Impact of Volcanic Slag on Cucumber Yield, Quality, and Rhizosphere Soil Environment
Abstract
1. Introduction
2. Results
2.1. Effects of Different Application Amounts of Volcanic Slag on Cucumber Yield
2.2. Effects of Different Application Amounts of Volcanic Slag on the Quality of Cucumber Fruits
2.3. Effects of Different Application Amounts of Volcanic Slag on the Physicochemical Properties of Cucumber Rhizosphere Soil
2.4. Analysis of the Composition and Relative Abundance of Soil Bacteria in Cucumber Rhizosphere Soil with Different Application Amounts of Volcanic Slag
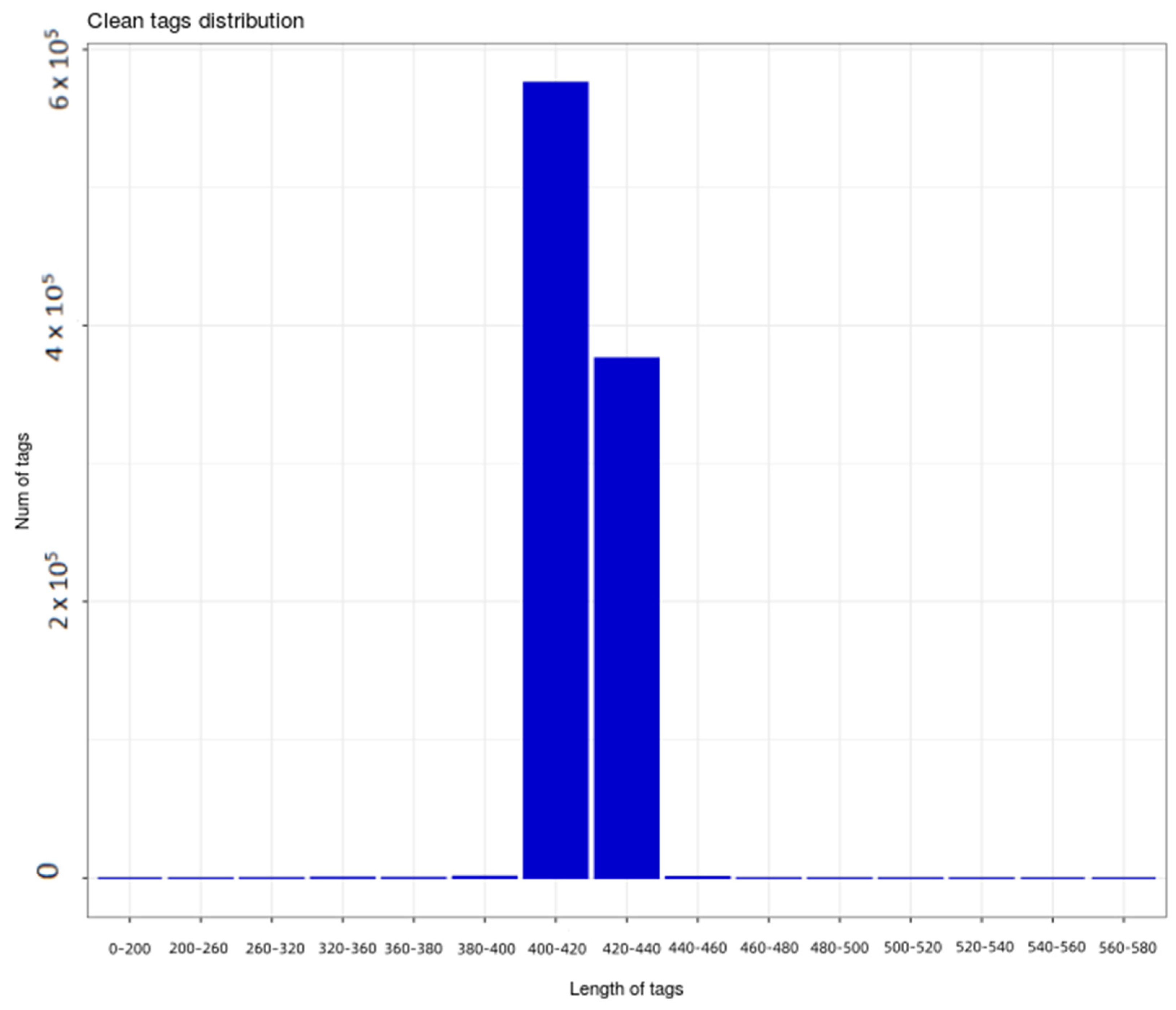
2.4.1. Analysis of Soil Sequencing Results
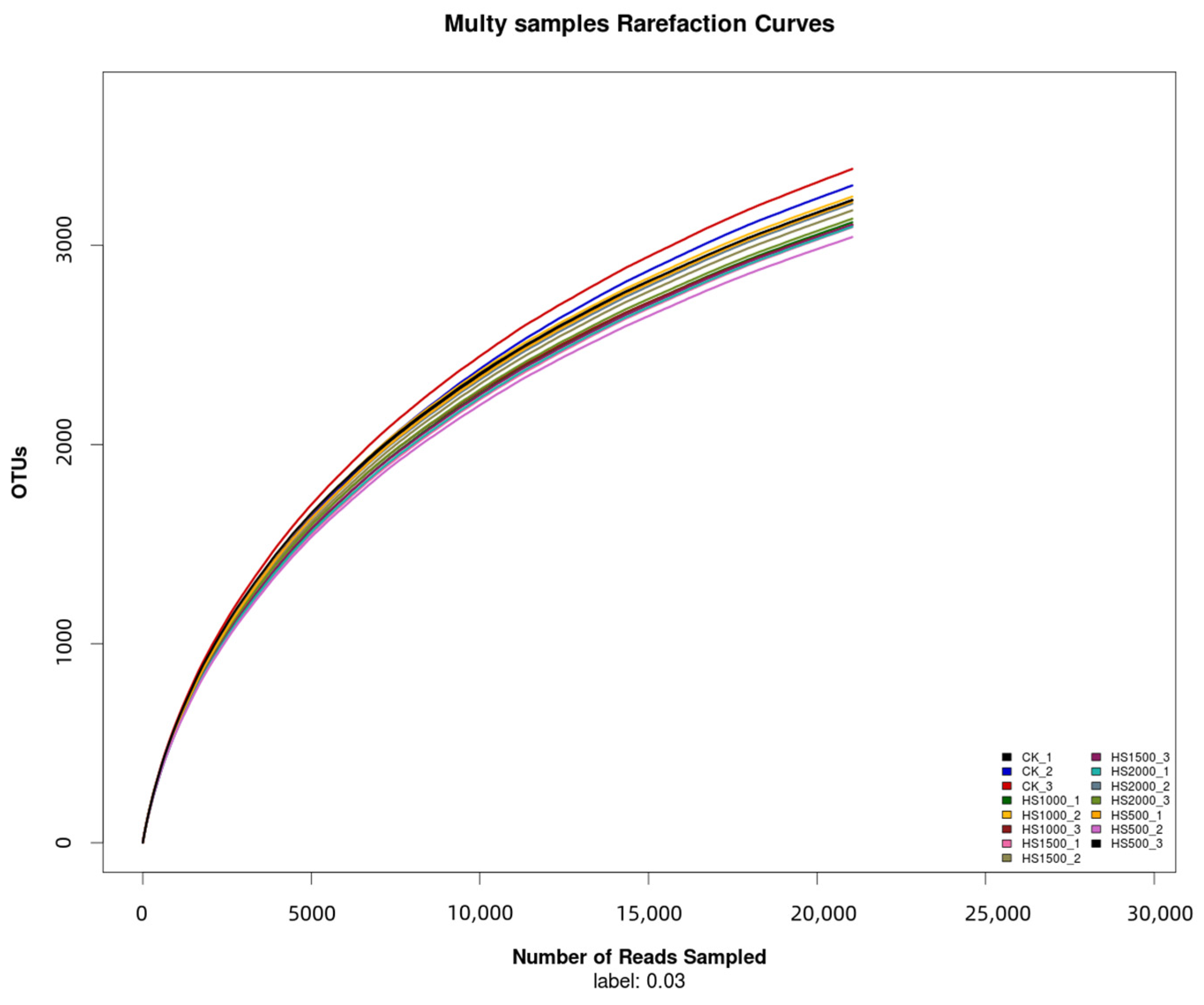
2.4.2. Distribution of OTUs in the Cucumber Root Soil with Different Application Amounts of Volcanic Slag
2.4.3. Effects of Different Application Amounts of Volcanic Slag on the Alpha Diversity of Soil Bacteria in the Cucumber Root Soil
2.4.4. Composition and Differential Analysis of Bacterial Communities in the Cucumber Root Soil with Different Application Amounts of Volcanic Slag
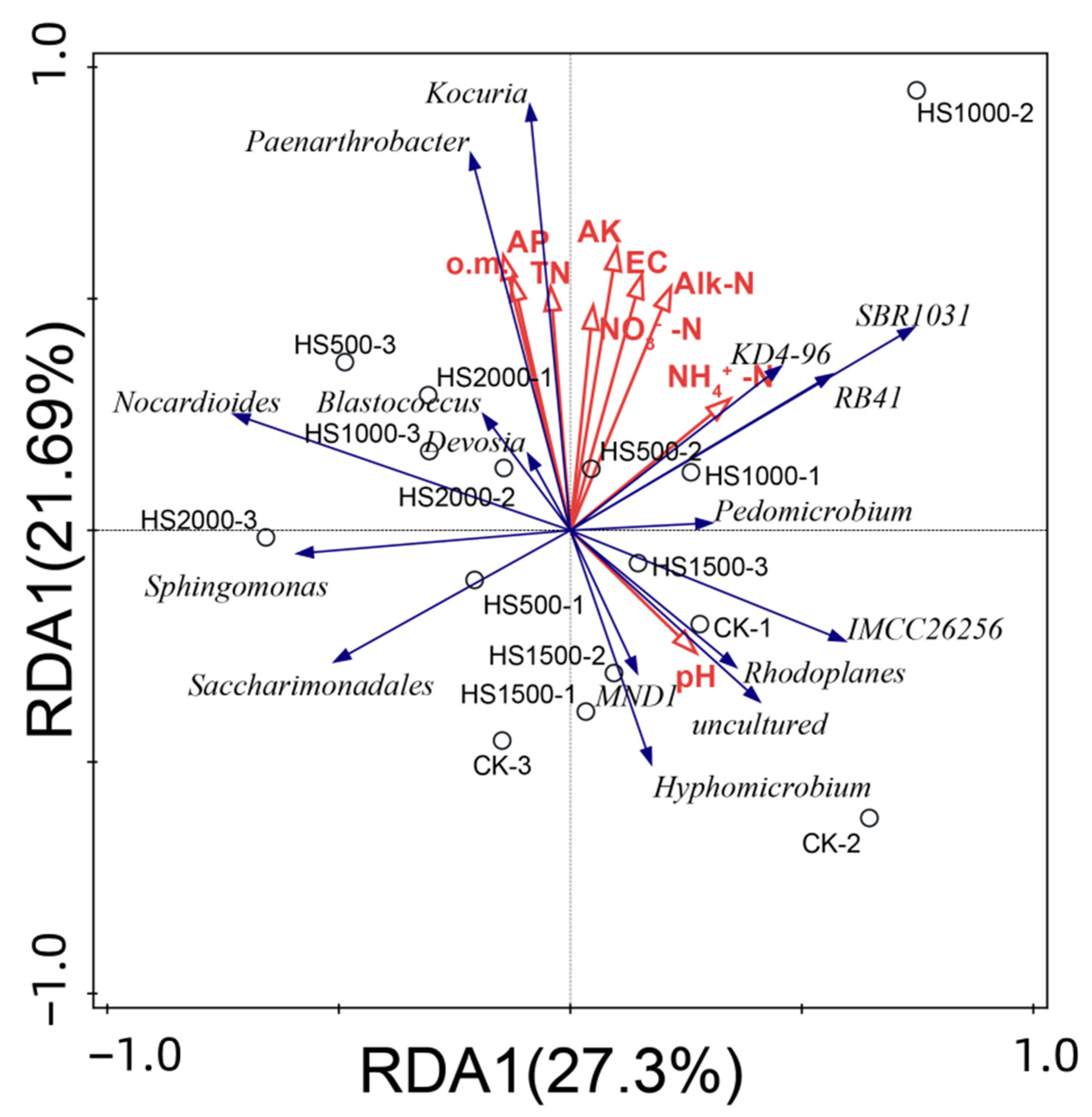
2.4.5. Impact of Environmental Factors on the Distribution of Bacterial Communities in Cucumber Rhizosphere Soil
3. Discussion
4. Experimental Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Determination of Cucumber Growth Indicators and Yield
4.2.2. Determination of Cucumber Fruit Quality
4.2.3. Collection of Soil Samples
4.2.4. Determination of Soil Physical and Chemical Properties
Soil Bacteria Sequencing Analysis with DNA Extraction
PCR Amplification and Purification, and MiSeq Sequencing
Statistical Analysis
5. Data Processing
6. Conclusions
- The appropriate addition of volcanic slag positively impacted the growth and development of cucumber plants, photosynthetic performance of leaves, fruit yield, and quality. All treatments promoted the growth of cucumber plants and improved the photosynthetic characteristics of cucumber leaves. The cucumber yield significantly increased by 24.28% under the HS1000 treatment. The soluble sugar, Vc, and soluble solid contents increased by 12.39%, 17.57%, and 24.33%, respectively, whereas the nitrate content decreased by 9.37% under the HS1000 treatment compared with the CK treatment.
