Abstract
The degradation of Pinus sylvestris var. mongholica plantations in Youyu County on the Loess Plateau has caused major ecological issues, though the mechanisms remain poorly understood. This study explores the effects of stand age and soil properties on the rhizosphere fungal community and their potential roles in plantation degradation. Soil samples were collected from plantations of different stand ages (13, 20, 25, and 35 years), and their fungal diversity and composition were analyzed using high-throughput sequencing. The results showed that soil organic carbon and total nitrogen declined with stand age due to high nutrient demand and limited litter input. The available phosphorus and available potassium (AK) contents were identified as key limiting factors, influencing ectomycorrhizal fungi abundance and the overall soil fungal diversity. With an increasing stand age, the fungal diversity decreased, the ectomycorrhizal fungi declined, and the pathogenic fungi increased, exacerbating plantation degradation. Regression analysis further indicated a significant negative correlation between AK content and stand age, suggesting potassium deficiency as a critical driver of tree health decline. This study highlights the pivotal role of soil nutrient availability in shaping rhizosphere fungal communities and sustaining P. sylvestris plantations, offering insights into degradation mechanisms and strategies to enhance forest resilience on the Loess Plateau.
1. Introduction
The Loess Plateau, spanning approximately 635,000 km2 in the upper and middle reaches of the Yellow River, represents the most severely eroded region in both China and the world [1,2]. This pervasive soil erosion has led to profound ecological degradation and has undermined sustainable socioeconomic development [3]. In particular, Youyu County, Shanxi Province, China, located in the northern Loess Plateau, exemplifies an ecologically fragile area that has experienced extensive soil erosion, and has been the focus of large-scale Grain for Green initiatives since the 1970s [4,5].
Pinus sylvestris var. mongholica, an evergreen conifer renowned for its robust resistance to environmental stress, has been extensively utilized in vegetation restoration and soil erosion mitigation efforts [6]. Consequently, it has been widely planted in Youyu County, playing a pivotal role in local soil conservation [4]. However, in recent years, these plantations have undergone marked degradation, characterized by stunted growth, a diminished resistance to cold, drought, and pest pressures, and a consequent rise in tree mortality. Such deterioration has substantially reduced the plantations’ capacity to control soil erosion. Although previous studies have primarily attributed the degradation of P. sylvestris to climatic variability, suboptimal management practices, imbalanced soil water utilization, pest infestations, and high stand density [7,8], these investigations have largely been confined by geographic and spatial limitations and have neglected the potential influence of soil fungi on the degradation process. Thus, the underlying mechanisms driving the degradation of P. sylvestris plantations remain incompletely understood.
Soil fungi, a diverse assemblage of microorganisms ubiquitous in terrestrial ecosystems, play a critical role in bridging aboveground and belowground processes [9,10,11]. These fungi exert significant influences on forest health; for instance, saprotrophic fungi accelerate litter decomposition and nutrient cycling in plantation ecosystems [12], while mycorrhizal fungi, through symbiotic associations with tree roots, enhance nutrient uptake and improve growth efficiency [13,14]. Given these functional roles, soil fungi are increasingly recognized as key factors in addressing the degradation of P. sylvestris plantations, underscoring the need for a comprehensive understanding of their diversity and community composition.
In monoculture plantations, stand age is a critical determinant influencing the structure and dynamics of the soil fungal communities [15]. Concurrently, the soil physicochemical properties—such as moisture content, pH, and soil organic carbon (SOC)—profoundly affect the diversity and successional trajectories of these communities [16,17]. Therefore, investigating the interplay between the stand age and soil properties in P. sylvestris plantations is essential for elucidating the dynamics of the soil fungal communities within the rhizosphere.
In this study, we collected rhizosphere soil samples from P. sylvestris plantations of varying stand ages—including degraded forests—in Youyu County. We subsequently analyzed both the soil fungal communities and the associated soil properties across these age classes. The objectives of the study were as follows: (1) elucidate the potential variations in the relationships between the stand age and soil physicochemical properties, (2) investigate the influence of these factors on key fungal communities (e.g., saprotrophic fungi and ectomycorrhizal fungi), and (3) delineate the microecological factors associated with plantation forest degradation in the region.
2. Results
2.1. Soil Properties
The results of a one-way analysis of variance (ANOVA) showed that the rhizosphere soil properties varied significantly with the stand age (Table 1). Generally, with an increasing stand age, the contents of the soil nutrients exhibited a decreasing trend, except for the pH. Notably, the ammonium nitrogen (AN) and nitrate nitrogen (NN) levels in 25-year-old stands (V25) were significantly higher than those in 20-year-old stands (V20). Conversely, the NN, AN, available phosphorus (AP), and available potassium (AK) contents in 35-year-old stands (V35) were significantly lower than those in V25. Notably, the regression analysis further indicated that AK content decreased significantly with increasing stand age (p < 0.05, R2 = 0.589).

Table 1.
The rhizosphere soil properties of P. sylvestris at different stand ages.
2.2. Composition of Soil Fungal Community
The rarefaction curves of the soil fungi across different stand ages are presented in Figure 1a. The Sobs index at the OTU level remained relatively stable with an increasing number of sequencing reads, indicating that the sequencing depth was sufficient and the dataset was reliable. Following Illumina MiSeq sequencing, a total of 495,708 fungal sequences were obtained, identifying 373 operational taxonomic units (OTUs). Among them, 144 OTUs were classified at the genus level, encompassing 7 phyla, 20 classes, 45 orders, and 92 families. The total number of soil fungal genera exhibited a decreasing trend with increasing stand age (Figure 1b).
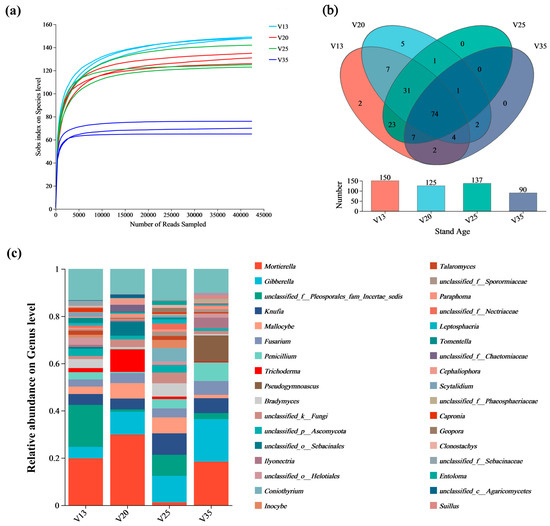
Figure 1.
