Effects of Water–Nitrogen Coupling on Root Distribution and Yield of Summer Maize at Different Growth Stages
Abstract
1. Introduction
2. Results
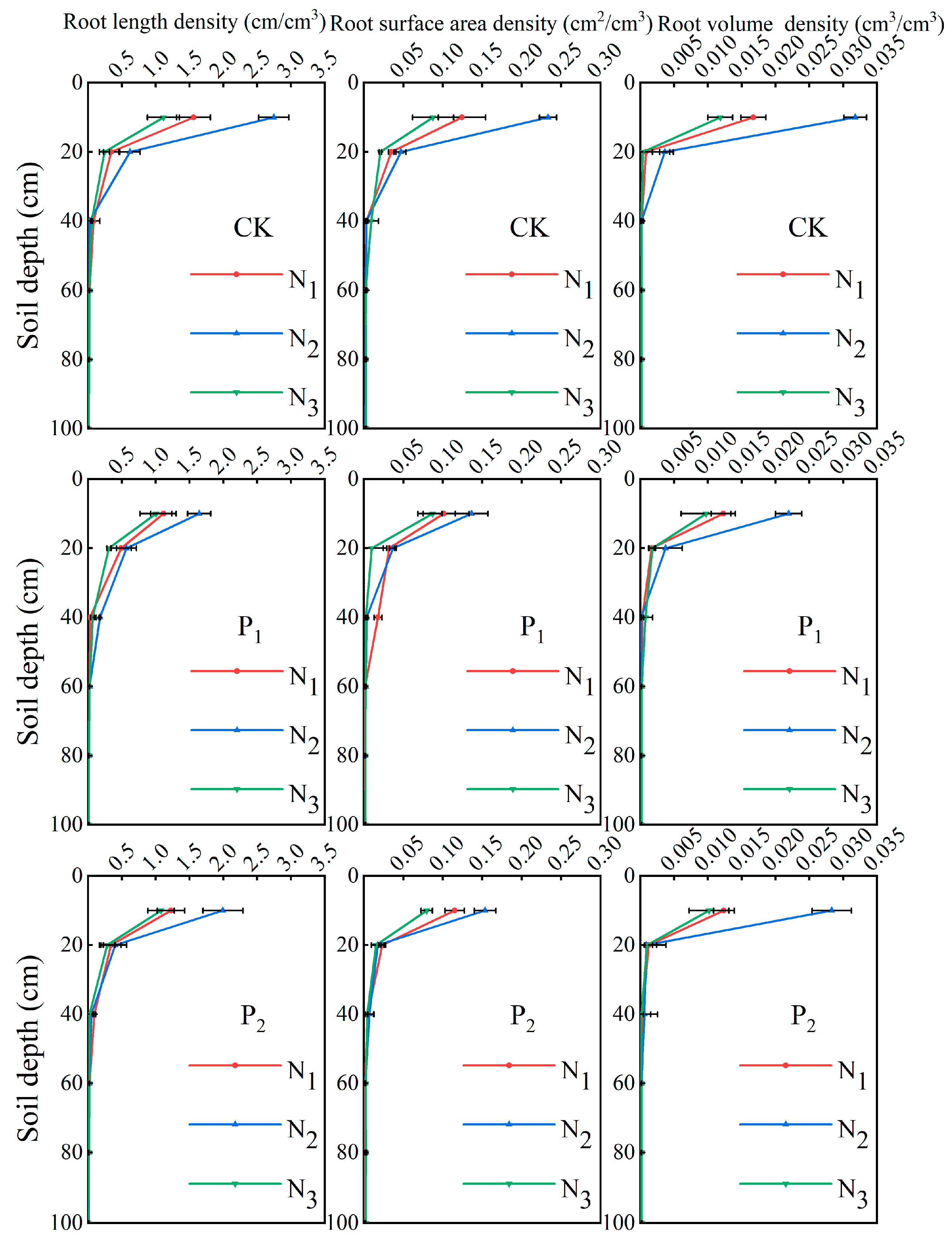
2.1. Root Spatial Distribution
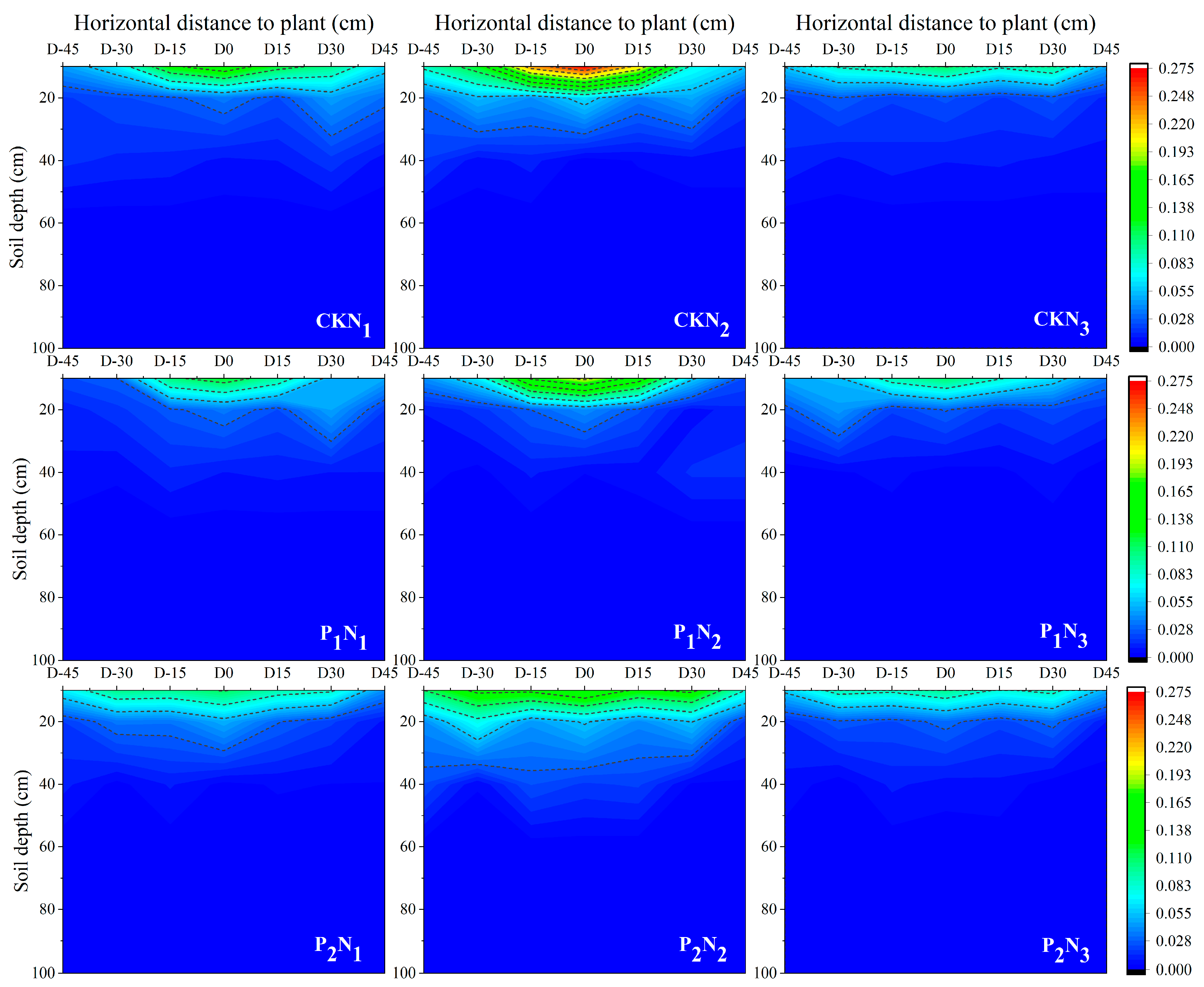
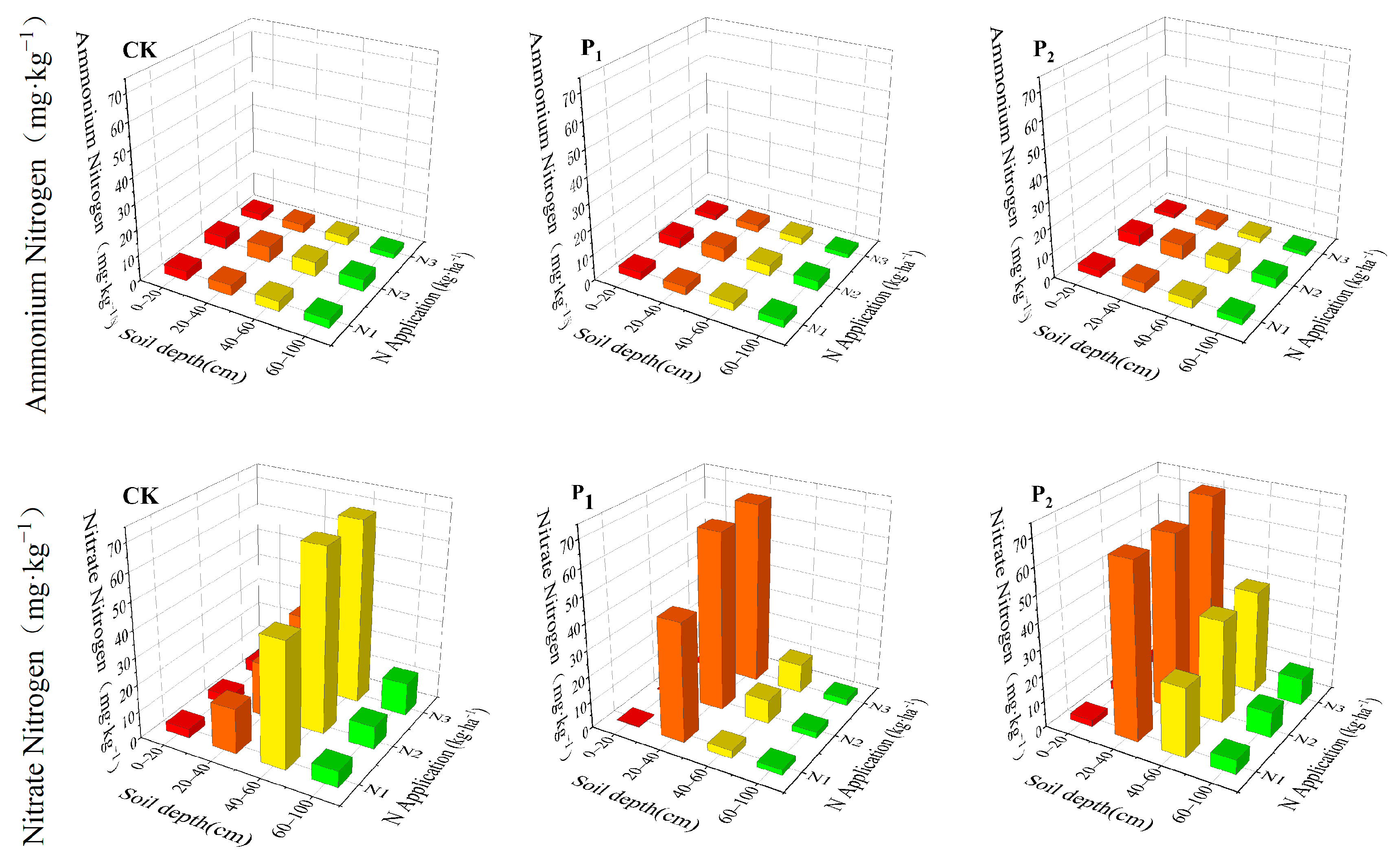
2.2. Nitrogen Spatial Distribution
2.3. Root Water Uptake
2.4. Yield and Its Component Factors
