J.; Uselis, N.; Liaudanskas, M.; Janulis, V.; Bielicki, P.; Univer, T.; Lepsis, J.; Kviklys, D. Triterpenic acid content in the fruit peel of Malus × domestica Borkh. depends on the growing technology. Zemdirb. Agric. 2018, 105, 71–78. [Google Scholar] [CrossRef]
- Ough, C.S.; Huang, Z.; An, D.; Stevens, D. Amino acid uptake by four commercial yeasts at two different temperatures of growth and fermentation: Effect. Am. J. Enol. Vitic. 1991, 42, 26–40. [Google Scholar] [CrossRef]
- Monteiro, F.F.; Bisson, L.F. Nitrogen supplementation of grape juice. II. Effect on amino acid and urea release following fermentation. Am. J. Enol. Vitic. 1992, 43, 11–17. [Google Scholar] [CrossRef]
- Valles, B.S.; García, N.P.; Madrera, R.R.; Lobo, A.P. Influence yeast strain and aging time on free amino acid changes in sparkling ciders. J. Agric. Food Chem. 2005, 53, 6408–6641. [Google Scholar] [CrossRef]
- Ling, Z.N.; Jiang, Y.F.; Ru, J.N.; Lu, J.H.; Ding, B.; Wu, J. Amino acid metabolism in health and disease. Signal Transduct. Target. Ther. 2023, 8, 345. [Google Scholar] [CrossRef]
- Akram, M.; Asif, H.M.; Uzair, M.; Akhtar, N.; Madni, A.; Shah, S.M.A.; Hasan, Z.U.; Ullah, A. Amino acids: A review article. J. Med. Plants Res. 2011, 5, 3997–4000. [Google Scholar]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Cargnin, S.T.; Gnoatto, S.B. Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic acid: An anti- and proinflammatory triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.; Clifford, M.; Morgan, L. Possible role for apple juice phenolic compounds in the acute modification of glucose tolerance and gastrointestinal hormone secretion in humans. J. Sci. Food Agric. 2002, 82, 1800–1805. [Google Scholar] [CrossRef]
- Avci, A.; Atli, T.; Eruder, I.; Varli, M.; Devrim, E.; Turgay, S.; Durak, I. Effects of apple consumption on plasma and erythrocyte antioxidant parameters in elderly subjects. Exp. Aging Res. 2007, 33, 429–437. [Google Scholar] [CrossRef]
- Braciuliene, A.; Janulis, V.; Petrikaite, V. The chemo-sensitizing effect of doxorubicin of apple extract-enriched triterpenic complex on human colon adenocarcinoma and human glioblastoma cell lines. Pharmaceutics 2022, 14, 2593. [Google Scholar] [CrossRef]
- Kukina, T.P.; Frolova, T.S.; Salnikova, O.I. Lipophilic constituents from Malus baccata. Chem. Nat. Compd. 2014, 6, 1096–1097. [Google Scholar] [CrossRef]
- Marks, S.C.; Mullen, W.; Borges, G.; Crozier, A. Absorption, metabolism, and excretion of cider dihydrochalcones in healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2009, 57, 2009–2015. [Google Scholar] [CrossRef]
- Rana, S.; Bhushan, S. Apple phenolics as nutraceuticals: Assessment, analysis and application. J. Food Sci. Technol. 2016, 53, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef]
- Amanor-Atiemoh, R.; Zhou, C.; Taiye, M.A.; Sarpong, F.; Chen, L.; Wahia, H.; Amoa-Owusu, A.; Ma, H. Effect of ultrasound-ethanol pretreatment on drying kinetics, quality parameters, functional group, and amino acid profile of apple slices using pulsed vacuum drying. J. Food Process. Eng. 2020, 43, e13347. [Google Scholar] [CrossRef]
- Holeček, M. Aspartic Acid in Health and Disease. Nutrients 2023, 15, 4023. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.-L.; Li, D.; Kim, S.W.; Wu, G. Amino Acids and Immune Function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Y.; Zhang, Q.; Zhao, Y.; Guo, L.; Wang, B.; Ma, H.; Chen, Y. Exogenous Aspartic Acid Improves Salt Tolerance in Plants. Front. Plant Sci. 2022, 13, 987641. [Google Scholar]
- Blachier, F.; Davila, A.M.; Benamouzig, R.; Tome, D. Channelling of Arginine in NO and Polyamine Pathways in Colonocytes and Consequences. Front. Biosci. 2011, 16, 1331–1343. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, G.W.; Wu, G.; Bazer, F.W. Functional Roles of Fructose. Proc. Natl. Acad. Sci. USA 2012, 109, E1619–E1628. [Google Scholar] [CrossRef]
- Kong, X.F.; Tan, B.E.; Yin, Y.L.; Gao, H.; Li, X.; Jaeger, L.A.; Bazer, F.W.; Wu, G. L-Arginine Stimulates the mTOR Signaling Pathway and Protein Synthesis in Porcine Trophectoderm Cells. J. Nutr. Biochem. 2012, 23, 1178–1183. [Google Scholar] [CrossRef]
- Wu, G. Amino Acids: Metabolism, Functions, and Nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Holeček, M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Burghardt, R.C.; Wu, G.; Johnson, G.A.; Spencer, T.E.; Bazer, F.W. Select Nutrients in the Ovine Uterine Lumen: VII. Effects of Arginine, Leucine, Glutamine, and Glucose on Trophectoderm Cell Signaling, Proliferation, and Migration. Biol. Reprod. 2011, 84, 62–69. [Google Scholar] [CrossRef]