- The appropriate amount of volcanic slag could improve the physical and chemical properties of cucumber rhizosphere soil. The electrical conductivity and organic matter, alkali nitrogen, nitrate nitrogen, and available potassium contents significantly increased under each treatment, whereas total nitrogen, available phosphorus, NH4+-N, and moisture contents increased to varying degrees. The pH of the soil increased with the increase in the amount of volcanic slag applied.
- Through Illumina MiSeq sequencing, clustering at a 97% similarity level, and analysis of alpha diversity and RDA, available phosphorus, available potassium, and NH4+-N were identified as the main influencing factors of changes in the bacterial community structure in cucumber rhizosphere soil.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Wu, Z.; Sun, G. Breeding and Field Management on New Cucumber Cultivar ‘Derit 917’. China Fruit Veg. 2023, 43, 43–47+77. [Google Scholar]
- Wang, X. Effects of Different Microbial Fertilizer Treatments Oncucumber Growth and Bacterial Stem Soft Rot. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2022. [Google Scholar]
- Yue, M.; Wang, P.; Jie, J.; Li, Y.; Zhang, J.; Fan, B.; Liu, Z.; Liu, X. Effects of liquid fertilizer prepared from kitchen wastewater on the growth of cucumber seedlings and soil environment. J. Shanxi Agric. Univ. Nat. Sci. Ed. 2023, 43, 93–101. [Google Scholar]
- Gómez-Castillo, G.; Mendoza, M.E.; Macías, J.L.; Vargas-Ramírez, N. Detailed geomorphology of debris avalanches of El Estribo volcanic complex (Central Mexico). J. Maps 2020, 16, 552–564. [Google Scholar] [CrossRef]
- Zhou, P. Triaxial Tests and Discrete-Continuum Coupled Numerical Investigation of Geocomposite-Encapsulated Volcanic Cinder Column. Master’s Thesis, Southwest Jiaotong University, Chengdu, China, 2021. [Google Scholar]
