The First Inventory of Sardinian Mining Vascular Flora
Abstract
1. Introduction
Aims of This Study
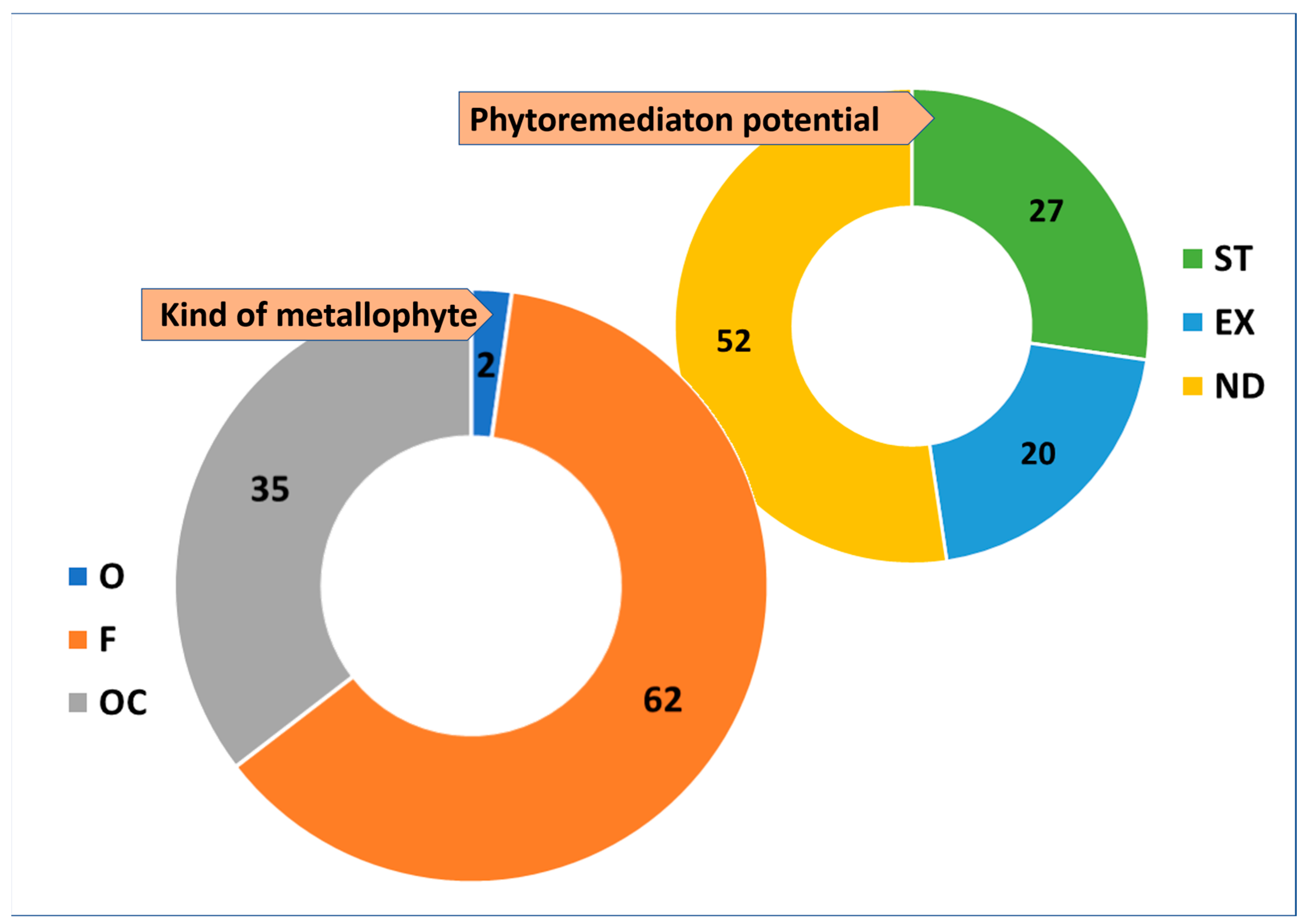
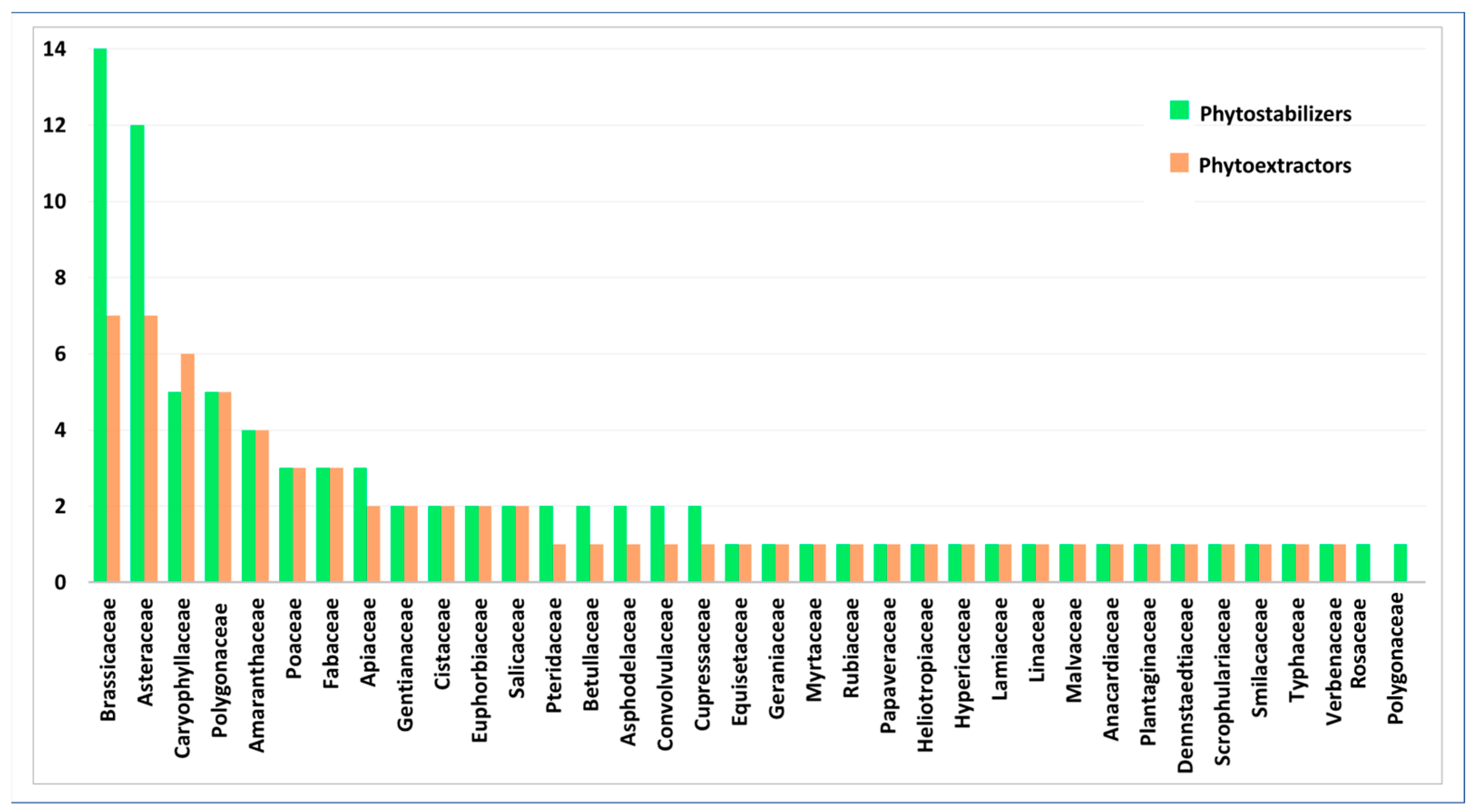
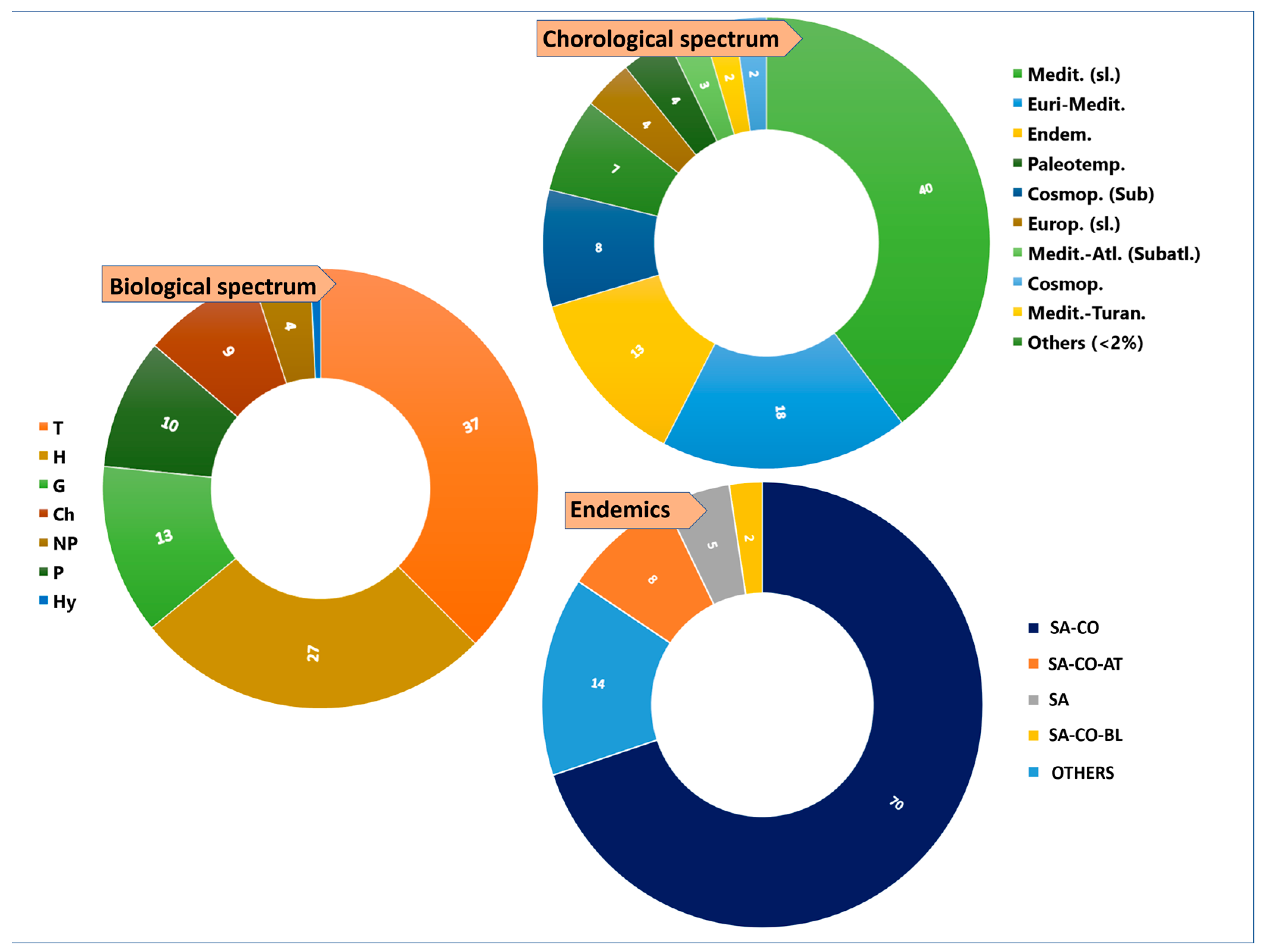
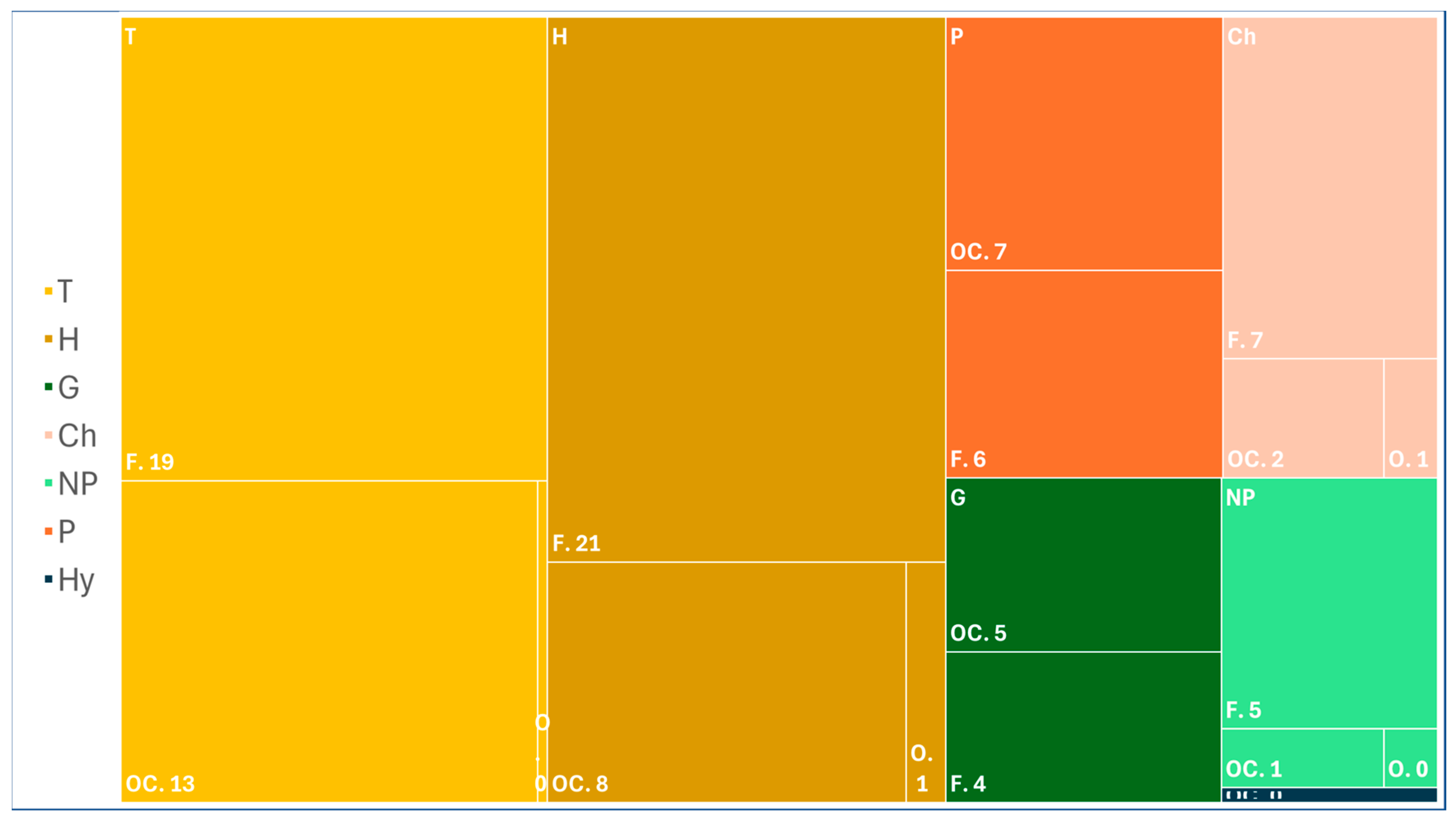
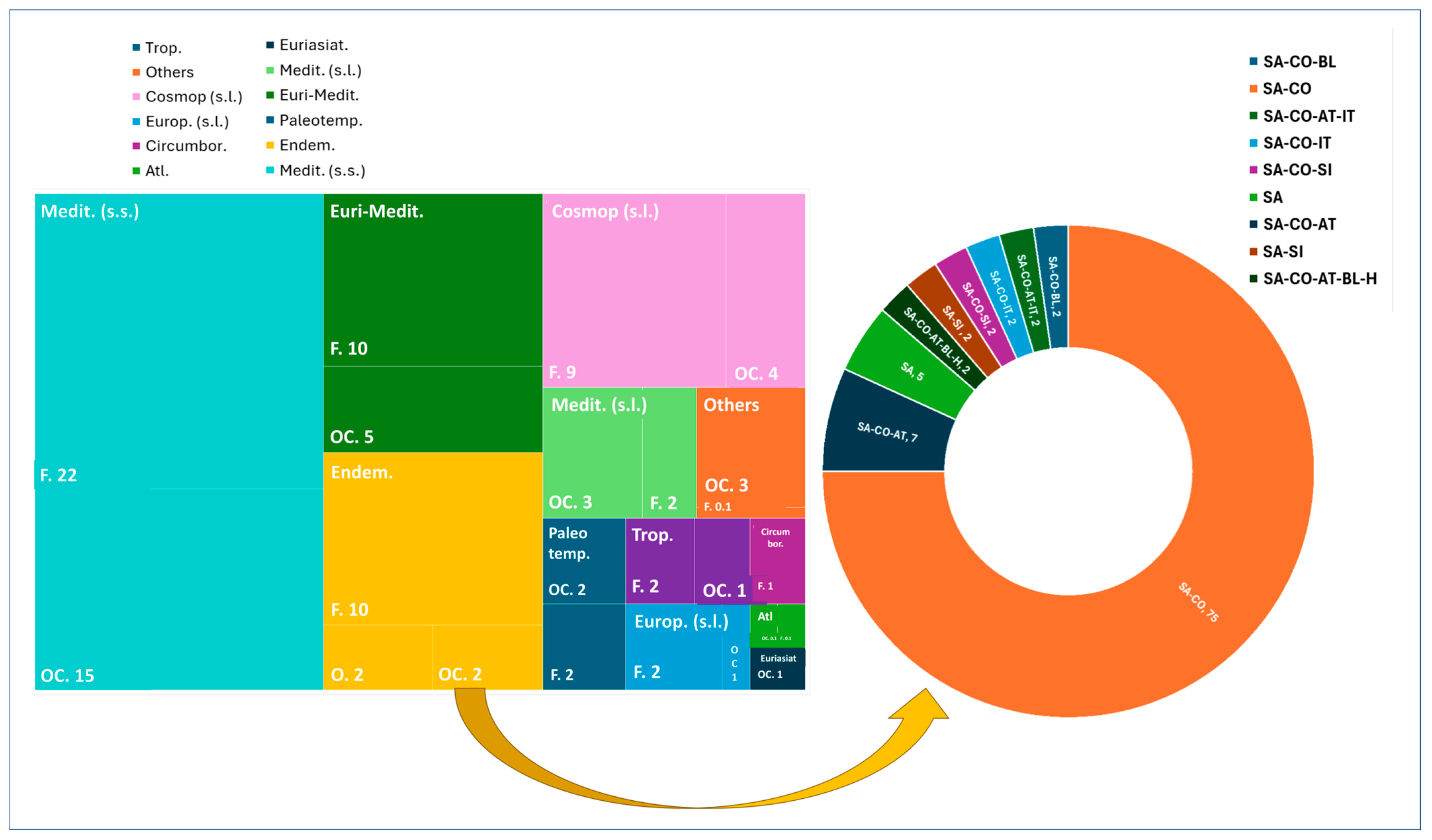
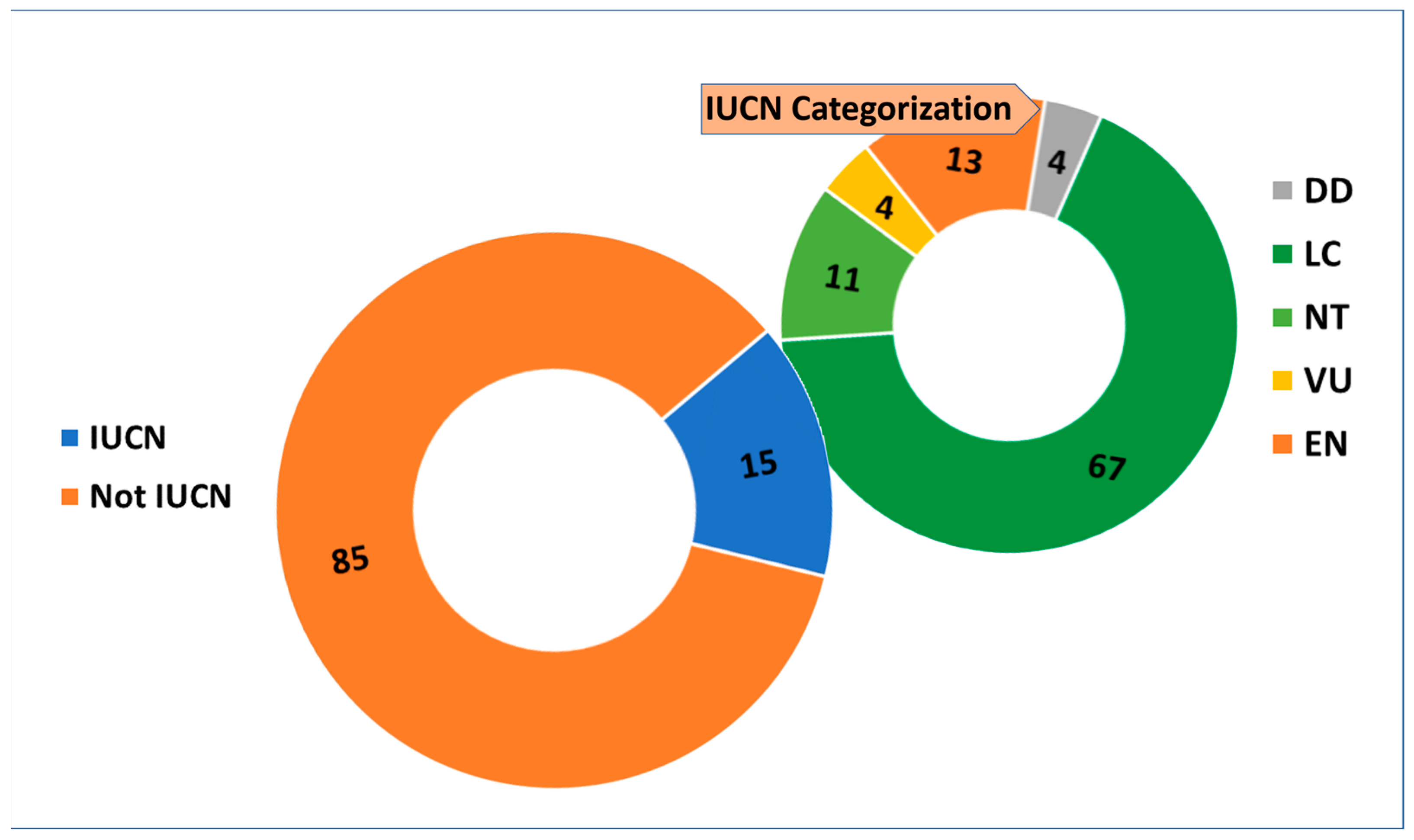
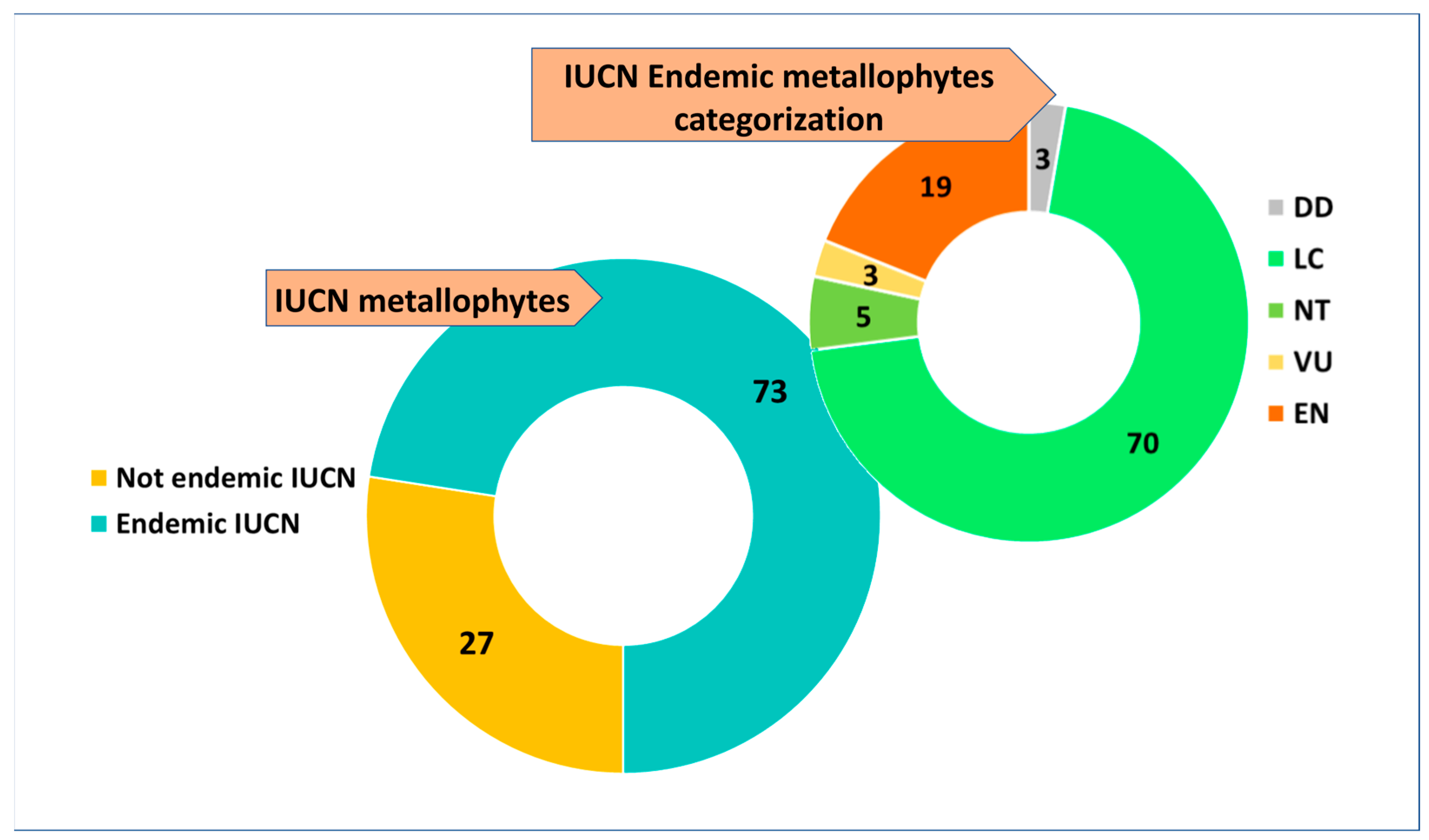
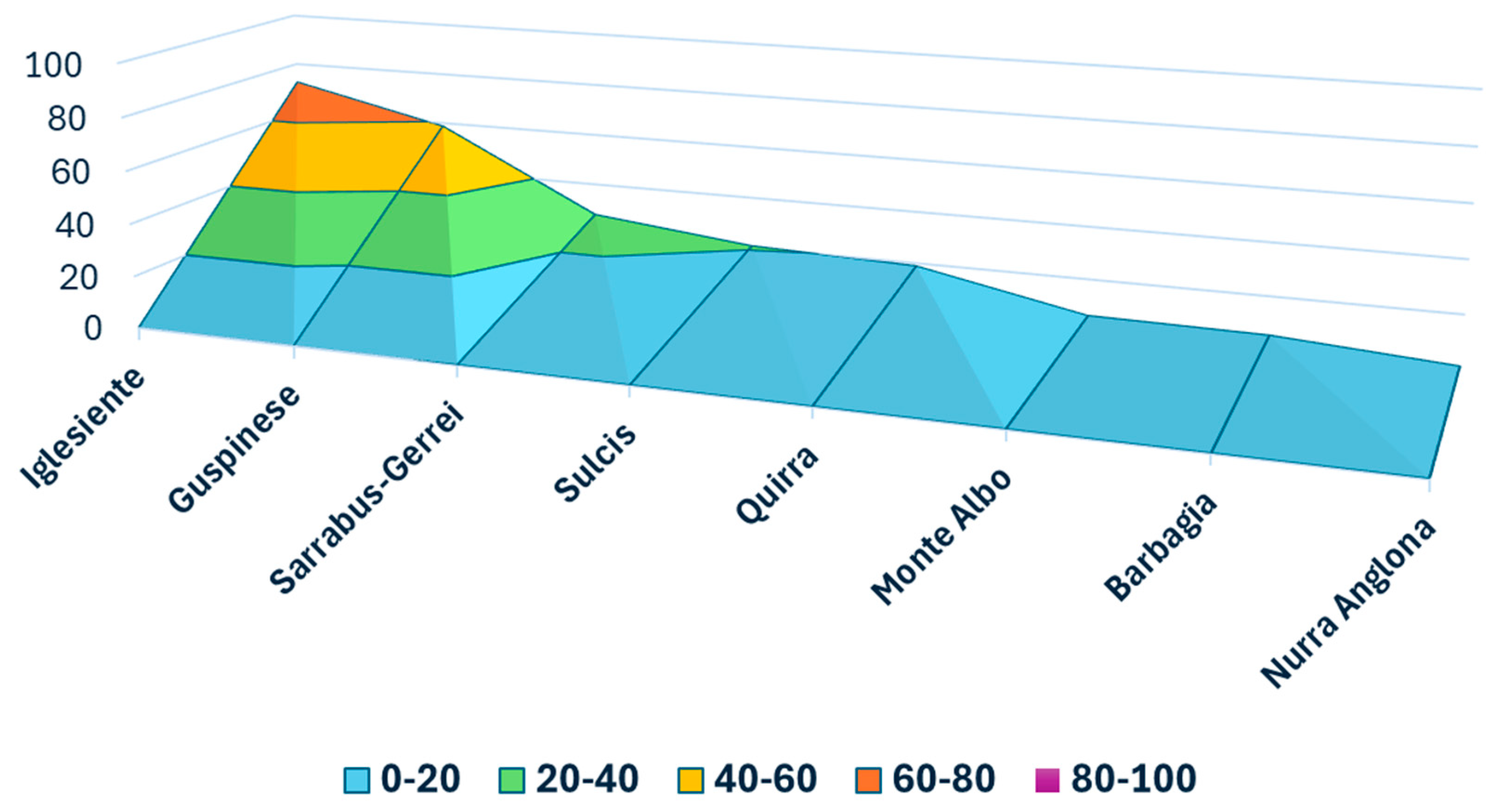
2. Results
3. Discussion
4. Materials and Methods
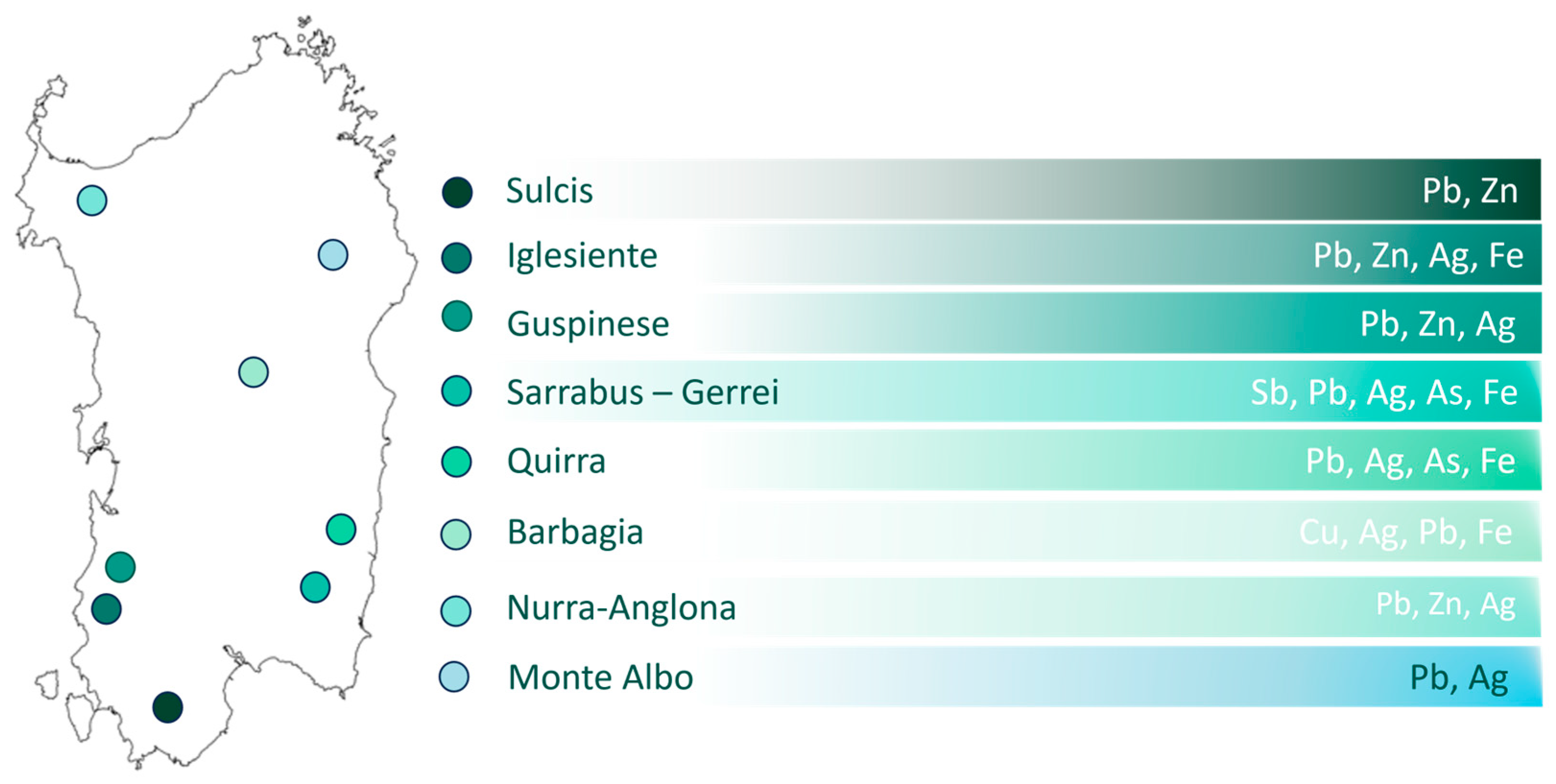
4.1. Study Area
4.2. Data Collection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Definition | |
|---|---|
| Accumulator species | Taxa where metals are highly translocated and accumulate in epigeal organs. Suitable for phytoextraction. |
| Excluder species | Taxa that exclude metal(loid)s in the rhizosphere region and prevent their translocation into epigeal organs. Suitable for phytostabilization. |
| Facultative metallophyte | Taxa able to grow in both metal-polluted/enriched and unpolluted substrates. |
| Metallophytes | Plants growing on metal(loid)-polluted substrates or substrates naturally enriched in metal(loid)s that have developed intrinsic resilience to metal(loid) stress. |