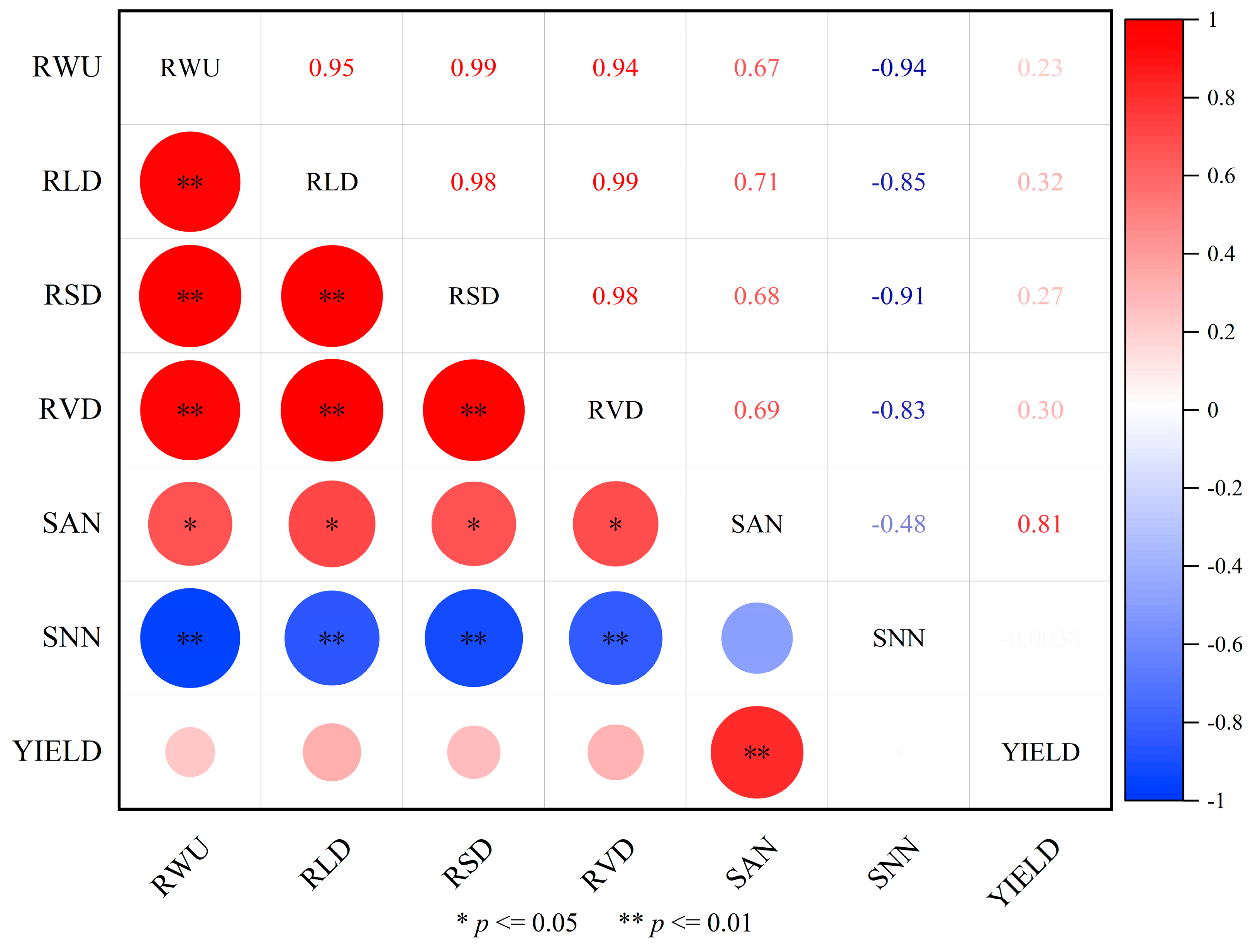
2.5. Correlation Analysis of Crop Roots, Soil Moisture, Nitrogen Content, and Yield
3. Discussion
4. Materials and Methods
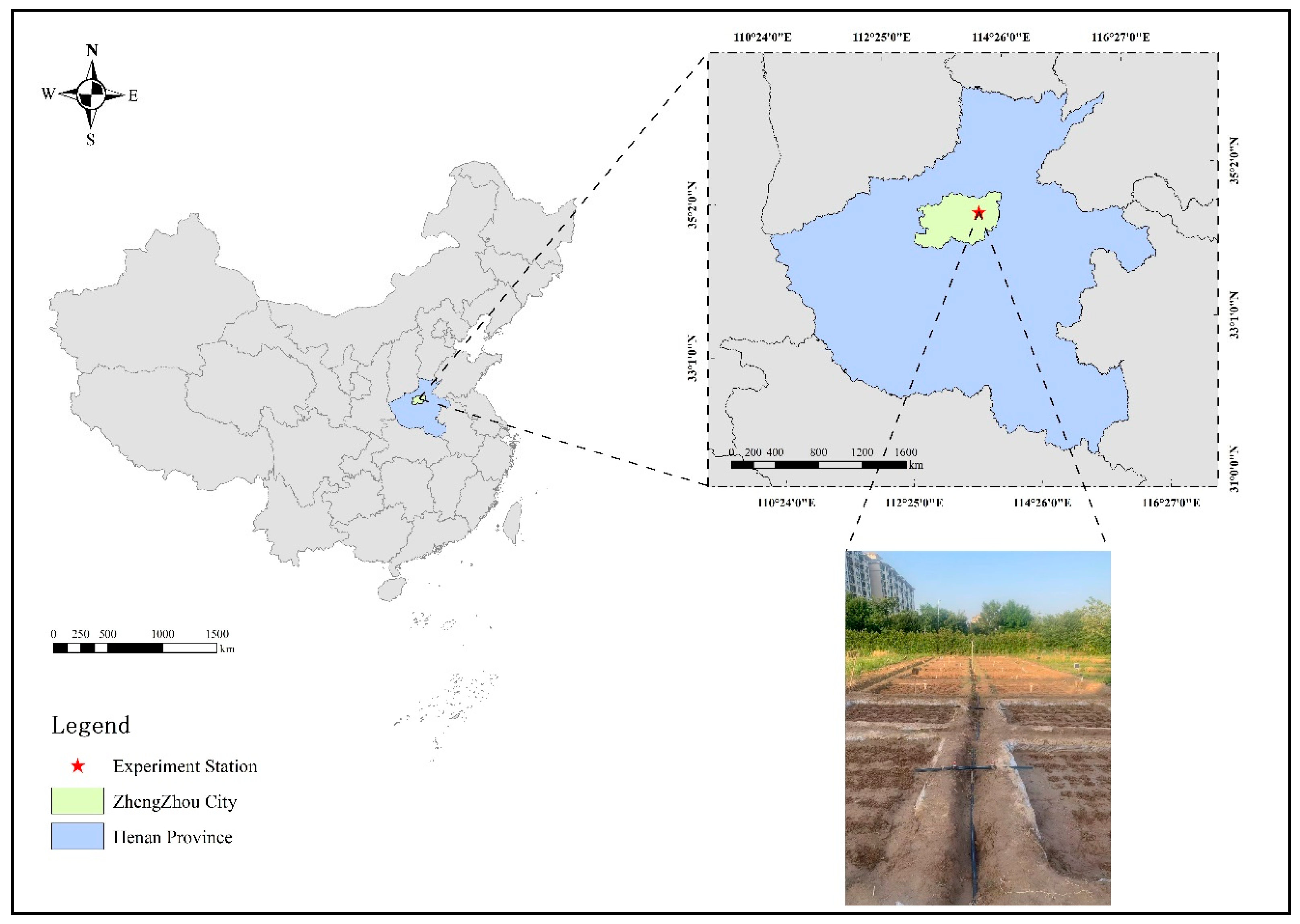
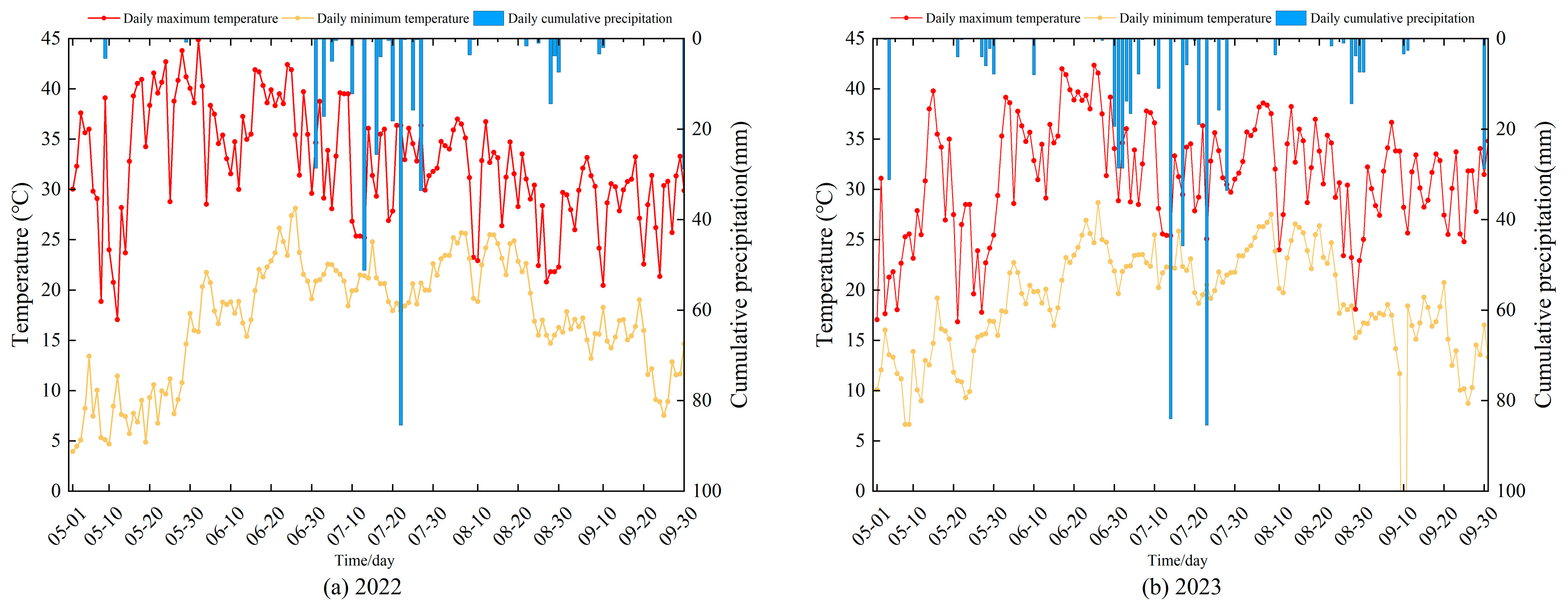
4.1. Overview of the Study Area
4.2. Experimental Materials
4.3. Experimental Design
4.4. Sampling, Measurement, and Calculation Methods