- Dong, T. Mechanism and Techniques of Microbial Purification of Nitrogen-Sulfonamides in Groundwater. Ph.D. Thesis, Jilin University, Changchun, China, 2020. [Google Scholar]
- Qian, C.; Cui, T.; Tang, Z.; Jiang, B.; Zhang, C.; Qin, T.; Lu, L.; Chen, H.; Wu, T. Stage division and genesis discussion of basaltic volcanism during the coneforming stage of Tianchi volcano in Changbaishan region. Geol. China 2016, 43, 1963–1976. [Google Scholar]
- Cheng, Y. Study on Integrative Technology of Treatment of Subgrade on Black Cotton Soil and Pavement Structre. Ph.D. Thesis, Southeast University, Nanjing, China, 2017. [Google Scholar]
- Ikeda, S.; Okazaki, K.; Tsurumaru, H.; Suzuki, T.; Hirafuji, M. Effects of Different Types of Additional Fertilizers on Root-associated Microbes of Napa Cabbage Grown in an Andosol Field in Japan. Microbes Environ. 2022, 37, ME22013. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Takebe, M.; Sato, F.; Komada, M.; Nagaoka, K.; Takenaka, M.; Urashima, Y.; Nishimua, S.; Takahashi, S.; Kato, N. Trends of lettuce and carrot yields and soil enzyme activities during transition from conventional to organic farming in an Andosol. Soil Sci. Plant Nutr. 2014, 61, 295–311. [Google Scholar] [CrossRef]
- Mulyono, N.; Maas, A.; Purwanto, B.H.; Sudira, P. Short Communication: Volcanic ash utilization as planting medium of curly lettuce with charcoal husk and urban waste compost as soil amendment. Asian J. Agric. 2018, 2, 39–43. [Google Scholar] [CrossRef]
- Theocharis, C.; Vasileios, T.; Anastasia, G.; Polyxeni, P. Inorganic and Organic Amendments Affect Soil Fertility, Nutrition, Photosystem II Activity, and Fruit Weight and May Enhance the Sustainability of Solanum lycopersicon L. (cv. ‘Mountain Fresh’) Crop. Sustainability 2020, 12, 9028. [Google Scholar] [CrossRef]
- Cadar, O.; Stupar, Z.; Senila, M.; Levei, L.; Moldovan, A.; Becze, A.; Ozunu, A.; Levei, E.A. Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables. Materials 2022, 15, 5657. [Google Scholar] [CrossRef]