| Obligate metallophytes | Taxa that can live and thrive only on metal(loid)-polluted substrates or substrates naturally enriched in metal(loid)s. |
| Occasional metallophytes | Taxa that are present at mining sites but are generally uncommon in polluted/metal-enriched substrates. |
| Phytoextractors | Taxa that are suitable for phytoextraction. They are generally metal accumulator and hyperaccumulator species. |
| Phytoextraction | Application of phytoremediation devoted to the economic recovery of metals from substrates. |
| Phytoremediation | Technology by which vascular plant species and their associated microbiota, in combination with amendments and different kind of agronomic strategies, are used to remove or limit contamination or make it as harmless as possible. |
| Phytostabilization | Application of phytoremediation suitable for stabilizing mine substrates from weathering, for creating a long-term plant canopy, and for reducing the visual impact of excavation and mine waste accumulation in dumps. |
| Phytostabilizer | Taxa that are suitable for phytostabilization. They are generally metal excluder species. |
References
- Coelho, P.; Costa, S.; Costa, C.; Silva, S.; Walter, A.; Ranville, J.; Pastorinho, M.R.; Harrington, C.; Taylor, A.; Dall’Armi, V.; et al. Biomonitoring of several toxic metal(loid)s in different biological matrices from environmentally and occupationally exposed populations from Panasqueira Mine Area, Portugal. Environ. Geochem. Health 2014, 36, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Varrica, D.; Tamburo, E.; Milia, N.; Vallascas, E.; Cortimiglia, V.; De Giudici, G.; Dongarrà, D.; Sanna, E.; Monna, F.; Losno, R. Metals and metalloids in hair samples of children living near the abandoned mine sites of Sulcis-Iglesiente (Sardinia, Italy). Environ. Res. 2014, 134, 366–374. [Google Scholar] [CrossRef]
- Mendez, M.O.; Maier, R.M. Phytostabilization of mine tailings in arid and semiarid environments. Rev. Environ. Sci. Biotechnol. 2008, 7, 47–59. [Google Scholar] [CrossRef]
- Boni, M.; Costabile, S.; Vivo, B.; Gasparrini, M. Potential environmental hazard in the mining district of southern Iglesiente (SW Sardinia, Italy). J. Geochem. Explor. 1999, 67, 417–430. [Google Scholar] [CrossRef]
- Boi, M.E.; Cappai, G.; Giudici, G.; Medas, D.; Piredda, M.; Porceddu, M.; Bacchetta, G. Ex Situ phytoremediation trial of Sardinian mine waste using a pioneer plant species. Environ. Sci. Pollut. Res. 2021, 28, 55736–55753. [Google Scholar] [CrossRef]
