Unveiling the Cold Acclimation of Alfalfa: Insights into Its Starch-Soluble Sugar Dynamic Transformation
Abstract
1. Introduction
2. Results
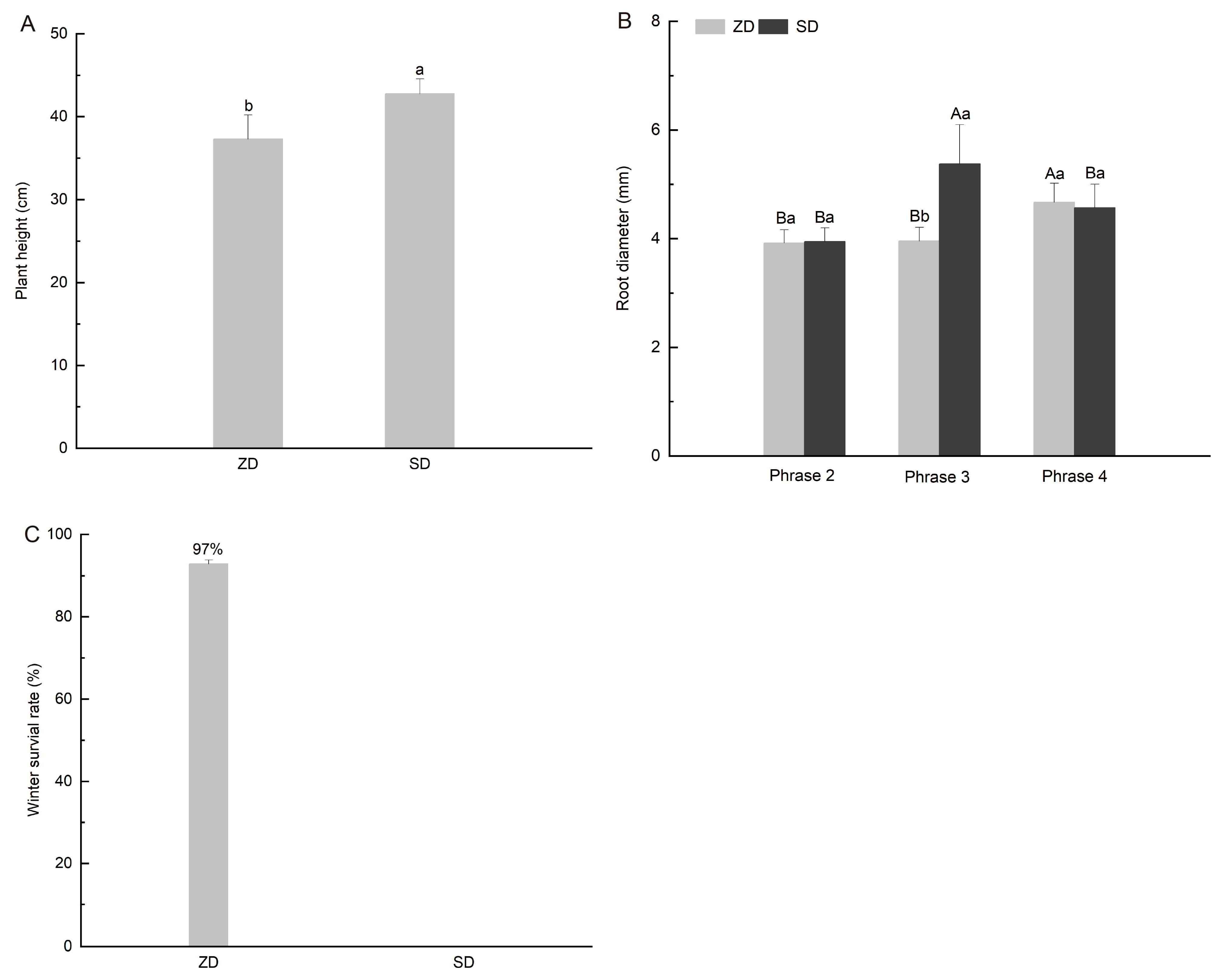
2.1. Comparison of Plant Growth Differences Between Two Alfalfa Cultivars
2.2. Comparison of Dry Matter and Starch Accumulation Between Two Alfalfa Cultivars
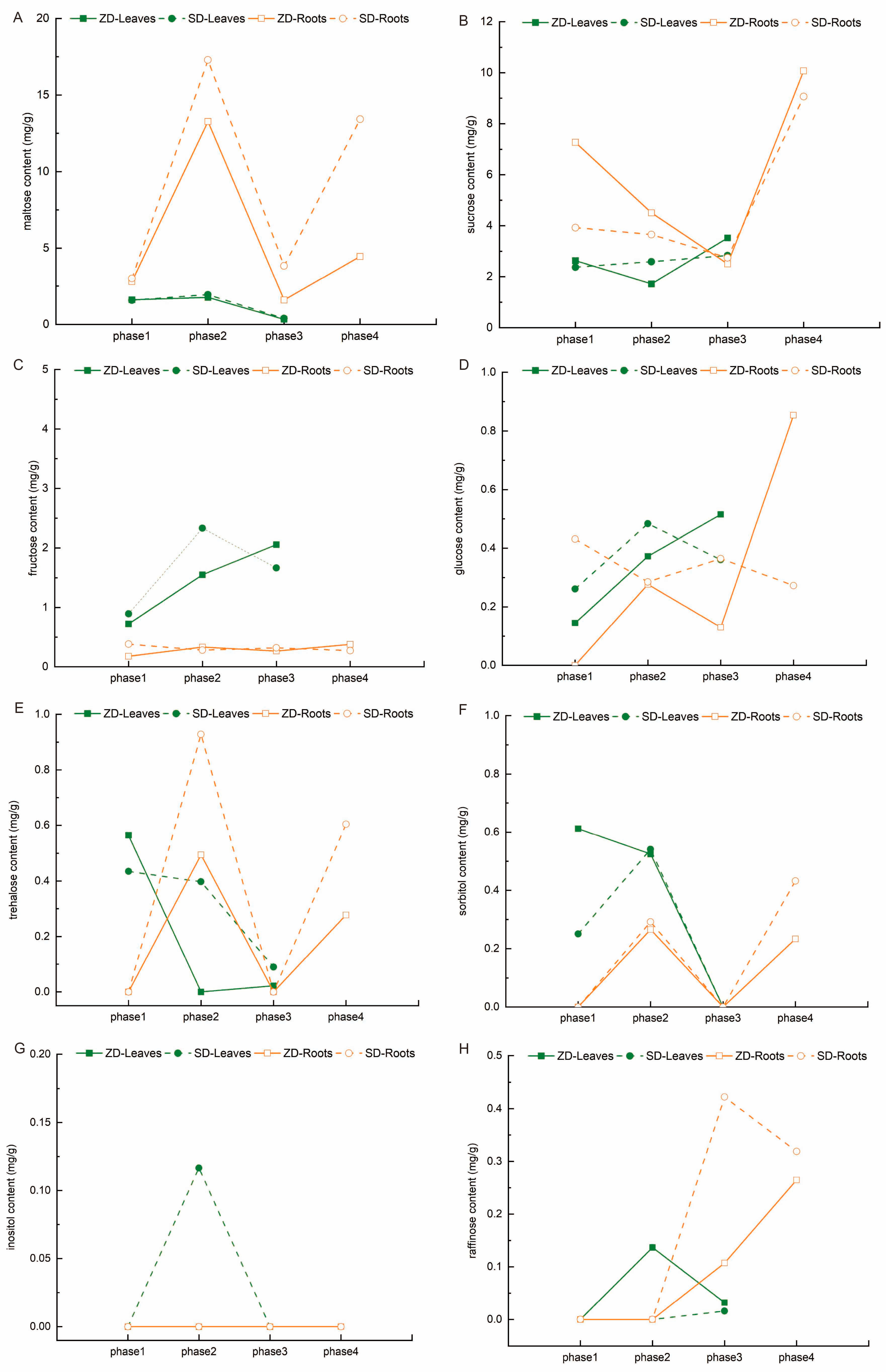
2.3. Comparison of Soluble Sugar Changes Between Two Alfalfa Cultivars
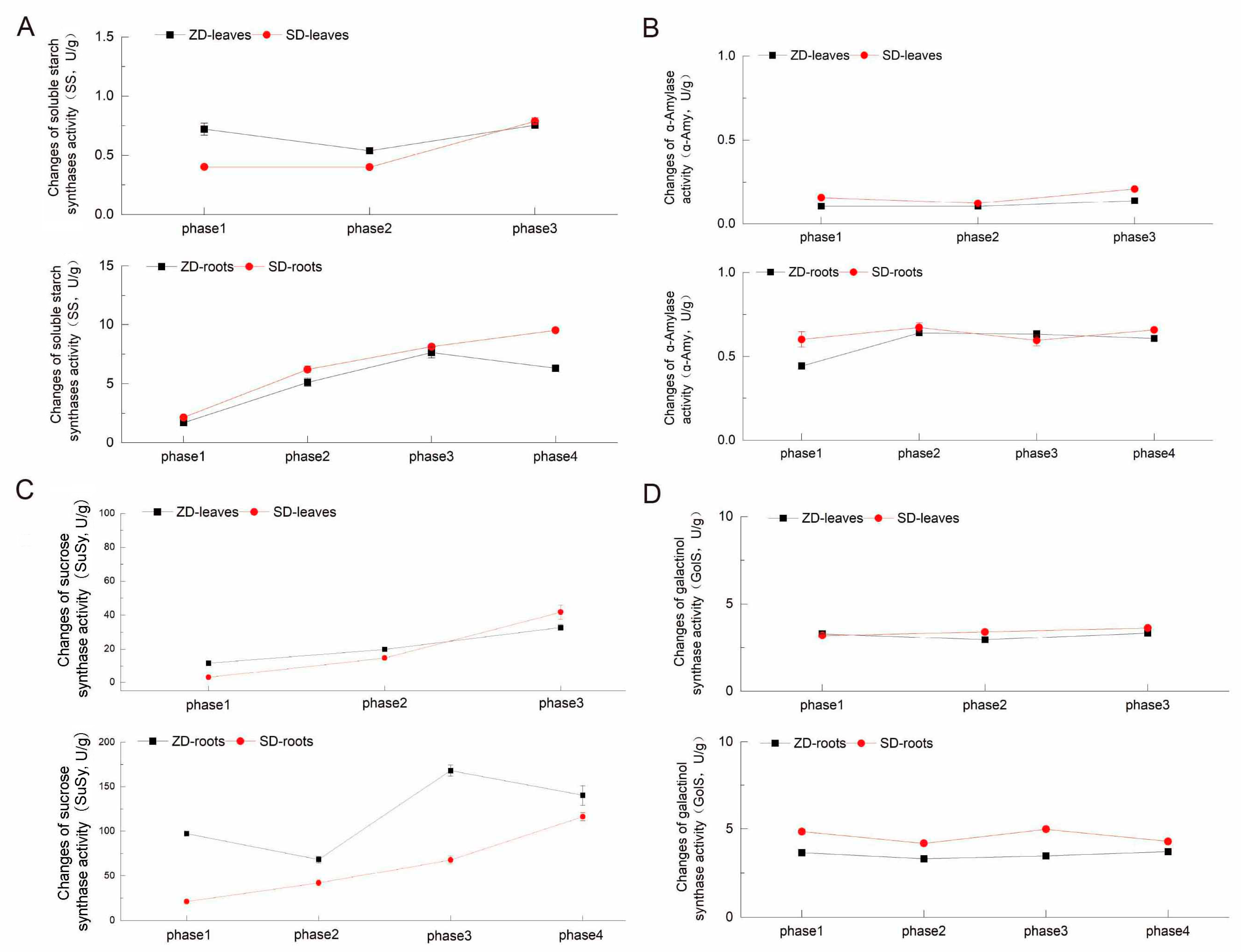
2.4. Comparison of Four Enzyme Activity Changes Between Two Alfalfa Cultivars
2.5. Comparison of Transcriptional Changes Between Two Alfalfa Cultivars
2.6. Differentially Expressed Gene (DEG) Enrichment Analysis of Two Alfalfa Cultivars
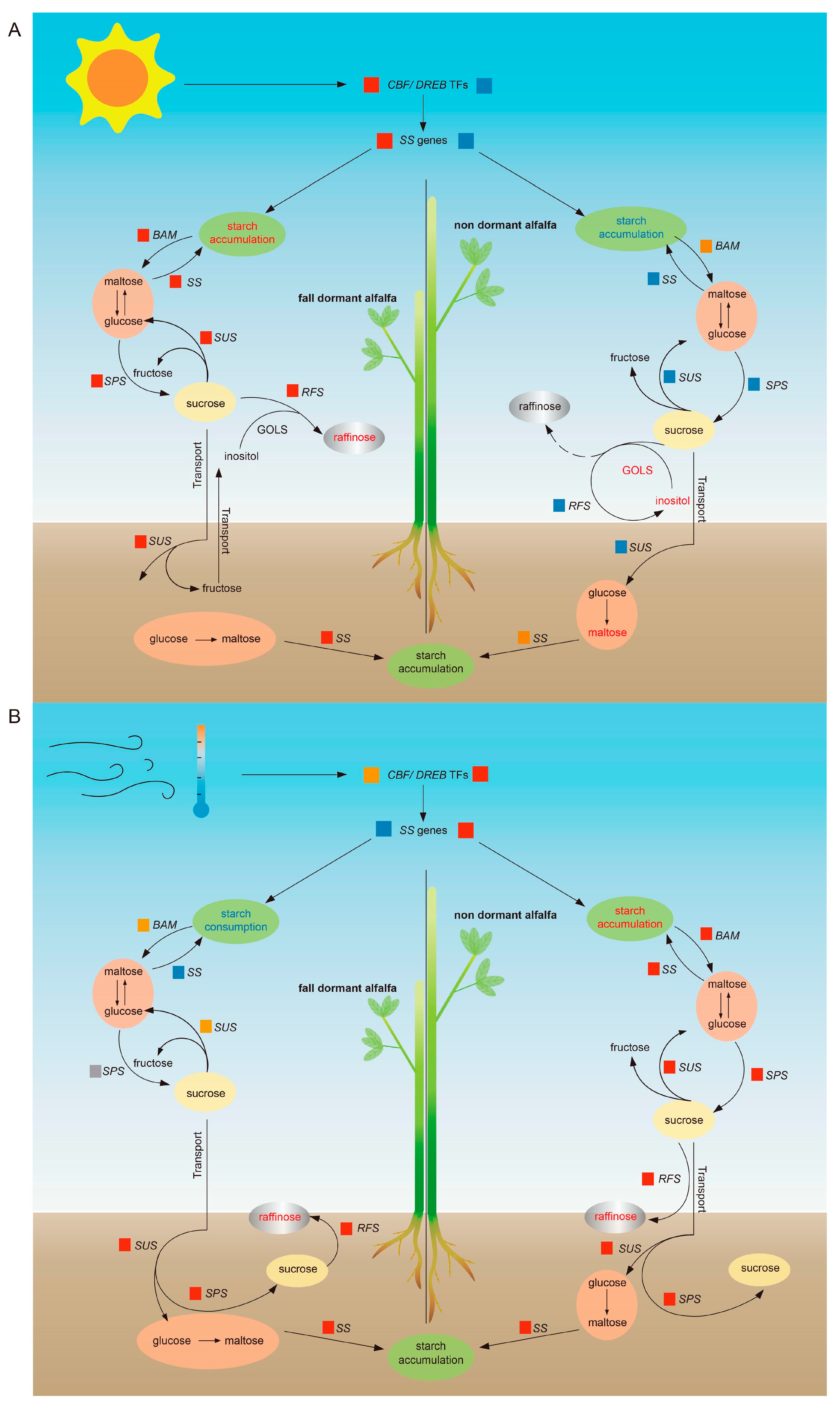
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. Physiological Index Determination
4.3. Enzymatic Activity Assay
4.4. Transcriptome Sequencing
4.5. Analysis of Gene Expression by Reverse-Transcription Quantitative PCR (qRT-PCR)
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ernest, S. Alfalfa and Relatives: Evolution and Classification of Medicago: The Other Species (Indicates a Species of Uncertain Status); NRC Research Press: Ottawa, ON, Canada, 2011. [Google Scholar]
- Russelle, M.P. Alfalfa. Am. Sci. 2001, 89, 252–261. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Martin, N.P.; Lamb, J.F.S.; Cuomo, G.R.; Jewett, J.G.; Quering, S.R. Leaf and stem properties of alfalfa entries. Agron. J. 2000, 92, 733–739. [Google Scholar] [CrossRef]