- Xue, D.; Wang, Y.; Sun, H.; Fu, L.; Zhu, L.; Liu, J.; Zhi, Z.; He, J.; Wang, W.; Wu, C. Effects of Soil Conditioner (Volcanic Ash) on Yield Quality and Rhizosphere Soil Characteristics of Melon. Plants 2024, 13, 1787. [Google Scholar] [CrossRef]
- Liu, C.; Luo, C.; Xu, X.; Wu, C.; Li, F.; Zhang, G. Effects of calcium peroxide on arsenic uptake by celery (Apium graveolens L.) grown in arsenic contaminated soil. Chemosphere 2012, 86, 1106–1111. [Google Scholar] [CrossRef]
- Yan, D.; Hhuang, Q.; Chen, H.; Chen, Z.; Huang, Z.; Guo, Y. Effects of oyster shell powder soil conditioner on soil physical and chemical properties and agronomic properties and quality of Chinese cabbage. China Cucurbits Veg. 2023, 36, 88–94. [Google Scholar]
- Qin, Y.; Shi, C.; DIing, Z.; Liu, Z.; Lian, X. Study on the effect of soil conditioner on soil improvement of facility pepper. Agric. Eng. Technol. 2022, 42, 79–81+90. [Google Scholar]
- Fu, L.; Tian, X.; Wang, W.; Wu, C. Metabolome and Transcriptome Analysis Reveals Molecular Mechanisms of Soil Amendment (Volcanic Ash) Alleviating Salt–Alkali Stress in Melons (Cucumis melo L.). Agronomy 2024, 14, 2478. [Google Scholar] [CrossRef]
- Wei, H. Effect of Soil Amendment on Garlic Growth and Cadmium Absorption. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2023. [Google Scholar]
- Zhang, F.; Xie, C.; Xiao, B.; Liu, S.; Wu, C.; Zhang, Y.; Wang, W. Effects of wood vinegar on physicochemical properties and bacterial community diversity of tomato rhizosphere soil. J. Jilin Agric. Univ. 2024, 1–8. [Google Scholar] [CrossRef]
- Yang, H.; Guo, Q.; Huang, B.; Chen, H.; Pan, X.; Fan, R.; Du, J. Effects of biochar-based soil conditioners on ameliorating acid soil in vegetable field. J. Agric. Resour. Environ. 2023, 40, 15–24. [Google Scholar]
- Ma, H.; Zhu, H.; Li, Y.; Huang, H.; Cai, Z.; Xue, Z.; Wen, Q. Characteristic Analysis of Microbial Community Structure After the Improvement of Continuous Cropping Soil with Greenhouse Pepper in Fujian Province. Fujian Agric. Sci. Technol. 2022, 53, 73–79. [Google Scholar]
- Chen, C.; Zhang, X.; Cai, X.; Wang, J.; Fu, R.; Lu, D. Effects of Modified Fertilization on Rhizosphere Microbial Community Structure of Chinese Cabbage. North. Hortic. 2018, 13, 108–113. [Google Scholar]