- Zine, H.; Midhat, L.; Hakkou, R.; El Adnani, M.; Ouhammou, A. Guidelines for a phytomanagement plan by the phytostabilization of mining wastes. Sci. Afr. 2020, 10, e00654. [Google Scholar] [CrossRef]
- Boi, M.E.; Fois, M.; Podda, L.; Porceddu, M.; Bacchetta, G. Using Mediterranean native plants for the phytoremediation of mining sites: An overview of the past and present, and perspectives for the future. Plants 2023, 12, 3823. [Google Scholar] [CrossRef]
- Doumas, P.; Munoz, M.; Banni, M.; Becerra, S.; Bruneel, O.; Casiot, C.; Cleyet Marel, J.C.; Gardon, J.; Noack, Y.; Sappin-Didier, V. Polymetallic pollution from abandoned mines in Mediterranean regions: A multidisciplinary approach to environmental risks. Reg. Environ. Chang. 2018, 18, 677–692. [Google Scholar] [CrossRef]
- Lindsay, W.L. Zinc in soils and plant nutrition. Adv. Agron. 1972, 24, 147–181. [Google Scholar]
- Shkolnik, M.J. Microelements in Plant Life; Izd. Nauka: Leningrad, Russia, 1974; p. 323. [Google Scholar]
- Macnicol, R.D.; Beckett, P.H.T. Critical tissue concentrations of potentially toxic elements. Plant Soil 1985, 85, 107. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2011; p. 534. [Google Scholar]