4.4.1. Root Sample Collection
4.4.2. Soil Nutrient Determination and Analysis
4.4.3. Yield and Components
4.5. Model Construction
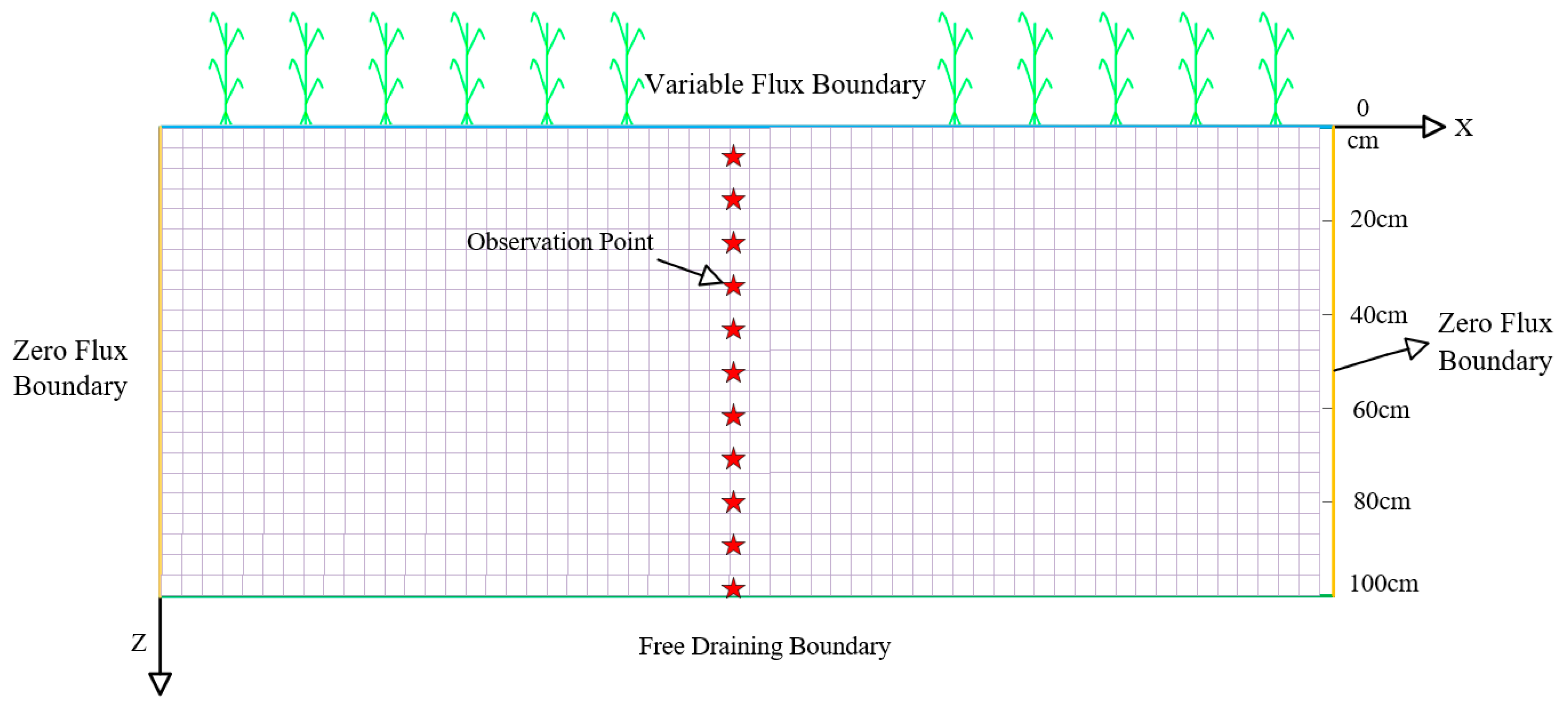
4.5.1. Soil Moisture Dynamics Model
4.5.2. Root Water Uptake Model
4.5.3. Evapotranspiration Model
4.5.4. Model Parameters
4.5.5. Boundary Conditions
4.6. Data Analysis
5. Conclusions
- The jointing stage represents a critical growth stage for maize root systems. Drought stress during this stage causes more severe impacts on root architecture compared to the tasseling-silking stage. Implementing combined precision irrigation and dynamic nitrogen supplementation strategies during jointing can optimize root configuration while balancing yield improvement with environmental sustainability.
- Maize roots employ a biphasic adaptive strategy, dynamically balancing vertical deepening and horizontal expansion under environmental stress, reinforcing vertical growth under water limitations while promoting horizontal expansion under nitrogen imbalance. This coordinated vertical–horizontal response mechanism demonstrates the plant’s adaptive capacity to challenging environmental conditions.