Sequencing results of rhizosphere soil fungi of P. sylvestris at different stand ages. (a) Rarefaction curves of soil fungi at different stand ages; (b) Venn diagram of rhizosphere soil fungal composition of P. sylvestris at genus level; (c) Composition of rhizosphere soil fungi of P. sylvestris at genus level and different stand ages. V13: 13-year-old forest, V20: 20-year-old forest, V25: 25-year-old forest, and V35: 35-year-old forest.
In terms of the fungal community composition, the genera Mortierella, Gibberella, Knufia, and Mallocybe were relatively abundant (Figure 1c). Specifically, Mortierella exhibited a relative abundance of 29.90% in V20, but sharply declined to 1.25% in V25. Conversely, Gibberella increased with the stand age, comprising 4.82% in V13, 9.60% in V20, 11.15% in V25, and reaching 17.99% in V35. The relative abundance of Knufia remained relatively stable, but increased to 9.08% in V25. Meanwhile, Trichoderma accounted for 9.34% in V20, but significantly decreased to 0.33% in V35. These results suggest distinct shifts in the fungal community composition with stand development, highlighting potential ecological changes in the rhizosphere fungal interactions.
2.3. Functional Groups of Soil Fungal Community
The FUNGuild analysis of the soil fungal communities across different stand ages revealed significant variations in functional group composition (Figure 2). Notably, the relative abundance of pathogenic fungi increased markedly with the stand age, peaking at 18.6% in the 35-year-old stands (V35). In contrast, the proportion of ectomycorrhizal fungi declined with the stand age, reaching a minimum of 3.6% in V35. The category labeled ‘other fungi’ exhibited its highest relative abundance in the 20-year-old stands (V20), accounting for 52.3%, while the proportion of saprotrophic fungi was low (14.2%) in the stands of this age.
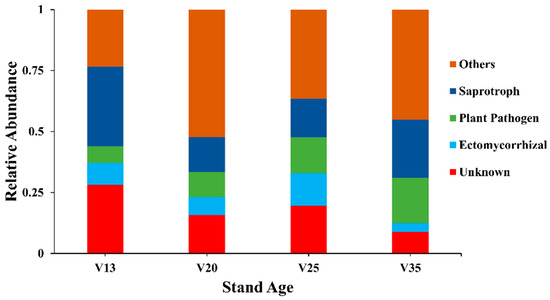
Figure 2.
Functional groups of rhizosphere soil fungal communities of P. sylvestris at different stand ages. V13: 13-year-old forest, V20: 20-year-old forest, V25: 25-year-old forest, and V35: 35-year-old forest.
2.4. Diversity of Soil Fungal Community
The Ace index (Figure 3a), Chao index (Figure 3b), and Sobs index (Figure 3c) indicated that the soil fungal community diversity was significantly influenced by the stand age. These diversity indices exhibited a decreasing trend with increasing stand age, with the values in the 35-year-old stands (V35) being significantly lower than those in the younger stands. Additionally, no significant differences were observed in the Sobs and Ace indices between the 20-year-old (V20) and 25-year-old (V25) stands, suggesting that the fungal diversity remained relatively stable within this age range.
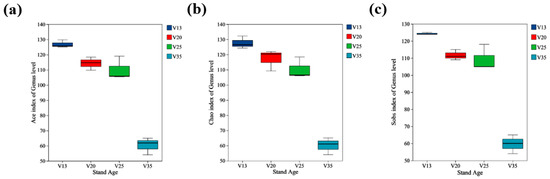
Figure 3.
Rhizosphere soil fungal diversity indices of P. sylvestris at different stand ages. (a) Ace index at genus level; (b) Chao index at genus level; (c) Sobs index at genus level. V13: 13-year-old forest, V20: 20-year-old forest, V25: 25-year-old forest, and V35: 35-year-old forest.
2.5. Relationship Between Soil Fungal Community Composition and Soil Physicochemical Properties
The results of a distance-based redundancy analysis (db-RDA) indicated that the soil physicochemical properties were significantly correlated with the soil fungal community structures across different stand ages (Figure 4). Specifically, the fungal community structure in V20 was positively correlated with the contents of AP, AN, NN, SOC, and AK. Meanwhile, the fungal community structure in V35 showed a positive correlation with AK, SOC, and Moi. Other soil properties did not exhibit significant effects on the fungal community structure.
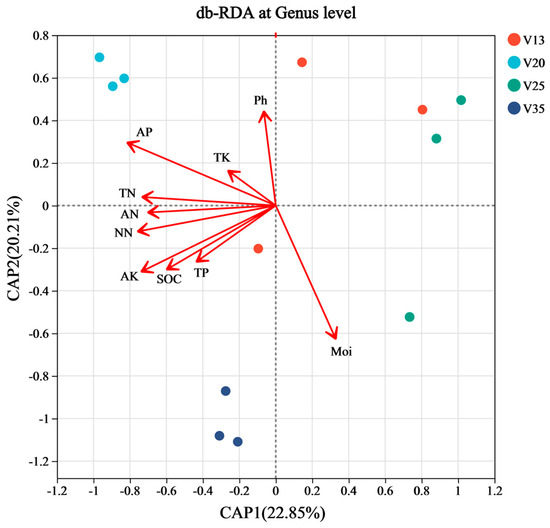
Figure 4.
Distance-based redundancy analysis (db-RDA) of rhizosphere soil fungal community structure of P. sylvestris at different stand ages. TN: total nitrogen, NN: nitrate nitrogen, AN: ammonium nitrogen, TP: total phosphorus, AP: available phosphorus, TK: total potassium, AK: available potassium, SOC: soil organic carbon, Moi: soil moisture content, V13: 13-year-old forest, V20: 20-year-old forest, V25: 25-year-old forest, and V35: 35-year-old forest.
The correlation analysis between the relative abundance of fungal genera from different functional groups and the soil properties (Figure 5) revealed distinct associations. Ectomycorrhizal fungi, including Tomentella, Inocybe, and Mallocybe, were positively correlated with AK, TK, and AP. In contrast, the pathogenic fungus Gibberella was negatively correlated with Moi, TP, NN, AN, TK, AP, SOC, TN, and AK, showing a particularly strong negative correlation with TK. Leptosphaeria exhibited a negative correlation with Moi, but a positive correlation with TP, NN, AN, TK, AP, SOC, TN, and AK. Regarding saprophytic fungi, Talaromyces and Scytalidium displayed a positive correlation with TP, NN, AN, AP, SOC, TN, and AK, while being negatively correlated with Moi. On the other hand, Trichoderma, Ilyonectria, and Penicillium were negatively correlated with TP, NN, and AN.