- Yuya, I.; Fukashi, M.; Atsushi, Y. Temporal change in eruption style during the basaltic explosive An’ei eruption of the Izu-Oshima volcano, Japan: Insights from stratigraphy and chemical composition analyses. Front. Earth Sci. 2023, 11, 1172615. [Google Scholar]
- Chen, Z.; Zhang, Y.; Si, C.; Chu, W.; Chen, Z.; Feng, K. Effect of Scoria on Adsorption of SO42− in Low Temperature Water. J. Jilin Univ. Sci. Ed. 2018, 56, 464–467. [Google Scholar]
- Prueher, L.M.; McBirney, A.R. Relations of cinder cones to the magmatic evolution of Mount Mazama, Crater Lake National Park, Oregon. J. Volcanol. Geotherm. Res. 1989, 58–67. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, M.; Ding, W.; Wang, L.; Chang, D. The Effect of Complex Enzyme Glycoside(CEG) on Cucumber Rooting and Yield. Vegetables 2024, 18–22. [Google Scholar]
- Xu, N.; Zhang, F.; Sun, X.; Guo, B.; Cao, N.; Wang, C. Effects of Exogenous Silicon on Growth, Yield and Quality of Continuous-cropped Cucumber. North. Hortic. 2022, 46–51. [Google Scholar]
- Dong, Y.; Guo, S.; Li, X.; Wang, J.; Wang, Y.; Shu, S. Effects of Disinfection, Accompanying Cultivation and Microbial Agent on the Properties of Continuous Cropping Substrate and Growth of Cucumbers. Acta Bot. Boreali-Occident. Sin. 2023, 43, 1136–1145. [Google Scholar]
- Soon, Y.K.; Arshad, M.A.; Rice, W.A.; Mills, P. Recovery of chemical and physical properties of boreal plain soils impacted by pipeline burial. Can. J. Soil Sci. 2000, 80, 489–497. [Google Scholar] [CrossRef]
- Coombs, L.M.; Eichelberger, C.J.; Rutherford, J.M. Magma storage and mixing conditions for the 1953–1974 eruptions of Southwest Trident volcano, Katmai National Park, Alaska. Contrib. Mineral. Petrol. 2000, 140, 99–118. [Google Scholar] [CrossRef]
- Bai, X.; Zhang, Y.; Chen, Z.; Liu, T.; Li, S. Application and adsorption characteristics of scoria for removing sulfate from a low-temperature groundwater environment in Longdong area, China. Environ. Earth Sci. 2021, 80, 729. [Google Scholar] [CrossRef]