- The HYDRUS-based one-dimensional vertical root water uptake model effectively simulated soil water, with all R2 values exceeding 0.8. This confirms its reliability in modeling soil water movement patterns.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, Y.; Liu, Y.; Yin, J.; Chang, Z.; Wang, Y.; Liu, L.; Tian, Y.; Chen, W.; Wang, L.; Hao, L. Effects of moisture and CO2 on photosynthetic performance and water utilisation in maize. J. Agric. Eng. 2023, 39, 71–81. [Google Scholar]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef]
- Xu, Z.; He, P.; Yin, X.; Struik, P.C.; Ding, W.; Liu, K.; Huang, Q. Simultaneously improving yield and nitrogen use efficiency in a double rice cropping system in China. Eur. J. Agron. 2022, 137, 126513. [Google Scholar] [CrossRef]
- Xia, H.; Qiao, Y.; Li, X.; Xue, Y.; Wang, N.; Yan, W.; Xue, Y.; Cui, Z.; van der Werf, W. Moderation of nitrogen input and integration of legumes via intercropping enable sustainable intensification of wheat-maize double cropping in the North China Plain: A four-year rotation study. Agric. Syst. 2023, 204, 103540. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; Gk, S.; Vadivel, R.; Tk, D. Nitrogen use efficiency—A key to enhance crop productivity under a changing climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, M.S.; Malhi, S.S. Interactions of nitrogen with other nutrients and water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and environmental pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar]
- Farhan, M.; Sathish, M.; Kiran, R.; Mushtaq, A.; Baazeem, A.; Hasnain, A.; Hakim, F.; Hasan Naqvi, S.A.; Mubeen, M.; Iftikhar, Y. Plant nitrogen metabolism: Balancing resilience to nutritional stress and abiotic challenges. Phyton 2024, 93, 581–609. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef]
- Wang, N.Q.; Kong, C.H.; Wang, P.; Meiners, S.J. Root exudate signals in plant–plant interactions. Plant Cell Environ. 2021, 44, 1044–1058. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Zhao, D.; Tang, Z.; Zhang, T.; Zhang, K.; Dong, J.; Zhang, H. Genetic regulation of lateral root development. Plant Signal. Behav. 2023, 18, 2081397. [Google Scholar] [CrossRef]
- Jia, Z.; Giehl, R.F.H.; von Wirén, N. Nutrient–hormone relations: Driving root plasticity in plants. Mol. Plant 2022, 15, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, J.; Khashi u Rahman, M.; Wu, F. The impact of root exudates, volatile organic compounds, and common mycorrhizal networks on root system architecture in root-root interactions. J. Plant Interact. 2022, 17, 685–694. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wang, C.Y.; He, D.X. Root of Wheat; China Agriculture Press: Beijing, China, 1999; pp. 1–10+136–142. [Google Scholar]
- Wang, C.; Gu, F.; Chen, J.; Yang, H.; Jiang, J.; Du, T.; Zhang, J. Assessing the response of yield and comprehensive fruit quality of tomato grown in greenhouse to deficit irrigation and nitrogen application strategies. Agric. Water Manag. 2015, 161, 9–19. [Google Scholar] [CrossRef]
- Gavrilescu, M. Water, soil, and plants interactions in a threatened environment. Water 2021, 13, 2746. [Google Scholar] [CrossRef]
- Wang, E.; Smith, C.J. Modelling the growth and water uptake function of plant root systems: A review. Aust. J. Agric. Res. 2004, 55, 501–523. [Google Scholar] [CrossRef]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant morphological, physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef]
- Sah, R.P.; Chakraborty, M.; Prasad, K.; Pandit, M.; Tudu, V.K.; Chakravarty, M.K.; Narayan, S.C.; Rana, M.; Moharana, D. Impact of water deficit stress in maize: Phenology and yield components. Sci. Rep. 2020, 10, 2944. [Google Scholar] [CrossRef]
- Wan, W.; Liu, Z.; Li, J.; Xu, J.; Wu, H.; Xu, Z. Spatiotemporal patterns of maize drought stress and their effects on biomass in the Northeast and North China Plain from 2000 to 2019. Agric. For. Meteorol. 2022, 315, 108821. [Google Scholar] [CrossRef]
- Zahra, N.; Hafeez, M.B.; Shaukat, K.; Wahid, A.; Hussain, S.; Naseer, R.; Raza, A.; Iqbal, S.; Farooq, M. Hypoxia and Anoxia Stress: Plant responses and tolerance mechanisms. J. Agron. Crop Sci. 2021, 207, 249–284. [Google Scholar] [CrossRef]
- Chen, K.; Yu, S.; Ma, T.; Ding, J.; He, P.; Dai, Y.; Zeng, G. Effects of water and nitrogen management on water productivity, nitrogen use efficiency and leaching loss in rice paddies. Water 2022, 14, 1596. [Google Scholar] [CrossRef]
- Javed, T.; Indu, I.; Singhal, R.K.; Shabbir, R.; Shah, A.N.; Kumar, P.; Jinger, D.; Dharmappa, P.M.; Shad, M.A.; Saha, D. Recent advances in agronomic and physio-molecular approaches for improving nitrogen use efficiency in crop plants. Front. Plant Sci. 2022, 13, 877544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cui, Z.; Chen, X.; Ju, X.; Shen, J.; Chen, Q.; Liu, X.; Zhang, W.; Mi, G.; Fan, M. Integrated nutrient management for food security and environmental quality in China. Adv. Agron. 2012, 116, 1–40. [Google Scholar]