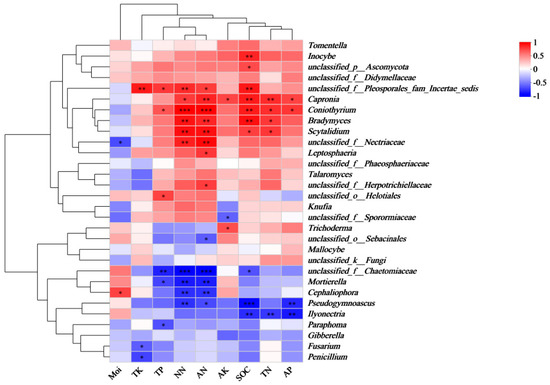
Figure 5.
Correlation heatmap of rhizosphere soil fungal genera and soil properties of P. sylvestris. TN: total nitrogen, NN: nitrate nitrogen, AN: ammonium nitrogen, TP: total phosphorus, AP: available phosphorus, TK: total potassium, AK: available potassium, SOC: soil organic carbon, Moi: soil moisture content, *: significant at p < 0.05, **: significant at p < 0.01, and ***: significant at p < 0.001.
Furthermore, the regression analysis demonstrated a significant linear relationship between the Sobs index and AK content (Figure 6c), suggesting that available potassium plays a crucial role in shaping soil fungal diversity. However, the TN, NN, AN, TP, AP, TK, Moi, pH, and SOC contents did not exhibit significant correlations with the Sobs index.
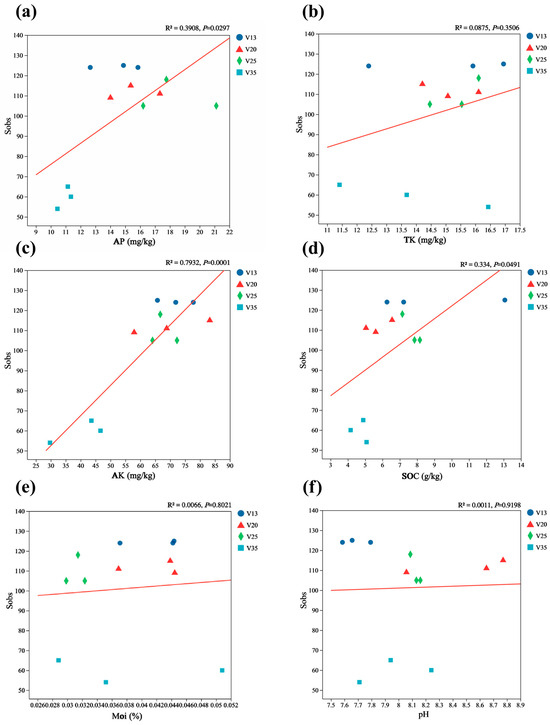
Figure 6.
The correlation of the rhizosphere soil fungal Sobs index with the soil properties of P. sylvestris at different stand ages. (a) AP: available phosphorus; (b) TK: total potassium; (c) AK: available potassium; (d) SOC: soil organic carbon; (e) Moi: soil moisture content; (f) pH. The level of fit and the significance of the relationship is shown in the top right corner of each panel. V13: 13-year-old forest, V20: 20-year-old forest, V25: 25-year-old forest, and V35: 35-year-old forest.
3. Discussion
The survival and growth of P. sylvestris depend on soil moisture and nutrient availability. Previous studies have shown that as stand age increases, P. sylvestris promotes local carbon (C) and nitrogen (N) cycling, thereby enhancing soil C and N storage [18]. However, in this study, the SOC and TN contents in the rhizosphere soil of P. sylvestris significantly decreased with the stand age. This phenomenon may stem from the early growth stage of plantations, where P. sylvestris has high carbon and nitrogen demands, but a limited capacity to fix them [19,20]. Additionally, litter accumulation, which contributes to soil C and N storage, has not reached sufficient levels [21,22]. Over time, the soil nutrients gradually decrease. Notably, a recovery trend in the soil nutrients was observed in mature plantations. For example, the SOC, TN, AN, and NN contents were significantly higher in V25 than in V20. This may be attributed to the increased stand density in V25, which promotes litter accumulation and enhances soil C and N storage. Furthermore, the reduced nutrient demands of mature plantations may also contribute to soil nutrient recovery [23].
The Sobs, Ace, and Chao diversity indices of the soil fungal community decreased with increasing stand age. This aligns with previous findings, indicating that an increasing stand age significantly reduces the diversity of rhizosphere soil fungal communities [24]. A long-term monoculture plantation pattern can exacerbate imbalances in the rhizosphere micro-ecosystem, significantly reducing the diversity and metabolic activity of the soil microbial communities [25]. During sampling, we observed a gradual decrease in the biomass and species richness of understory herbs with increasing stand age, which is consistent with previous studies. Understory herbs play a crucial role in maintaining soil microbial communities by producing litter that provides physical protection for the microbes and affects the soil properties, which serve as key resources for microbial growth [26,27,28]. Therefore, the reduction in understory herbs may be a key factor contributing to the decline in soil fungal diversity. As stand age increases, the degradation of the pine plantations results in decreased stand density, and trees serve as the primary source of soil litter [29,30]. Since most soil microbes are saprophytic and rely on litter for growth, a reduction in stand density leads to a decrease in litter quantity, negatively impacting the soil fungal diversity. Although the canopy thinning in older stands might theoretically improve light conditions and promote the growth of understory herbs, our field observations revealed a consistent decline in the understory biomass and species richness with increasing stand age. This suggests that light availability alone may not be sufficient to stimulate understory regeneration, particularly under conditions of declining soil fertility and increasing tree root competition. Consequently, the reduction in tree litter input was not compensated for by herbaceous vegetation, leading to an overall decline in litter contribution to the soil and, in turn, indirectly reducing the soil fungal community diversity.