- Han, C.; Kang, T.; Miao, H.; Lv, X.; Jiang, Z. Morphological and Mineralogical Characteristics of Basaltic Volcanic Ballast Soils under Different Hydrothermal Conditions. Chin. J. Soil Sci. 2019, 50, 1038–1044. [Google Scholar]
- Al-Swaidani, M.A. Use of micro and nano volcanic scoria in the concrete binder: Study of compressive strength, porosity and sulfate resistance. Case Stud. Constr. Mater. 2019, 11, e00294. [Google Scholar] [CrossRef]
- Zou, J.; Dai, Y.; Sun, T.; Li, Y.; Li, G.; Li, Q. Effect of amended soil and hydraulic load on enhanced biological nitrogen removal in lab-scale SWIS. J. Hazard. Mater. 2008, 163, 816–822. [Google Scholar] [CrossRef]
- LI, J.; SHI, X.; LI, S.; Wang, Z.; Wang, J.; Zou, B.; Wang, S.; Huang, Z. Effects of stand ages on soil enzyme activities in Chinese fir plantations and natural secondary forests. J. Jilin Univ. Sci. Ed. 2024, 35, 339–346. [Google Scholar]
- Li, L.; He, X.Z.; Wang, M.; Huang, L.; Wang, Z.; Zhang, X.; Hu, J.; Hou, F. Grazing-driven shifts in soil bacterial community structure and function in a typical steppe are mediated by additional N inputs. Sci. Total Environ. 2023, 9, 169488. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Meng, T.; Qi, B.; Zhang, W.; Zhang, Z.; Lin, D.; Lin, Z.; Liu, Y. Effect of different JUNCAO genus on soil enzyme activities and microbial community diversity. J. Fujian Agric. For. Univ. Nat. Sci. Ed. 2023, 52, 48–58. [Google Scholar]
- Shannon, C.E. A Mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 623–656. [Google Scholar] [CrossRef]
- Xu, H.; Wang, S.; Zhang, Y.; Li, S.; Yin, Y. Effects of biocompost derived from spent mushroom substrates on soil microbial activity, abundance and diversity in cucumber field. Chin. J. Ecol. 2024, 43, 1279–1290. [Google Scholar]
- Hu, Y.; Liu, J.; Li, M.; Wang, X.; Zhang, Q.; Liu, L. Effect of Biochar on Bacterial Abundance in Cucumber Rhizosphere under Continuous Cropping. Mol. Plant Breed. 2023, 21, 2396–2402. [Google Scholar]