- Wang, C.Z.; Ma, B.L.; Han, J.F.; Wang, Y.H.; Gao, Y.G.; Hu, X.F.; Zhang, C.M. Photoperiod effect on phytochrome and abscisic acid in alfalfa varieties differing in fall dormancy. J. Plant Nutr. 2008, 31, 1257–1269. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Tong, Z.; He, F.; Li, X. Metabolomic analyses reveal substances that contribute to the increased freezing tolerance of alfalfa (Medicago sativa L.) after continuous water deficit. BMC Plant Biol. 2020, 20, 15. [Google Scholar] [CrossRef]
- Uemura, M.; Steponkus, P.L. Modification of the intracellular sugar content alters the incidence of freeze-induced membrane lesions of protoplasts isolated from Arabidopsis thaliana leaves. Plant Cell Environ. 2003, 26, 1083–1096. [Google Scholar] [CrossRef]
- Uemura, M.; Tominaga, Y.; Nakagawara, C.; Shigematsu, S.; Minami, A.; Kawamura, Y. Responses of the plasma membrane to low temperatures. Physiol. Plant. 2006, 126, 81–89. [Google Scholar] [CrossRef]
- Armstrong, J.J.; Takebayashi, N.; Wolf, D.E. Cold tolerance in the genus Arabidopsis. Am. J. Bot. 2020, 107, 489–497. [Google Scholar] [CrossRef]
- Xin, Z.; Browse, J. Cold comfort farm: The acclimation of plants to freezing temperatures. Plant Cell Environ. 2000, 23, 893–902. [Google Scholar] [CrossRef]
- Hincha, D.K.; Zuther, E. Introduction: Plant cold acclimation and winter survival. Methods Mol. Biol. 2020, 2156, 1–7. [Google Scholar] [CrossRef]
- Fürtauer, L.; Weiszmann, J.; Weckwerth, W.; Nägele, T. Dynamics of plant metabolism during cold acclimation. Int. J. Mol. Sci. 2019, 20, 5411. [Google Scholar] [CrossRef]