Soil properties are closely related to the distribution and diversity of soil fungal communities [31]. In this study, we found a significant correlation between the soil properties and the composition and diversity of the soil fungal communities. Mortierella and Gibberella were the two most abundant genera in the soil fungal community. Previous studies have indicated that Mortierella is a core keystone taxon at different growth stages of P. sylvestris [32], while Gibberella is a common pathogenic genus [33,34]. In our study, the relative abundances of Mortierella and Gibberella were negatively correlated with SOC, TN, AN, NN, AP, TP, and TK contents. As stand age increases, these soil properties decrease, maintaining the dominance of these fungal genera in the community. The increasing relative abundance of Gibberella with stand age further supports this conclusion.
Moreover, in V25, the relative abundance of Mortierella dramatically decreased, losing its dominance, while Knufia, which was positively correlated with SOC, TN, AN, NN, and AP contents, significantly increased. This shift may be attributed to the significant increase in the SOC, TN, AN, NN, and AP contents in V25 compared to V20 (even exceeding those in V13). Additionally, the number of fungal genera was higher in V25 than in V20, which we speculate is due to the substantial increase in soil nutrients. Soil physicochemical properties and nutrient availability are crucial limiting factors for fungal growth, and profoundly influence the composition of soil fungi [35,36]. Therefore, the recovery of the soil physicochemical properties in V25 provided favorable conditions for the restoration of the soil fungi [18].
Meanwhile, microbial communities are key shapers of the rhizosphere microenvironment [37]. The functional groups of soil fungal communities, particularly the ectomycorrhizal fungi and plant pathogenic fungi, significantly impact tree growth [38]. Previous studies have shown that in healthy P. sylvestris plantations, the diversity of the ectomycorrhizal fungi in the rhizosphere soil increases with stand age, promoting nutrient absorption and providing physical protection to the roots [39,40]. However, in our study, the relative abundance of ectomycorrhizal fungi in the soil fungal community decreased with stand age, weakening the resistance of the pine trees to stresses such as cold and drought [41] and increasing the risk of pests like Bursaphelenchus xylophilus [42]. Meanwhile, the relative abundance of pathogenic fungi increased significantly with stand age, raising the risk of disease infection. Pathogenic fungi such as Sphaeropsis sapinea can cause stress-induced diseases such as shoot blight and canker disease, damaging the wood and phloem of infected trees and producing toxic metabolites that result in tree death [43,44,45]. Therefore, the decrease in ectomycorrhizal fungi and the increase in pathogenic fungi with stand age may collectively result in habitat deterioration, accelerating plantation degradation.
Notably, ectomycorrhizal fungi such as Tomentella, Mallocybe, and Inocybe were positively correlated with AP content, while pathogenic fungi such as Gibberella were negatively correlated with AP content. Therefore, supplementing the local soil with phosphate fertilizer may relieve P stress in P. sylvestris and improve the functional composition of the soil fungal community, aiding in plantation disease management. Additionally, the AP content in the degraded plantations (V35) was significantly lower than in the normal plantations (V13, V20, V25). Cao et al. (2024) found that AP content is significantly negatively correlated with plant disease incidence, and under adequate P levels, plant disease-suppressive genes increase significantly during pathogen infection. Therefore, increasing the AP content in the soil may help relieve survival stress in P. sylvestris.
Furthermore, we speculate that the increasing stand age, soil potassium (K) stress, and changes in the soil fungal functional groups led to a significant deterioration of the rhizosphere microenvironment, profoundly impacting P. sylvestris degradation. The linear regression analysis indicated a significant negative correlation between AK content and stand age. K is an essential nutrient for tree growth, playing a key role in regulating physiological processes, particularly in alleviating drought stress [46,47]. K stress weakens the metabolic activity and growth ability of pine trees. For instance, K deficiency reduces lateral root elongation in pines, impairing nutrient and water uptake [48], and causes needle yellowing [49]. During sampling, we observed increasing needle yellowing with stand age, and in some plots, entire needles turned yellow. Additionally, older pine trees had a significantly reduced lateral root biomass. These observations are consistent with K stress, suggesting that K deficiency may be a critical driver of P. sylvestris degradation in this region.
Although our findings emphasized the key roles of AP and AK, we acknowledge that the nitrogen indices (TN, AN, NN) also exhibited pronounced fluctuations and ecological significance. Specifically, the declines in TN, AN, and NN observed in the older plantations (V35) suggest a broader pattern of diminished microbial mineralization capacity and nutrient turnover. As nitrogen is critical for microbial biomass and function, the decreased nitrogen availability likely contributes indirectly to the shifts in fungal community composition and plantation degradation. This phenomenon warrants further investigation in future studies.
It is worth noting that, although this study focused solely on the rhizosphere soil and excluded the surface litter layer during sampling, the quantity and composition of the litter likely influence long-term nutrient cycling and fungal community development. Future studies may benefit from integrated sampling of both litter and soil to better capture the aboveground–belowground interactions.
4. Conclusions
This study reveals that the P. sylvestris rhizosphere soil nutrients, particularly the SOC and TN, declined with stand age due to high nutrient demands and limited litter accumulation. However, the mature plantations exhibited nutrient recovery, likely due to an increased stand density. The soil fungal diversity decreased with stand age, driven by the understory herb loss and reduced litter input. The ectomycorrhizal fungi declined, weakening tree resistance, while pathogenic fungi like Gibberella increased, raising disease risks. Mortierella declined in the mature stands, while Knufia, associated with higher nutrients, became dominant. Available phosphorus (AP) and potassium (AK) were critical for soil health. The AP supported the ectomycorrhizal fungi and suppressed pathogens, while AK deficiency contributed to P. sylvestris degradation. Given these findings, we preliminarily recommend phosphorus and potassium fertilization, appropriate stand density management, and the promotion of understory vegetation as potential strategies to mitigate the degradation of P. sylvestris plantations. However, their long-term effectiveness and ecological impact should be further validated through multi-site and longitudinal studies.
5. Materials and Methods
5.1. Study Site Description
The study was conducted in Youyu County, Shuozhou City, Shanxi Province, China (coordinates: 40°25′ N, 112°32′ E). The sampling site is situated at the northwestern edge of Shanxi Province, on the periphery of the Loess Plateau. The region exhibits an average annual precipitation of 319.5 mm and an average annual temperature of 13 °C. Characterized as a gentle-slope hilly sandstorm area, Youyu County has an average elevation of approximately 1400 m. The local climate is marked by strong winds, abundant sand, low temperatures, limited rainfall, and severe wind and water erosion, all of which contribute to a highly degraded ecological environment. With a total area of 1969 km2, approximately 1498.85 km2 of Youyu County is affected by soil erosion, underscoring its critical role in regional soil and water loss control on the Loess Plateau.