- Qiao, M.; Hong, C.; Jiao, Y.; Hou, S.; Gao, H. Impacts of drought on photosynthesis in major food crops and the related mechanisms of plant responses to drought. Plants 2024, 13, 1808. [Google Scholar] [CrossRef] [PubMed]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of fertilizer nitrogen in cereal production: Retrospects and prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar]
- Ladha, J.K.; Jat, M.L.; Stirling, C.M.; Chakraborty, D.; Pradhan, P.; Krupnik, T.J.; Sapkota, T.B.; Pathak, H.; Rana, D.S.; Tesfaye, K. Achieving the sustainable development goals in agriculture: The crucial role of nitrogen in cereal-based systems. Adv. Agron. 2020, 163, 39–116. [Google Scholar]
- Jin, Z.; Tao, Y.; Yue, R.; Ma, Z.; Cheng, S.; Khan, M.N.; Nie, L. Trade-off between grain yield and bioactive substance content of colored rice under coupled water and nitrogen conditions. Field Crops Res. 2024, 309, 109312. [Google Scholar] [CrossRef]
- Jin, Z.; Yue, R.; Ma, Z.; Cheng, S.; Khan, M.N.; Nie, L. Effect of water and nitrogen coupling on energy balance and production efficiency in rice production. Energy 2024, 288, 129739. [Google Scholar] [CrossRef]
- Li, P.; Zhang, F.; Jia, Y. Coupling effects of water and nitrogen on Cotton under different capillary arrangement modes of drip irrigation. Sci. Agric. Sin. 2009, 42, 1672–1681. [Google Scholar]
- Huang, Y.; Gao, F.; Khan, R.; Ali, S.; Zhou, X.B. Synergistic effects of irrigation and nitrogen fertilisation on maize photosynthetic performance and yield of rainfed systems in drought-prone environments. J. Agron. Crop Sci. 2024, 210, e12782. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Sheng, D.; Zhang, S.; Gu, S.; Yan, Y.; Zhao, F.; Wang, P.; Huang, S. Optimizing root system architecture to improve root anchorage strength and nitrogen absorption capacity under high plant density in maize. Field Crops Res. 2023, 303, 109109. [Google Scholar] [CrossRef]
- Liu, Y.; von Wirén, N. Integration of nutrient and water availabilities via auxin into the root developmental program. Curr. Opin. Plant Biol. 2022, 65, 102117. [Google Scholar] [CrossRef] [PubMed]
- Lagunas, B.; Dodd, I.C.; Gifford, M.L. A ‘nodemap’ to sustainable maize roots: Linking nitrogen and water uptake improvements. J. Exp. Bot. 2019, 70, 5036–5039. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Gong, P.; He, X.; Liu, H.; Li, L.; Wang, C.; Li, P.; Wei, J.; Yu, X. Enhanced irrigation volume reduces salinity and improves deep root zone soil nutrients, phosphatase activity and changes root traits of fruit trees. Agric. Water Manag. 2024, 302, 109001. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, F.; Li, Y.; Fan, J.; Ji, Q.; Jiang, F.; He, Z. Optimizing drip irrigation and nitrogen fertilization regimes to reduce greenhouse gas emissions, increase net ecosystem carbon budget and reduce carbon footprint in saline cotton fields. Agric. Ecosyst. Environ. 2024, 366, 108912. [Google Scholar] [CrossRef]
- Wang, S.J.; Yin, G.H.; Li, Z.; Gu, J.; Ma, N.; Feng, H.; Liu, Y. Effects of water-fertilizer coupling on the yield of spring maize under shallow-buried drip irrigation in semi-arid region of western Liaoning Province. J. Appl. Ecol. 2020, 31, 139–147. [Google Scholar]
- Li, G.; Niu, W.; Sun, J.; Zhang, W.; Zhang, E.; Wang, J. Soil moisture and nitrogen content influence wheat yield through their effects on the root system and soil bacterial diversity under drip irrigation. Land Degrad. Dev. 2021, 32, 3062–3076. [Google Scholar] [CrossRef]
- Ahmad, N.; Jiang, Z.; Zhang, L.; Hussain, I.; Yang, X. Insights on phytohormonal crosstalk in plant response to nitrogen stress: A focus on plant root growth and development. Int. J. Mol. Sci. 2023, 24, 3631. [Google Scholar] [CrossRef]
- Nadeem, M.; Yahya, M.; Tong, J.; Shah, L.; Khan, S.U.; Ali, A.; Sher, A.; Ullah, N.; Waheed, A. Improving nitrogen acquisition and utilization through root architecture remodelling: Insight from legumes. J. Plant Growth Regul. 2023, 42, 5295–5310. [Google Scholar] [CrossRef]
- Leitner, D.; Meunier, F.; Bodner, G.; Javaux, M.; Schnepf, A. Impact of contrasted maize root traits at flowering on water stress tolerance–a simulation study. Field Crops Res. 2014, 165, 125–137. [Google Scholar] [CrossRef]
- York, L.M.; Silberbush, M.; Lynch, J.P. Spatiotemporal variation of nitrate uptake kinetics within the maize (Zea mays L.) root system is associated with greater nitrate uptake and interactions with architectural phenes. J. Exp. Bot. 2016, 67, 3763–3775. [Google Scholar] [CrossRef]
- Wang, X.; Cai, H.; Zheng, Z.; Yu, L.; Wang, Z.; Li, L. Modelling root water uptake under deficit irrigation and rewetting in Northwest China. Agron. J. 2020, 112, 158–174. [Google Scholar] [CrossRef]