- Chellaiah, E.R. Cadmium (heavy metals) bioremediation by Pseudomonas aeruginosa: A mini review. Appl. Water Sci. 2018, 8, 154. [Google Scholar] [CrossRef]
- Jali, P.; Pradhan, C.; Das, A.B. Effects of cadmium toxicity in plants: A review. Acad. J. Biosci. 2016, 4, 1074–1081. [Google Scholar]
- Hermans, C.; Chen, J.; Coppens, F.; Inzé, D.; Verbruggen, N. Low magnesium status in plants enhances tolerance to cadmium exposure. New Phytol. 2011, 192, 428–436. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Adrees, A.; Zia-ur-Rehman, M.; Qayyum, M.F.; Ok, Y.S.; Murtaza, G. Effect of biochar on alleviation of cadmium toxicity in wheat (Triticum aestivum L.) grown on Cd-contaminated saline soil. Environ. Sci. Pollut. Res. 2017, 25, 25668–25680. [Google Scholar] [CrossRef]
- Angiolini, C.; Bacchetta, G.; Brullo, S.; Casti, M.; Giusso del Galdo, G.; Guarino, R. The vegetation of mining dumps in SW-Sardinia. Feddes Repert. 2005, 116, 243–276. [Google Scholar] [CrossRef]
- Abreu, M.M.; Tavares, M.T.; Batista, M.J. Potential Use of Erica andevalensis and Erica australis in Phytoremediation of Sulphide Mine Environments: São Domingos, Portugal. J. Geochem. Explor. 2008, 96, 210–222. [Google Scholar] [CrossRef]