The selected plantation stands were established under similar initial conditions. The study sites were all located on gentle slopes (less than 5° gradients) with comparable aspects and elevations to minimize the variations caused by topography. The soil type across all selected stands was a typical loessial soil, characterized by uniform parent materials (loess deposits), textures (predominantly silt loam), and similar initial nutrient levels.
The initial planting densities across the selected plantations were standardized according to the local forestry management guidelines, typically adopting a spacing of approximately 2 m × 3 m (around 1650 trees per hectare). While minor variations in the planting density might exist due to historical management practices, such differences were within the normal operational range and unlikely to significantly affect the comparability of the sites. Moreover, the sampling plots within each age class were carefully chosen to be representative of the typical stand conditions, thereby minimizing the potential biases arising from any initial planting discrepancies.
These plantations were established as part of local ecological restoration initiatives, including the afforestation of barren slopes and forest regeneration projects. Consequently, extensive areas of P. sylvestris plantations at varying stand ages now exist throughout the study region. However, these plantations currently exhibit differing degrees of degradation, making this area ideal for examining the factors influencing forest health and sustainability.
5.2. Sample Collection
This study employed a time–space substitution approach by selecting P. sylvestris plantations representing four stand ages (13, 20, 25, and 35 years) as the research subjects. All sampling sites were located within the same ecological region and shared similar topographic features and loessial soil conditions. The distance between plantations of different age classes ranged from approximately 0.5 to 2 km, depending on the site availability and accessibility.
For each age class, three independent sampling plots (15 m × 15 m) were established within a single stand and spaced at least 50 m apart to ensure plot independence while maintaining the environmental consistency. Within each plot, a five-point composite sampling method was applied: rhizosphere soil (sensu lato) was collected from five healthy and representative trees located at the four corners and the center of each plot. For each tree, the soil was excavated from the root zone (approximately 1 m from the trunk) in four directions (east, west, south, and north) to a depth of 40 cm. The subsamples were first combined at the tree level, and then all five tree-level samples were mixed to generate one composite soil sample per plot, resulting in three biological replicates per stand age.
Each composite sample was divided into two subsamples: one was immediately flash-frozen in liquid nitrogen and stored at −80 °C for soil fungal community analysis, while the other was air-dried and used for the determination of the soil physicochemical properties. To ensure consistency, only trees with typical canopy structures, heights, and diameters at breast height (DBHs) were selected, while those exhibiting disease symptoms, mechanical damage, or abnormal growth were excluded.
The understory vegetation was primarily composed of native herbaceous species such as Artemisia capillaris and Setaria viridis. Field observations showed that both the understory coverage and the species richness declined with increasing stand age, with the 35-year-old stands exhibiting noticeably sparse ground vegetation.
Although the soil erosion was not quantitatively measured, qualitative field observations indicated minor signs of erosion—such as surface runoff traces, shallow root exposure, and localized soil crusting—primarily in the older plantations. However, no severe erosion features (e.g., gully erosion) were observed.
All field sampling was conducted in August, during the local peak growing season, which ensured high microbial activity and minimized the influence of seasonal variation on the soil nutrient levels and fungal community composition.
5.3. Analysis of Soil Samples
The total nitrogen (TN) and nitrate nitrogen (NN) were determined using the Kjeldahl method and UV spectrophotometry, respectively [50]. Ammonium nitrogen (AN) was quantified via a colorimetric method [51]. The soil organic carbon (SOC) was measured by dichromate oxidation [52]. The total phosphorus (TP) was assessed using molybdenum antimony blue colorimetry [53], while the available phosphorus (AP) was extracted with sodium bicarbonate and analyzed spectrophotometrically [54]. The total potassium (TK) was determined after digestion with hydrofluoric and perchloric acids, and the available potassium (AK) was extracted using 1 N ammonium acetate and quantified by atomic absorption spectroscopy [18]. The soil moisture content was evaluated via thermogravimetric analysis [55], and the soil pH was measured using colorimetric methods [56].
5.4. Total DNA Extraction from Rhizosphere Soil Samples
The soil samples after pure culture were weighed, and the total DNA was extracted from the soil with an EZNA Soil DNA Kit (D5625-01, Omega, Macon, GA, USA) [57]. To verify successful extraction, agarose gel electrophoresis with a 1% concentration was employed for DNA separation. The concentration and purity of the DNA were assessed using a NanoDrop 2000 UV–vis spectrophotometer (Thermo Fisher Scientific, Wilmington, NC, USA), 1 µL DNA solution was used to measure the absorbance, and the data were recorded [58]. The quality of the total DNA was detected by agarose gel electrophoresis; 3 µL DNA solution was evenly mixed with 0.5 µL loading buffer (10×) and added into 1% agar gel for electrophoresis under the following conditions: 1 × TAE electrophoresis buffer (20 mM Tris, 10 mM Acetate, and 0.5 mM Na2EDTA, pH 8.0) at 130 V for 15 min. The total DNA extracted above was amplified by PCR using the fungi universal primers ITS1-F (5′-CTTGGTCATTTAGAG-GAAGTAA-3′) and ITS2-R (5′-CTTGGTCATTTAGAGGAAGTAA-3′). The volume for the PCR was 20 µL, containing 10 μL of PCR SuperMix (TransGen Biotech, Beijing, China), template DNA (1 µL), 0.5 μL of each primer, and 8 μL of ddH2O [59]. The PCR amplification was carried out as follows: initial denaturation at 95 °C for 5 min, followed by 30 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min. After completing all cycles, a final extension was performed at 72 °C for 5 min. The PCR was carried out in triplicate. Agarose gel electrophoresis was used to detect the amplified fragments: 3 µL PCR product was loaded onto a 1% agarose gel and electrophoresed at 130 V for 15 min in 1 × TAE buffer. The PCR products were then sent to Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) (https://www.majorbio.com) for Illumina MiSeq sequencing.