- Wu, G.; Bazer, F.W.; Johnson, G.A.; Knabe, D.A.; Burghardt, R.C.; Spencer, T.E.; Li, X.L.; Wang, J.J. Important Roles for L-Glutamine in Swine Nutrition and Production. J. Anim. Sci. 2011, 89, 2017–2030. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.D.; Wu, X.; Yin, Y.L.; Liu, Y.Q.; Geng, M.M.; Yang, H.S.; Blachier, F.; Wu, G.Y. Effects of Dietary L-Arginine or N-Carbamylglutamate Supplementation During Late Gestation of Sows on the miR-15b/16, miR-221/222, VEGFA and eNOS Expression in Umbilical Vein. Amino Acids 2012, 42, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Bazer, F.W.; Song, G.H.; Kim, J.Y.; Erikson, D.W.; Johnson, G.A.; Burghardt, R.C.; Gao, H.; Satterfield, M.C.; Spencer, T.E.; Wu, G. Mechanistic Mammalian Target of Rapamycin (MTOR) Cell Signaling: Effects of Select Nutrients and Secreted Phosphoprotein 1 on Development of Mammalian Conceptuses. Mol. Cell. Endocrinol. 2012, 354, 22–33. [Google Scholar] [CrossRef]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino Acid Signalling Upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef]
- Tan, B.E.; Li, X.G.; Wu, G.; Kong, X.; Liu, Z.; Li, T.; Yin, Y. Dynamic Changes in Blood Flow and Oxygen Consumption in the Portal-Drained Viscera of Growing Pigs Receiving Acute Administration of L-Arginine. Amino Acids 2012, 43, 2481–2489. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, J.; Wu, G. Glycine Metabolism in Animals and Humans: Implications for Nutrition and Health. Amino Acids 2013, 45, 463–477. [Google Scholar] [CrossRef]
- Wu, Z.L.; Satterfield, M.C.; Bazer, F.W.; Wu, G. Regulation of Brown Adipose Tissue Development and White Fat Reduction by L-Arginine. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 529–538. [Google Scholar] [CrossRef]
- Hou, Y.Q.; Wang, L.; Zhang, W.; Yang, Z.; Ding, B.; Zhu, H.; Liu, Y.; Qiu, Y.; Yin, Y.; Wu, G. Protective Effects of N-Acetylcysteine on Intestinal Functions of Piglets Challenged with Lipopolysaccharide. Amino Acids 2012, 43, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Luo, W.; Wu, M.; Liu, G.; Yu, X.; Fang, J.; Li, T.; Yin, Y.; Wu, G. Dietary L-Glutamine Supplementation Improves Pregnancy Outcome in Mice Infected with Type-2 Porcine Circovirus. Amino Acids 2013, 45, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Uneyama, H.; Kobayashi, H.; Tonouchi, N. New Functions and Potential Applications of Amino Acids. Adv. Biochem. Eng. Biotechnol. 2017, 159, 273–287. [Google Scholar] [PubMed]
- Lee, J.T.; Rochell, S.J.; Kriseldi, R.; Kim, W.K.; Mitchell, R.D. Functional properties of amino acids: Improve health status and sustainability. Poult. Sci. 2023, 102, 102288. [Google Scholar] [CrossRef]
- Butkeviciute, A.; Viskelis, J.; Liaudanskas, M.; Viskelis, P.; Janulis, V. Variation of Triterpenes in Apples Stored in a Controlled Atmosphere. Molecules 2021, 26, 3639. [Google Scholar] [CrossRef]
- Šimoliūnienė, R.; Tomkevičiūtė, J.; Jokšienė, Ž.; Šimatonienė, V.; Kriščiukaitis, A.; Šaferis, V. Basics of Biostatistics; Kauno Medicinos Universiteto Leidykla: Kaunas, Lithuania, 2015; pp. 133–142. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bračiulienė, A.; Žvikas, V.; Liaudanskas, M.; Janulis, V. A Preliminary Study on the Qualitative and Quantitative Changes of Amino Acids in Whole Apple, Apple Peel, and Flesh Samples Grown in Lithuania. Plants 2025, 14, 1330. https://doi.org/10.3390/plants14091330
Bračiulienė A, Žvikas V, Liaudanskas M, Janulis V. A Preliminary Study on the Qualitative and Quantitative Changes of Amino Acids in Whole Apple, Apple Peel, and Flesh Samples Grown in Lithuania. Plants. 2025; 14(9):1330. https://doi.org/10.3390/plants14091330
Chicago/Turabian StyleBračiulienė, Aurita, Vaidotas Žvikas, Mindaugas Liaudanskas, and Valdimaras Janulis. 2025. "A Preliminary Study on the Qualitative and Quantitative Changes of Amino Acids in Whole Apple, Apple Peel, and Flesh Samples Grown in Lithuania" Plants 14, no. 9: 1330. https://doi.org/10.3390/plants14091330
APA StyleBračiulienė, A., Žvikas, V., Liaudanskas, M., & Janulis, V. (2025). A Preliminary Study on the Qualitative and Quantitative Changes of Amino Acids in Whole Apple, Apple Peel, and Flesh Samples Grown in Lithuania. Plants, 14(9), 1330. https://doi.org/10.3390/plants14091330