- Sun, S.; Ma, B.; Wang, Y.; Hao, S. Effects of Humic Acid on Soil Nutrients and Microbial Community Structure of Greenhouse Cucumber Rhizosphere Under Salt Stress. North. Hortic. 2022, 83–90. [Google Scholar]
- Du, J.; Nie, L.; Wei, L.; Wang, R.; Gong, W. Characterization of Inter-root Bacterial Community of Oilseed Rape in Nyingchi, Tibet. J. Plateau Agric. 2022, 6, 16–28. [Google Scholar]
- Zhang, Z.; Chen, Z. Experimental Techniques of Plant Physiology; Jilin University Press: Changchun, China, 2008. [Google Scholar]
- Boston. Soil Agrochemical Analysis; China Agricultural Publishing House: Beijing, China, 2005. [Google Scholar]


| Treatment | Average Ear Weight (g) | Yield (kg/ha) |
|---|---|---|
| CK | 195.46 ± 3.08 c | 42,759.44 ± 114.15 c |
| HS500 | 206.35 ± 1.8 b | 45,693.13 ± 103.89 b |
| HS1000 | 210.93 ± 1.61 a | 50,057.37 ± 116.36 a |
| HS1500 | 208.72 ± 3.18 ab | 47,211.19 ± 47.54 ab |
| HS2000 | 195.88 ± 0.68 c | 42,780.11 ± 87.56 c |
| Treatment | Soluble Sugar (%) | Titratable Acid (%) | Soluble Protein (mg/g−1) | Vitamin C (mg/100 g−1) | Soluble Solid Content (%) | Nitrate (μg·g−1 FW) |
|---|---|---|---|---|---|---|
| CK | 5.81 ± 0.13 c | 2.23 ± 0.01 d | 1.75 ± 0.04 c | 6.43 ± 1.13 d | 3.00 ± 0.11 c | 202.72 ± 0.21 a |
| HS500 | 6.16 ± 0.06 b | 2.42 ± 0.01 c | 2.30 ± 0.12 a | 7.11 ± 2.26 c | 3.43 ± 0.07 b | 194.24 ± 0.17 b |
| HS1000 | 6.53 ± 0.06 a | 3.01 ± 0.01 a | 2.29 ± 0.04 a | 7.56 ± 2.83 a | 3.73 ± 0.17 a | 183.73 ± 0.21 d |
| HS1500 | 6.22 ± 0.04 b | 2.82 ± 0.01 b | 2.17 ± 0.01 ab | 7.37 ± 1.17 b | 3.57 ± 0.07 b | 187.51 ± 0.39 c |
| HS2000 | 6.13 ± 0.02 b | 2.44 ± 0.03 c | 2.14 ± 0.03 b | 6.83 ± 1.03 c | 3.10 ± 0.11 c | 191.75 ± 0.46 b |
| Treatment | pH | Electrical Conductivity (ms/cm−1) | Total Nitrogen (g/kg−1) | Alkaline-Hydrolyzable Nitrogen (mg/kg−1) | Ammonium Nitrogen (mg/kg−1) | Organic Matter (g/kg−1) | Nitrate Nitrogen (mg/kg−1) | Quick-Acting Phosphorus (mg/kg−1) | Quick-Acting Potassium (mg/kg−1) | Moisture Content (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| CK | 6.806 d | 1.67 | 1.05 | 156.79 | 11.43 | 94.20 | 25.66 | 132.26 | 153.42 | 1.93 |