5.5. Data Processing
The raw sequencing data from Illumina MiSeq were quality-controlled using fastp (Version 0.20.0; https://github.com/OpenGene/fastp, accessed on 12 May 2023) and assembled with FLASH (Version 1.2.7; http://www.cbcb.umd.edu/software/flash, accessed on 19 May 2023). The operational taxonomic units (OTUs) were clustered at a 97% similarity threshold, and chimeric sequences were removed using UPARSE (Version 7.1; http://drive5.com/uparse/, accessed on 24 May 2024). The taxonomic assignments were performed by comparing individual sequences against the UNITE fungal ITS database (Version 8.0) using the RDP Classifier (Version 2.2; http://rdp.cme.msu.edu/, accessed on 25 May 2024). The functional categorization of the fungal sequences, particularly for the identification of the ectomycorrhizal fungi, was achieved using the FUNGuild database (https://github.com/UMNFuN/FUNGuild, accessed on 17 July 2024).
5.6. Statistical Analysis
The statistical significance of the differences in the soil properties and soil fungal diversity and composition among the stand ages was determined by a one-way analysis of variance (ANOVA) using SPSS (Version 25). Prior to conducting the ANOVA and regression analysis, the data were tested for normality using the Shapiro–Wilk test and for homogeneity of variance using Levene’s test. These tests confirmed that the data met the assumptions required for the parametric analyses. Duncan multiple range tests were performed to assess the statistically significant differences between the values at a 95% confidence level. The Venn diagram was drawn by using R software (Version 3.3.1, Vegan package). Alpha diversity (α-diversity) was used to examine the complexity of the species diversity in the sample using three indices (Ace, Chao, and Sobs), which were used to analyze the fungal community diversity, and the rarefaction curves were drawn. A distance-based redundancy analysis (db-RDA) was used to investigate the importance of environmental factors in explaining the distribution patterns of the fungal communities for different stand ages using R software (Version 4.0.4, Vegan package). A heatmap correlation analysis was performed to evaluate the relationships between the soil properties and the soil fungal communities using R software (Version 4.0.4, Pheatmap package). The interactions between the Sobs index and soil properties were analyzed by the regression analysis using R software (Version 4.0.4).
Author Contributions
Conceptualization, J.W.; methodology, X.S.; software, Y.Z.; validation, D.W.; formal analysis, X.S., Y.L., and Y.Z.; investigation, Y.W. and J.G.; resources, D.W.; data curation, Y.L. and Y.Z.; writing—original draft preparation, X.S.; writing—review and editing, J.W., X.S., J.G., and Y.L.; visualization, X.S. and Y.L.; supervision, J.W.; project administration, J.G. and D.W.; funding acquisition, Y.W., J.W., and D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the China Agriculture Research System of MOF and MARA, CARS-21; the Natural Science Foundation of Shanxi Province, 202303021212121; the Scientific Research Fund of the Yunnan Provincial Department of Education, 2024J0474; the Doctoral Research Startup Project at Shanxi Agricultural University, SXBYKY2022132; the Research Project for High-level Talent at Shanxi Agricultural University, 2023BQ02; and the College Students Innovation Training Program of Shanxi Agricultural University, 20240266.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Jin, F.; Yang, W.; Fu, J.; Li, Z. Effects of vegetation and climate on the changes of soil erosion in the Loess Plateau of China. Sci. Total Environ. 2021, 773, 145514. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, J.; Zhao, G.; Holden, J.; Liu, B.; Chan, F.K.S.; Hu, J.; Wu, P.; Mu, X. Determining the drivers and rates of soil erosion on the Loess Plateau since 1901. Sci. Total Environ. 2022, 823, 153674. [Google Scholar] [CrossRef]
- Bai, R.; Wang, X.; Li, J.; Yang, F.; Shangguan, Z.; Deng, L. The impact of vegetation reconstruction on soil erosion in the Loess plateau. J. Environ. Manag. 2024, 363, 121382. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Zeng, H.; Zhong, X.; Kuang, S. Analysis of Carbon Sink Benefits from Comprehensive Soil and Water Conservation in the Loess Hilly Gently Slope Aeolian Sand Region. Water 2024, 16, 3434. [Google Scholar] [CrossRef]
- Luan, Z.; Wei, G.; Suqing, L.; Xiaojun, W. Distribution pattern and species diversity of artificial vegetation communities in sandy-hilly region of Northwest Shanxi Province, China. For. Grassl. Resour. Res. 2017, 6, 60–66. [Google Scholar] [CrossRef]
- Dang, H.; Zhang, X.; Han, H.; Shi, C.; Ge, Y.; Ma, Q.; Chen, S.; Liu, C. Research advances on forest-water relationships in Pinus sylvestris var. mongolica plantations for sand dune immobilization and guidance to forest management practices. Chin. J. Plant Ecol. 2022, 46, 971–983. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Han, R.; Zhang, X.; Ge, J.; Hao, G. Increased temperatures contribute to early aging of plantation-grown Mongolian pine in introduced areas at lower latitudes. J. For. Res. 2024, 35, 122. [Google Scholar] [CrossRef]
- Liu, J. Degeneration of Pinus sylvestris var. mongolica Plantations at Different Densities in Zhanggutai Area. Prot. For. Sci. Technol. 2019, 5, 57–58. [Google Scholar] [CrossRef]
- Castaño, C.; Lindahl, B.D.; Alday, J.G.; Hagenbo, A.; de Aragón, J.M.; Parladé, J.; Pera, J.; Bonet, J.A. Soil microclimate changes affect soil fungal communities in a Mediterranean pine forest. New Phytol. 2018, 220, 1211–1221. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; De Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Tedersoo, L.; Mikryukov, V.; Zizka, A.; Bahram, M.; Hagh-Doust, N.; Anslan, S.; Prylutskyi, O.; Delgado-Baquerizo, M.; Maestre, F.T.; Pärn, J. Global patterns in endemicity and vulnerability of soil fungi. Glob. Change Biol. 2022, 28, 6696–6710. [Google Scholar] [CrossRef]