- Albasha, R.; Mailhol, J.C.; Cheviron, B. Compensatory uptake functions in empirical macroscopic root water uptake models–experimental and numerical analysis. Agric. Water Manag. 2015, 155, 22–39. [Google Scholar] [CrossRef]
- Sharma, B.; Molden, D.; Cook, S. Water Use Efficiency in Agriculture: Measurement, Current Situation and Trends; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2015. [Google Scholar]
- Zhang, Y.F.; Li, Y.P.; Sun, J.; Huang, G.H. Optimizing water resources allocation and soil salinity control for supporting agricultural and environmental sustainable development in Central Asia. Sci. Total Environ. 2020, 704, 135281. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, W.D.; Salvagiotti, F.; Pagani, A.; Reussi Calvo, N.I.; Eyherabide, M.; Sainz Rozas, H.R.; Ciampitti, I.A. Nitrogen and sulfur interaction on nutrient use efficiencies and diagnostic tools in maize. Eur. J. Agron. 2020, 116, 126045. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, Y.; Cai, M.; Zhou, J. Coupled effects of mulching and nitrogen fertilization on crop yield, residual soil nitrate, and water use efficiency of summer maize in the Chinese Loess Plateau. Environ. Sci. Pollut. Res. 2017, 24, 25849–25860. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xia, H.; Li, Y.; Ren, N. Effects of irrigation and nitrogen coupling on growth, yield and quality of cherry tomato under greenhouse cultivation during the whole growth period. J. Chin. Agric. Mech. 2024, 45, 200–207. [Google Scholar]
- Liu, X.; Lu, J.; QI, Y. The Effects of Water-nitrogen Coupling on Uptake and Transformation of Nitrogen in Soil. J. Irrig. Drain. 2022, 41, 1. [Google Scholar]
- Ko, J.; Piccinni, G.; Marek, T.; Howell, T. Determination of growth-stage-specific crop coefficients (Kc) of cotton and wheat. Agric. Water Manag. 2009, 96, 1691–1697. [Google Scholar] [CrossRef]

| Treatment | MAE | RMSE | R2 |
|---|---|---|---|
| CKN1 | 0.0164 | 0.0191 | 0.874 |
| CKN2 | 0.0109 | 0.0289 | 0.915 |
| CKN3 | 0.0342 | 0.0153 | 0.892 |
| P1N1 | 0.0119 | 0.0169 | 0.874 |
| P1N2 | 0.0209 | 0.0177 | 0.892 |
| P1N3 | 0.0087 | 0.0262 | 0.895 |
| P2N1 | 0.0134 | 0.0154 | 0.903 |
| P2N2 | 0.0121 | 0.0175 | 0.840 |
| P2N3 | 0.0374 | 0.0189 | 0.863 |
| Treatment | Ear Weight (g) | Number of Grains | Thousand-Grain Weight (g) | Yield (kg·ha−1) |
|---|---|---|---|---|
| CKN1 | 96.9 b | 394.0 c | 310.5 b | 9785 b |
| CKN2 | 129.2 a | 524.2 a | 316.8 a | 13,336 a |
| CKN3 | 78.9 c | 334.1 cd | 293.1 c | 7751 c |
| P1N1 | 66.5 cd | 287.0 d | 292.2 c | 6692 d |
| P1N2 | 111.5 a | 436.3 b | 329.6 a | 11,489 b |
| P1N3 | 48.5 d | 298.0 d | 205.6 e | 4892 e |
| P2N1 | 90.9 b | 325.6 cd | 341.1 a | 9049 c |
| P2N2 | 122.5 a | 454.4 b | 339.2 a | 12,216 a |
| P2N3 | 75.2 c | 420.0 b | 211.7 e | 7042 d |
| Soil Layer (cm) | Soil Bulk Density (g·cm−3) | Soil Organic Matter (g·kg−1) | Field Water Retention (V/V%) | Total N (g·kg−1) | Ammonium Nitrogen (mg·kg−1) | Nitrate Nitrogen (mg·kg−1) | Total P (g·kg−1) | pH |
|---|---|---|---|---|---|---|---|---|
| 0–20 | 1.46 | 16.1 | 34.2 | 0.89 | 7.43 | 8.15 | 0.75 | 6.7 |
| 20–40 | 1.48 | 14.5 | 35.1 | 0.93 | 6.71 | 7.75 | 0.94 | |
| 40–60 | 1.51 | 5.6 | 34.7 | 0.67 | 5.72 | 7.03 | 0.56 | |
| 60–80 | 1.55 | 3.4 | 34.5 | 0.45 | 5.55 | 6.51 | 0.38 | |
| 80–100 | 1.55 | 2.1 | 34.6 | 0.22 | 4.21 | 6.14 | 0.19 |
| Treatment | Soil Moisture | N Application (kg·ha−1) | |
|---|---|---|---|
| P1 | P2 | ||
| CKN1 | 75–100% | 75–100% | 100 |
| CKN2 | 200 | ||
| CKN3 | 300 | ||
| P1N1 | 50–60% | 75–100% | 100 |
| P1N2 | 200 | ||
| P1N3 | 300 | ||
| P2N1 | 75–100% | 50–60% | 100 |
| P2N2 | 200 | ||
| P2N3 | 300 | ||
| Soil Layer (cm) | (cm3·cm−3) | (cm3·cm−3) | (1·cm−1) | (cm·d−1) | |
|---|---|---|---|---|---|
| 0–20 | 0.0371 | 0.3105 | 0.0128 | 1.3941 | 35.60 |
| 20–40 | 0.0386 | 0.3209 | 0.0136 | 1.1392 | 25.13 |
| 40–60 | 0.0394 | 0.3752 | 0.0181 | 1.0633 | 35.56 |
| 60–80 | 0.0342 | 0.3578 | 0.0134 | 1.3855 | 34.19 |
| 80–100 | 0.0360 | 0.3809 | 0.0152 | 1.5630 | 45.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wang, Q.; Gao, S.; Wang, X.; He, A.; He, P. Effects of Water–Nitrogen Coupling on Root Distribution and Yield of Summer Maize at Different Growth Stages. Plants 2025, 14, 1278. https://doi.org/10.3390/plants14091278
Li Y, Wang Q, Gao S, Wang X, He A, He P. Effects of Water–Nitrogen Coupling on Root Distribution and Yield of Summer Maize at Different Growth Stages. Plants. 2025; 14(9):1278. https://doi.org/10.3390/plants14091278
Chicago/Turabian StyleLi, Yanbin, Qian Wang, Shikai Gao, Xiaomeng Wang, Aofeng He, and Pengcheng He. 2025. "Effects of Water–Nitrogen Coupling on Root Distribution and Yield of Summer Maize at Different Growth Stages" Plants 14, no. 9: 1278. https://doi.org/10.3390/plants14091278
APA StyleLi, Y., Wang, Q., Gao, S., Wang, X., He, A., & He, P. (2025). Effects of Water–Nitrogen Coupling on Root Distribution and Yield of Summer Maize at Different Growth Stages. Plants, 14(9), 1278. https://doi.org/10.3390/plants14091278