| HS500 | 6.881 d | 1.78 | 1.11 | 177.66 | 13.52 | 104.63 | 29.98 | 234.30 | 165.45 | 1.91 |
| HS1000 | 6.999 c | 1.81 | 1.22 | 207.01 | 16.78 | 135.62 | 44.11 | 245.62 | 189.19 | 2.11 |
| HS1500 | 7.003 b | 1.83 | 1.09 | 182.54 | 13.66 | 120.87 | 36.35 | 214.22 | 175.29 | 2.00 |
| HS2000 | 7.193 a | 1.87 | 1.06 | 164.68 | 9.35 | 128.53 | 32.18 | 134.11 | 168.32 | 1.93 |
| Treatment | Chao Index | ACE Index | Shannon Index | Simpson Index | Coverage (%) |
|---|---|---|---|---|---|
| CK | 4473.58 ± 62.71 b | 4549.91 ± 36.81 b | 9.84 ± 0.04 b | 1.00 ± 0.01 a | 94 a |
| HS500 | 4508.98 ± 27.89 ab | 4613.49 ± 17.91 ab | 9.97 ± 0.01 ab | 1.00 ± 0.01 a | 94 a |
| HS1000 | 4705.34 ± 57.51 a | 4786.83 ± 41.89 a | 10.11 ± 0.02 a | 0.99 ± 0.02 a | 94 a |
| HS1500 | 4479.91 ± 43.56 ab | 4603.72 ± 37.91 ab | 9.93 ± 0.03 ab | 1.00 ± 0.02 a | 94 a |
| HS2000 | 4479.58 ± 47.59 ab | 4581.04 ± 26.51 ab | 9.93 ± 0.02 ab | 1.00 ± 0.02 a | 94 a |
| Process Name | Volcanic Slag Addition (kg/667 sq.m) |
|---|---|
| HS0 (CK) | 0 |
| HS500 | 500 |
| HS1000 | 1000 |
| HS1500 | 1500 |
| HS2000 | 2000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Li, X.; Zhang, W.; Xue, D.; Sun, Q.; Xing, H.; Wang, W.; Wu, C. Impact of Volcanic Slag on Cucumber Yield, Quality, and Rhizosphere Soil Environment. Plants 2025, 14, 1328. https://doi.org/10.3390/plants14091328
Chen Q, Li X, Zhang W, Xue D, Sun Q, Xing H, Wang W, Wu C. Impact of Volcanic Slag on Cucumber Yield, Quality, and Rhizosphere Soil Environment. Plants. 2025; 14(9):1328. https://doi.org/10.3390/plants14091328
Chicago/Turabian StyleChen, Qi, Xiaohong Li, Wanwu Zhang, Dongxu Xue, Qiyuan Sun, Hangtao Xing, Wei Wang, and Chunyan Wu. 2025. "Impact of Volcanic Slag on Cucumber Yield, Quality, and Rhizosphere Soil Environment" Plants 14, no. 9: 1328. https://doi.org/10.3390/plants14091328
APA StyleChen, Q., Li, X., Zhang, W., Xue, D., Sun, Q., Xing, H., Wang, W., & Wu, C. (2025). Impact of Volcanic Slag on Cucumber Yield, Quality, and Rhizosphere Soil Environment. Plants, 14(9), 1328. https://doi.org/10.3390/plants14091328