- Li, J.; Zhang, T.; Meng, B.; Rudgers, J.A.; Cui, N.; Zhao, T.; Chai, H.; Yang, X.; Sternberg, M.; Sun, W. Disruption of fungal hyphae suppressed litter-derived C retention in soil and N translocation to plants under drought-stressed temperate grassland. Geoderma 2023, 432, 116396. [Google Scholar] [CrossRef]
- Adnan, M.; Islam, W.; Gang, L.; Chen, H.Y. Advanced research tools for fungal diversity and its impact on forest ecosystem. Environ. Sci. Pollut. Res. 2022, 29, 45044–45062. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qu, Z.; Zhang, Y.; Ge, Y.; Sun, H. Soil fungal community and potential function in different forest ecosystems. Diversity 2022, 14, 520. [Google Scholar] [CrossRef]
- Marčiulynienė, D.; Marčiulynas, A.; Mishcherikova, V.; Lynikienė, J.; Gedminas, A.; Franic, I.; Menkis, A. Principal drivers of fungal communities associated with needles, shoots, roots and adjacent soil of Pinus sylvestris. J. Fungi 2022, 8, 1112. [Google Scholar] [CrossRef]
- Povilaitienė, A.; Gedminas, A.; Varnagirytė-Kabašinskienė, I.; Marčiulynienė, D.; Marčiulynas, A.; Lynikienė, J.; Mishcherikova, V.; Menkis, A. Changes in chemical properties and fungal communities of mineral soil after clear-cutting and reforestation of Scots pine (Pinus sylvestris L.) sites. Forests 2022, 13, 1780. [Google Scholar] [CrossRef]
- Bastida, F.; López-Mondéjar, R.; Baldrian, P.; Andrés-Abellán, M.; Jehmlich, N.; Torres, I.; García, C.; López-Serrano, F. When drought meets forest management: Effects on the soil microbial community of a Holm oak forest ecosystem. Sci. Total Environ. 2019, 662, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, J.; Zhang, H.; Tang, M. Changes in rhizosphere soil fungal communities of Pinus tabuliformis plantations at different development stages on the loess plateau. Int. J. Mol. Sci. 2022, 23, 6753. [Google Scholar] [CrossRef]
- Trentini, C.P.; Campanello, P.I.; Villagra, M.; Ferreras, J.; Hartmann, M. Thinning partially mitigates the impact of Atlantic forest replacement by pine monocultures on the soil microbiome. Front. Microbiol. 2020, 11, 1491. [Google Scholar] [CrossRef]
- Uri, V.; Kukumägi, M.; Aosaar, J.; Varik, M.; Becker, H.; Aun, K.; Lõhmus, K.; Soosaar, K.; Astover, A.; Uri, M. The dynamics of the carbon storage and fluxes in Scots pine (Pinus sylvestris) chronosequence. Sci. Total Environ. 2022, 817, 152973. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Ma, Y.; Fu, S.; Chen, H.Y. Increased litterfall contributes to carbon and nitrogen accumulation following cessation of anthropogenic disturbances in degraded forests. For. Ecol. Manage. 2019, 432, 832–839. [Google Scholar] [CrossRef]
- Huang, Z.; Cui, Z.; Liu, Y.; Wu, G.L. Carbon accumulation by Pinus sylvestris forest plantations after different periods of afforestation in a semiarid sandy ecosystem. Land Degrad. Dev. 2021, 32, 2094–2104. [Google Scholar] [CrossRef]
- Niu, S.; Zhou, Y.; Liu, L.; Qin, S.; Yin, Y.; Song, X.; Xiao, W. Soil properties in Pinus sylvestris var mongolica plantation of different ages. J. Northeast For. Univ. 2015, 43, 47–50. [Google Scholar] [CrossRef]
- Dang, P.; Yu, X.; Le, H.; Liu, J.; Shen, Z.; Zhao, Z. Effects of stand age and soil properties on soil bacterial and fungal community composition in Chinese pine plantations on the Loess Plateau. PLoS ONE 2017, 12, e0186501. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, J.; Zheng, J.; Liu, J.; Liu, S.; Lin, W.; Wu, C. Soil microbial community structure and catabolic activity are significantly degenerated in successive rotations of Chinese fir plantations. Sci. Rep. 2017, 7, 6691. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Zhang, L.; Chen, D.; Tian, Y.; Zhang, F.; Wen, M.; Yuan, C. Understory herb layer exerts strong controls on soil microbial communities in subtropical plantations. Sci. Rep. 2016, 6, 27066. [Google Scholar] [CrossRef]
- Chen, K.; Hu, L.; Wang, C.; Yang, W.; Zi, H.; Manuel, L. Herbaceous plants influence bacterial communities, while shrubs influence fungal communities in subalpine coniferous forests. For. Ecol. Manage. 2021, 500, 119656. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Zhang, C.; Wang, H.; Fu, X.; Chen, F.; Wan, S.; Sun, X.; Wen, X.; Wang, J. Understory vegetation plays the key role in sustaining soil microbial biomass and extracellular enzyme activities. Biogeosciences 2018, 15, 4481–4494. [Google Scholar] [CrossRef]
- Krishna, M.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Weißbecker, C.; Wubet, T.; Lentendu, G.; Kühn, P.; Scholten, T.; Bruelheide, H.; Buscot, F. Experimental evidence of functional group-dependent effects of tree diversity on soil fungi in subtropical forests. Front. Microbiol. 2018, 9, 2312. [Google Scholar] [CrossRef]
- Tedersoo, L.; Anslan, S.; Bahram, M.; Drenkhan, R.; Pritsch, K.; Buegger, F.; Padari, A.; Hagh-Doust, N.; Mikryukov, V.; Gohar, D. Regional-scale in-depth analysis of soil fungal diversity reveals strong pH and plant species effects in Northern Europe. Front. Microbiol. 2020, 11, 1953. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, P.; Gao, G.; Ren, Y.; Ding, G.; Zhang, Y. Growing season stage determines the stability of root symbiotic and pathogenic fungi associated with Pinus sylvestris var. mongolica in a semi-arid desert. Appl. Soil Ecol. 2023, 190, 104993. [Google Scholar] [CrossRef]
- Ferrigo, D.; Mondin, M.; Ladurner, E.; Fiorentini, F.; Causin, R.; Raiola, A. Effect of seed biopriming with Trichoderma harzianum strain INAT11 on Fusarium ear rot and Gibberella ear rot diseases. Biol. Control 2020, 147, 104286. [Google Scholar] [CrossRef]