- Monaci, F.; Leidi, E.O.; Mingorance, M.D.; Valdés, B.; Rossini Oliva, S.; Bargagli, R. Selective Uptake of Major and Trace Elements in Erica andevalensis, an Endemic Species to Extreme Habitats in the Iberian Pyrite Belt. J. Environ. Sci. 2011, 23, 444–452. [Google Scholar] [CrossRef]
- De la Fuente, V.; Rufo, L.; Rodríguez, N.; Amils, R.; Zuluaga, J. Metal Accumulation Screening of the Río Tinto Flora (Huelva, Spain). Biol. Trace Element. Res. 2010, 134, 318–341. [Google Scholar] [CrossRef]
- Cecchi, L.; Coppi, A.; Selvi, F. Evolutionary Dynamics of Serpentine Adaptation in Onosma (Boraginaceae) as Revealed by ITS Sequence Data. Plant Syst. Evol. 2011, 297, 185–199. [Google Scholar] [CrossRef]
- Cecchi, L.; Španiel, S.; Bianchi, E.; Coppi, A.; Gonnelli, C.; Federico, S. Odontarrhena stridii (Brassicaceae), a new nickel hyperaccumulating species from mainland Greece. Plant Syst. Evol. 2020, 306, 69. [Google Scholar] [CrossRef]
- Laplaze, L.; Doumas, P.; Smouni, A.; Brhada, F.; Ater, M. Phytoremédiation du Plomb par Cistus libanotis: Demande de Brevet Prioritaire. Patentscope. 2009. Available online: https://patentscope.wipo.int/search/fr/detail.jsf?docId=WO2010130730 (accessed on 31 March 2025).