- Theocharis, A.; Clément, C.; Barka, E. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Seydel, C.; Kitashova, A.; Fürtauer, L.; Nägele, T. Temperature-induced dynamics of plant carbohydrate metabolism. Physiol. Plant 2022, 174, e13602. [Google Scholar] [CrossRef]
- Cunningham, S.M.; Nadeau, P.; Castonguay, Y.; Laberge, S.; Volenec, J.J. Raffinose and stachyose accumulation, galactinol synthase expression, and winter injury of contrasting alfalfa germplasms. Crop Sci. 2003, 43, 562–570. [Google Scholar] [CrossRef]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef]
- Scarth, G.W.; Levitt, J. The frost-hardening mechanism of plant cells. Plant Physiol. 1937, 12, 51–78. [Google Scholar] [CrossRef]
- Song, L.; Jiang, L.; Chen, Y.; Shu, Y.; Bai, Y.; Guo, C. Deep-sequencing transcriptome analysis of field-grown Medicago sativa L. crown buds acclimated to freezing stress. Funct. Integr. Genom. 2016, 16, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Tong, Z.; He, F.; Li, X. Response of alfalfa (Medicago sativa L.) to abrupt chilling as reflected by changes in freezing tolerance and soluble sugars. Agronomy 2020, 10, 255. [Google Scholar] [CrossRef]
- Dong, R.; Luo, B.; Tang, L.; Wang, Q.X.; Lu, Z.J.; Chen, C.; Yang, F.; Wang, S.; He, J. A comparative transcriptomic analysis reveals a coordinated mechanism activated in response to cold acclimation in common vetch (Vicia sativa L.). BMC Genom. 2022, 23, 814. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.Y.; Cao, Y.; Arora, R.; Zhou, H.; Xia, Y.P. Factors affecting freezing tolerance: A comparative transcriptomics study between field and artificial cold acclimations in overwintering evergreens. Plant J. 2020, 103, 2279–2300. [Google Scholar] [CrossRef]
- Yano, R.; Nakamura, M.; Yoneyama, T.; Nishida, I. Starch-related alpha-glucan/water dikinase is involved in the cold-induced development of freezing tolerance in Arabidopsis. Plant Physiol. 2005, 138, 837–846. [Google Scholar] [CrossRef]
- Bilska-Kos, A.; Mytych, J.; Suski, S.; Magoń, J.; Ochodzki, P.; Zebrowski, J. Sucrose phosphate synthase (SPS), sucrose synthase (SUS) and their products in the leaves of Miscanthus × giganteus and Zea mays at low temperature. Planta 2020, 252, 23. [Google Scholar] [CrossRef]
- Nägele, T.; Kandel, B.A.; Frana, S.; Meissner, M.; Heyer, A.G. A systems biology approach for the analysis of carbohydrate dynamics during acclimation to low temperature in Arabidopsis thaliana. FEBS J. 2011, 278, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Nägele, T.; Heyer, A.G. Approximating subcellular organisation of carbohydrate metabolism during cold acclimation in different natural accessions of Arabidopsis thaliana. New Phytol. 2013, 198, 777–787. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Zhang, C.; Zhang, W.; Deng, N.; Dirk, L.M.A.; Downie, A.B.; Zhao, T. Raffinose positively regulates maize drought tolerance by reducing leaf transpiration. Plant J. 2023, 114, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, Y.; Liu, Y.; Li, X.; Hao, G.; Han, Q.; Dirk, L.M.A.; Downie, A.B.; Ruan, Y.L.; Wang, J.; et al. Raffinose synthase enhances drought tolerance through raffinose synthesis or galactinol hydrolysis in maize and Arabidopsis plants. J. Biol. Chem. 2020, 295, 8064–8077. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Baoyin, T.; Li, X.L.; Wang, Z.L. How fall dormancy benefits alfalfa winter-survival? Physiologic and transcriptomic analyses of dormancy process. BMC Plant Biol. 2019, 19, 205. [Google Scholar] [CrossRef]
- Kitashova, A.; Adler, S.O.; Richter, A.S.; Eberlein, S.; Dziubek, D.; Klipp, E.; Nägele, T. Limitation of sucrose biosynthesis shapes carbon partitioning during plant cold acclimation. Plant Cell Environ. 2023, 46, 464–478. [Google Scholar] [CrossRef]
- Zhao, J.M.; Alamusi, L.Y.; Sun, J.J.; Chang, C.; Liu, H.L.; Li, W.; Wei, Y. Study on clod resistance and growth of Medicago sativa under simulated cold hardening. Chin. J. Grassl. 2020, 42, 30–36. [Google Scholar] [CrossRef]
- Smith, A.; Zeeman, S. Quantification of starch in plant tissues. Nat. Protoc. 2006, 1, 1342–1345. [Google Scholar] [CrossRef]
- Lin, Q.; Yang, J.; Wang, Q.; Zhu, H.; Chen, Z.; Dao, Y.; Wang, K. Overexpression of the trehalose-6-phosphate phosphatase family gene AtTPPF improves the drought tolerance of Arabidopsis thaliana. BMC Plant Biol. 2019, 19, 381. [Google Scholar] [CrossRef]



| Treatment | Upregulated | Downregulated | Total DEGs |
|---|---|---|---|