- Elvira-Recuenco, M.; Cacciola, S.O.; Sanz-Ros, A.V.; Garbelotto, M.; Aguayo, J.; Solla, A.; Mullett, M.; Drenkhan, T.; Oskay, F.; Aday Kaya, A.G. Potential interactions between invasive Fusarium circinatum and other pine pathogens in Europe. Forests 2020, 11, 7. [Google Scholar] [CrossRef]
- Adamo, I.; Castano, C.; Bonet, J.A.; Colinas, C.; de Aragon, J.M.; Alday, J.G. Soil physico-chemical properties have a greater effect on soil fungi than host species in Mediterranean pure and mixed pine forests. Soil Biol. Biochem. 2021, 160, 108320. [Google Scholar] [CrossRef]
- Rodríguez-Navarro, A. Potassium transport in fungi and plants. Biochim. Biophys. Acta 2000, 1469, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Si, P.; Shao, W.; Yu, H.; Yang, X.; Gao, D.; Qiao, X.; Wang, Z.; Wu, G. Rhizosphere microenvironments of eight common deciduous fruit trees were shaped by microbes in northern China. Front. Microbiol. 2018, 9, 3147. [Google Scholar] [CrossRef]
- Jiang, S.; Xing, Y.; Liu, G.; Hu, C.; Wang, X.; Yan, G.; Wang, Q. Changes in soil bacterial and fungal community composition and functional groups during the succession of boreal forests. Soil Biol. Biochem. 2021, 161, 108393. [Google Scholar] [CrossRef]
- Kyaschenko, J.; Clemmensen, K.E.; Hagenbo, A.; Karltun, E.; Lindahl, B.D. Shift in fungal communities and associated enzyme activities along an age gradient of managed Pinus sylvestris stands. ISME J. 2017, 11, 863–874. [Google Scholar] [CrossRef]
- Wang, Q.; He, X.; Guo, L. Ectomycorrhizal fungus communities of Quercus liaotungensis Koidz of different ages in a northern China temperate forest. Mycorrhiza 2012, 22, 461–470. [Google Scholar] [CrossRef]
- Modesto, I.; Mendes, A.; Carrasquinho, I.; Miguel, C.M. Molecular defense response of pine trees (Pinus spp.) to the parasitic nematode Bursaphelenchus xylophilus. Cells 2022, 11, 3208. [Google Scholar] [CrossRef]
- Chu, H.; Wang, H.; Zhang, Y.; Li, Z.; Wang, C.; Dai, D.; Tang, M. Inoculation with ectomycorrhizal fungi and dark septate endophytes contributes to the resistance of Pinus spp. to pine wilt disease. Front. Microbiol. 2021, 12, 687304. [Google Scholar] [CrossRef]
- Blumenstein, K.; Bußkamp, J.; Langer, G.J.; Langer, E.J.; Terhonen, E. The diplodia tip blight pathogen Sphaeropsis sapinea is the most common fungus in Scots pines’ mycobiome, irrespective of health status—A case study from Germany. J. Fungi 2021, 7, 607. [Google Scholar] [CrossRef]
- Bußkamp, J.; Langer, G.J.; Langer, E.J. Sphaeropsis sapinea and fungal endophyte diversity in twigs of Scots pine (Pinus sylvestris) in Germany. Mycol. Prog. 2020, 19, 985–999. [Google Scholar] [CrossRef]
- Spear, E.R.; Broders, K.D. Host-generalist fungal pathogens of seedlings may maintain forest diversity via host-specific impacts and differential susceptibility among tree species. New Phytol. 2021, 231, 460–474. [Google Scholar] [CrossRef]
- Santos, E.F.; Mateus, N.S.; Rosario, M.O.; Garcez, T.B.; Mazzafera, P.; Lavres, J. Enhancing potassium content in leaves and stems improves drought tolerance of eucalyptus clones. Physiol. Plant. 2021, 172, 552–563. [Google Scholar] [CrossRef]
- Johnson, R.; Vishwakarma, K.; Hossen, M.S.; Kumar, V.; Shackira, A.; Puthur, J.T.; Abdi, G.; Sarraf, M.; Hasanuzzaman, M. Potassium in plants: Growth regulation, signaling, and environmental stress tolerance. Plant Physiol. Biochem. 2022, 172, 56–69. [Google Scholar] [CrossRef]
- Triboulot, M.B.; Pritchard, J.; Levy, G. Effects of potassium deficiency on cell water relations and elongation of tap and lateral roots of maritime pine seedlings. New Phytol. 1997, 135, 183–190. [Google Scholar] [CrossRef]
- Hytönen, J.; Wall, A. Foliar colour as indicator of nutrient status of Scots pine (Pinus sylvestris L.) on peatlands. For. Ecol. Manag. 2006, 237, 156–163. [Google Scholar] [CrossRef]
- Liu, J.; Cai, H.; Chen, S.; Pi, J.; Zhao, L. A review on soil nitrogen sensing technologies: Challenges, progress and perspectives. Agriculture 2023, 13, 743. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic forms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1982; pp. 643–698. [Google Scholar]
- Beltrame, K.K.; Souza, A.M.; Coelho, M.R.; Winkler, T.C.; Souza, W.E.; Valderrama, P. Soil organic carbon determination using NIRS: Evaluation of dichromate oxidation and dry combustion analysis as reference methods in multivariate calibration. J. Braz. Chem. Soc. 2016, 27, 1527–1532. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Stevenson, F.J. Nitrogen—Organicforms. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1982; pp. 625–641. [Google Scholar]
- Bittelli, M. Measuring soil water content: A review. HortTechnology 2011, 21, 293–300. [Google Scholar] [CrossRef]
- Sparks, D.L.; Page, A.L.; Helmke, P.A.; Loeppert, R.H. Methods of Soil Analysis, Part 3: Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; The Soil Science Society of America, Inc.: Madison, WI, USA; American Society of Agronomy, Inc.: Madison, WI, USA, 1996; Volume 14, pp. 475–490. [Google Scholar]
- Gobert, A.; Evers, M.S.; Morge, C.; Sparrow, C.; Delafont, V. Comparison of DNA purification methods for high-throughput sequencing of fungal communities from wine fermentation. MicrobiologyOpen 2022, 11, e1321. [Google Scholar] [CrossRef]
- García-Alegría, A.M.; Anduro-Corona, I.; Pérez-Martínez, C.J.; Corella-Madueño, M.A.G.; Rascón-Durán, M.L.; Astiazaran-Garcia, H. Quantification of DNA through the NanoDrop spectrophotometer: Methodological validation using standard reference material and Sprague Dawley rat and human DNA. Int. J. Anal. Chem. 2020, 2020, 8896738. [Google Scholar] [CrossRef]
- Aamir, S.; Sutar, S.; Singh, S.; Baghela, A. A rapid and efficient method of fungal genomic DNA extraction, suitable for PCR based molecular methods. Plant Pathol. Quar. 2015, 5, 74–81. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).