- Fois, M.; Murgia, L.; Bacchetta, G. Plant diversity and species composition of the abandoned mines of the Iglesiente mining district (Sardinia, Italy): A restoration perspective. Ecol. Eng. 2023, 188, 106879. [Google Scholar] [CrossRef]
- Rodríguez, N.; Amils, R.; Jiménez-Ballesta, R.; Rufo, L.; Fuente, V. Heavy Metal Content in Erica andevalensis: An endemic plant from the extreme acidic environment of Tinto River and its soils. Arid. Land. Res. Manag. 2007, 21, 51–65. [Google Scholar] [CrossRef]
- Fois, M.; Farris, E.; Calvia, G.; Campus, G.; Fenu, G.; Porceddu, M.; Bacchetta, G. The endemic vascular flora of Sardinia: A dynamic checklist with an overview of biogeography and conservation status. Plants 2022, 11, 601. [Google Scholar] [CrossRef]
- Whiting, S.N.; Reeves, R.D.; Richards, D.; Johnson, M.S.; Cooke, J.A.; Malaisse, F.; Paton, A.; Smith, J.A.C.; Angle, J.S.; Chaney, R.L.; et al. Research priorities for conservation of metallophyte biodiversity and their potential for restoration and site remediation. Restor. Ecol. 2004, 12, 106–116. [Google Scholar] [CrossRef]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A.C. The genetic basis of metal hyperaccumulation in plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Ballesteros, M.; Cañadas, E.M.; Foronda, A.; Peñas, J.; Valle, F.; Lorite, J. Central role of bedding materials for gypsum-quarry restoration: An experimental planting of gypsophile species. Ecol. Eng. 2014, 70, 470–476. [Google Scholar] [CrossRef]
- Mota, J.F.; Garrido-Becerra, J.A.; Merlo, M.E.; Medina-Cazorla, J.M.; Sánchez-Gómez, P. The Edaphism: Gypsum, Dolomite and Serpentine Flora and Vegetation. In The Vegetation of the Iberian Peninsula, Plant and Vegetation Series; Loidi, J., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 277–354. [Google Scholar]
- Mota, J.; Merlo, E.; Martínez-Hernández, F.; Mendoza-Fernández, A.J.; Pérez-García, F.J.; Salmerón-Sánchez, E. Plants on Rich-Magnesium Dolomite Barrens: A Global Phenomenon. Biology 2021, 10, 38. [Google Scholar] [CrossRef]
- Eibes, P.M.; Schaffrath, F.; Oldeland, J.; Thormahlen, W.; Schmiedel, U.; Irl, S.D.H. Testing the concept of edaphism for the quartz island flora of the Knersvlakte, South Africa. S. Afr. J. Bot. 2021, 151, 555–564. [Google Scholar] [CrossRef]
- Dore, E.; Fancello, D.; Rigonat, N.; Medas, D.; Cidu, R.; Da Pelo, S.; De Giudici, G. Natural attenuation can lead to environmental resilience in mine environment. Appl. Geochem. 2020, 117, 104597. [Google Scholar] [CrossRef]
- Bencala, K.E. Stream-groundwater interactions. In Treatise on Water Science; Elsevier: Newnes, Australia, 2011; pp. 537–546. [Google Scholar]
- Medas, D.; De Giudici, G.; Pusceddu, C.; Casu, M.A.; Birarda, G.; Vaccari, L.; Meneghini, C. Impact of Zn excess on biomineralization processes in Juncus acutus grown in mine polluted sites. J. Hazard. Mater. 2019, 370, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Caldelas, C.; Weiss, D.J. Zinc homeostasis and isotopic fractionation in plants: A review. Plant Soil 2017, 411, 17–46. [Google Scholar] [CrossRef]
- De Giudici, G.; Medas, D.; Meneghini, C.; Casu, M.A.; Gianoncelli, A.A.A.S.P.; Iadecola, A.; Lattanzi, P. Microscopic biomineralization processes and Zn bioavailability: A synchrotron-based investigation of Pistacia lentiscus L. roots. Environ. Sci. Pollut. Res. 2015, 22, 19352–19361. [Google Scholar] [CrossRef]
- Poláková, M.; Straka, M.; Polášek, M.; Němejcová, D. 2022. Unexplored freshwater communities in post-mining ponds: Effect of different restoration approaches. Restor. Ecol. 2022, 30, e13679. [Google Scholar] [CrossRef]
- Řehounková, K.; Vítovcová, K.; Prach, K. 2020. Threatened vascular plant species in spontaneously revegetated post-mining sites. Restor. Ecol. 2020, 28, 679–686. [Google Scholar] [CrossRef]
- Pat-Espadas, A.M.; Loredo Portales, R.; Amabilis-Sosa, L.E.; Gómez, G.; Vidal, G. Review of Constructed Wetlands for Acid Mine Drainage Treatment. Water 2018, 10, 1685. [Google Scholar] [CrossRef]
- Zavattero, L.; Casti, M.; Bacchetta, G.; Di Pietro, R. Analisi multitemporale del paesaggio del distretto minerario di Monteponi (Sardegna sud-occidentale). Ital. J. Remote Sens. 2006, 37, 137–146. [Google Scholar]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Dybowska, A.; Farago, M.; Valsami-Jones, E.; Thornton, I. Remediation strategies for historical mining and smelting sites. Sci. Prog. 2006, 89, 71–138. [Google Scholar] [CrossRef]
- Bacchetta, G.; Cappai, G.; Carucci, A.; Tamburini, E. Use of native plants for the remediation of abandoned mine sites in Mediterranean semiarid environments. Bull. Environ. Contam. Toxicol. 2015, 94, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, C.N.; Yong, R.N.; Gibbs, B.F. Remediation technologies for metal-contaminated soils and groundwater: An evaluation. Eng. Geol. 2001, 60, 193–207. [Google Scholar] [CrossRef]
- Podar, D.; Maathuis, F.J. The role of roots and rhizosphere in providing tolerance to toxic metals and metalloids. Plant Cell Environ. 2022, 45, 719–736. [Google Scholar] [CrossRef]