| ZD Leaf phase 1 vs. SD Leaf phase 1 | 1018 | 1628 | 2646 |
| ZD Root phase 1 vs. SD Root phase 1 | 4980 | 6822 | 11,802 |
| ZD Leaf phase 2 vs. SD Leaf phase 2 | 4199 | 3171 | 7370 |
| ZD Root phase 2 vs. SD Root phase 2 | 4260 | 3408 | 7668 |
| ZD Leaf phase 3 vs. SD Leaf phase 3 | 3629 | 1410 | 5039 |
| ZD Root phase 3 vs. SD Root phase 3 | 5033 | 3285 | 8318 |
| ZD Root phase 4 vs. SD Root phase 4 | 8731 | 7198 | 15,929 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Li, Z.; Zhang, X.; Yin, G.; Liu, S.; Zhao, J.; Yun, Y.; Guo, M.; Zhang, J. Unveiling the Cold Acclimation of Alfalfa: Insights into Its Starch-Soluble Sugar Dynamic Transformation. Plants 2025, 14, 1313. https://doi.org/10.3390/plants14091313
Zhu L, Li Z, Zhang X, Yin G, Liu S, Zhao J, Yun Y, Guo M, Zhang J. Unveiling the Cold Acclimation of Alfalfa: Insights into Its Starch-Soluble Sugar Dynamic Transformation. Plants. 2025; 14(9):1313. https://doi.org/10.3390/plants14091313
Chicago/Turabian StyleZhu, Lin, Zhiyong Li, Xiaoqing Zhang, Guomei Yin, Siqi Liu, Jinmei Zhao, Ying Yun, Maowei Guo, and Jiaqi Zhang. 2025. "Unveiling the Cold Acclimation of Alfalfa: Insights into Its Starch-Soluble Sugar Dynamic Transformation" Plants 14, no. 9: 1313. https://doi.org/10.3390/plants14091313
APA StyleZhu, L., Li, Z., Zhang, X., Yin, G., Liu, S., Zhao, J., Yun, Y., Guo, M., & Zhang, J. (2025). Unveiling the Cold Acclimation of Alfalfa: Insights into Its Starch-Soluble Sugar Dynamic Transformation. Plants, 14(9), 1313. https://doi.org/10.3390/plants14091313