- Tamburini, E.; Mandaresu, M.; Lussu, R.; Sergi, S.; Vitali, F.; Carucci, A.; Cappai, G. Metal phytostabilization by mastic shrub (Pistacia lentiscus L.) and its root-associated bacteria in different habitats of Sardinian abandoned mining areas (Italy). Environ. Sci. Pollut. Res. 2023, 30, 122107–122120. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Reeves, R.D.; Haiar, A.S.M. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl. (Brassicaceae). New Phytol. 1994, 127, 61–68. [Google Scholar]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Rogel, J.; Peñalver-Alcalá, A.; Nazaret González-Alcaraz, M. Spontaneous vegetation colonizing abandoned metal(loid) mine tailings consistently modulates climatic, chemical and biological soil conditions throughout seasons. Sci. Total Environ. 2022, 838, 155945. [Google Scholar] [CrossRef]
- Mang, K.C.; Ntushelo, K. Phytoextraction and phytostabilisation approaches of heavy metal remediation in acid mine drainage with case studies: A review. Appl. Ecol. Environ. Res. 2019, 17, 6129–6149. [Google Scholar] [CrossRef]
- Solomou, A.D.; Germani, R.; Proutsos, N.; Petropoulou, M.; Koutroumpilas, P.; Galanis, C.; Maroulis, G.; Kolimenakis, A. Utilizing Mediterranean plants to remove contaminants from the soil environment: A short review. Agriculture 2022, 12, 238. [Google Scholar] [CrossRef]
- Bacchetta, G.; Casti, M.; Zavattero, L. Integration of vegetational and multitemporal analysis: A case study in the abandoned mine district of Montevecchio (South-western Sardinia). Ann. Botanic. 2007, 7, 163–174. [Google Scholar]
- Navarro-Cano, J.A.; Verdú, M.; Goberna, M. Trait-based selection of nurse plants to restore ecosystem functions in mine tailings. J. Appl. Ecol. 2018, 55, 1041–1565. [Google Scholar] [CrossRef]
- Vacca, A.; Aru, F.; Ollesch, G. Short-term impact of coppice management on soil in a Quercus ilex L. Stand of Sardinia. Land. Degrad. Dev. 2017, 28, 553–565. [Google Scholar] [CrossRef]
- Cristaldi, A.; Oliveri Conti, G.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello Fiore, P.M.; Restuccia, C.; Ferrante, M. Phytoremediation potential of Arundo donax (Giant Reed) in contaminated soil by heavy metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef]
- Iiriti, G. Flora e Paesaggio Vegetale del Sarrabus Gerrei (Sardegna Sud Orientale). Ph.D. Thesis, Università degli Studi di Cagliari, Cagliari, Italy, 2006. [Google Scholar]
- Bacchetta, G.; Casti, M.; Mossa, L.; Piras, M.L. La flora del distretto minerario di Montevecchio (Sardegna sud-occidentale). Webbia 2007, 62, 27–52. [Google Scholar] [CrossRef]
- Fois, M.; Bacchetta, G.; Caria, M.C.; Cogoni, D.; Farris, E.; Fenu, G.; Manca, M.; Pinna, M.S.; Pisanu, S.; Rivieccio, G.; et al. Proposals for improvement of annex I of Directive 92/43/EEC: Sardinia. Plant Sociol. 2021, 58, 65–76. [Google Scholar] [CrossRef]
- Bacchetta, G.; Cao, A.; Cappai, G.; Carucci, A.; Casti, M.; Fercia, M.L.; Lonis, R.; Mola, F. A field experiment on the use of Pistacia lentiscus L. and Scrophularia canina L. subsp. bicolor (Sibth. et Sm.) Greuter for the phytoremediation of abandoned mining areas. Plant Biosyst. 2012, 146, 1054–1063. [Google Scholar]
- Boi, M.E.; Angotzi, M.S.; Porceddu, M.; Musu, E.; Mameli, V.; Bacchetta, G.; Cannas, C. Germination and early seedling development of Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso in the presence of arsenates and arsenites. Heliyon 2022, 8, e10693. [Google Scholar]
- Caldelas, C.; Dong, S.; Araus, J.L.; Jakob Weiss, D. Zinc isotopic fractionation in Phragmites australis in response to toxic levels of zinc. J. Exp. Bot. 2011, 62, 2169–2178. [Google Scholar] [CrossRef]
- Medas, D.; De Giudici, G.; Casu, M.A.; Musu, E.; Gianoncelli, A.; Iadecola, A.; Lattanzi, P. Microscopic processes ruling the bioavailability of Zn to roots of Euphorbia pithyusa L. pioneer plant. Environ. Sci. Technol. 2015, 49, 1400–1408. [Google Scholar] [CrossRef]
- Mirete, S.; de Figueras, C.G.; González-Pastor, J.E. Novel Nickel Resistance Genes from the Rhizosphere Metagenome of Plants Adapted to Acid Mine Drainage. Appl. Environ. Microbiol. 2007, 73, 6001–6011. [Google Scholar] [CrossRef]
- De Agostini, A.; Caltagirone, C.; Caredda, A.; Cicatelli, A.; Cogoni, A.; Farci, D.; Cortis, P. Heavy metal tolerance of orchid populations growing on abandoned mine tailings: A case study in Sardinia Island (Italy). Ecotoxicol. Environ. Saf. 2020, 189, 110018. [Google Scholar] [CrossRef]
- Cuena-Lombraña, A.; Fois, M.; Calvia, G.; Bacchetta, G. An updated checklist of the vascular flora of Montarbu massif (CE Sardinia, Italy). Flora Medit. 2023, 33, 251–268. [Google Scholar]
- Djordjević, V.; Tsiftsis, S.; Lakušić, D.; Jovanović, S.; Jakovljević, K.; Stevanović, V. Patterns of distribution, abundance and composition of forest terrestrial orchids. Biodivers. Conserv. 2020, 29, 4111–4134. [Google Scholar] [CrossRef]
- Directive, H. Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Union 1992, 206, 50. [Google Scholar]
- Baker, A.J.; Ernst, W.H.; van der Ent, A.; Malaisse, F.; Ginocchio, R. Metallophytes: The unique biological resource, its ecology and conservational status in Europe, central Africa and Latin America. Ecol. Ind. Poll. 2010, 18, 7–40. [Google Scholar]
- Brown, G. The heavy-metal vegetation of north-western mainland Europe. Bot. Jahrb. Fur Syst. 2001, 123, 63–110. [Google Scholar]
- Pontecorvo, C. La Flora dell’Iglesiente (Sardegna SW). Ph.D. Thesis, Università degli Studi di Cagliari, Cagliari, Italy, 2006. [Google Scholar]
- El Aafi, N.; Saidi, N.; Maltouf, A.F.; Perez-Palacios, P.; Dary, M.; Brhada, F.; Pajuelo, E. Prospecting Metal-Tolerant Rhizobia for Phytoremediation of Mining Soils from Morocco Using Anthyllis vulneraria L. Environ. Sci. Pollut. Res. 2015, 22, 4500–4512. [Google Scholar] [CrossRef]
- Cecchi, L.; Bettarini, I.; Colzi, I.; Coppi, A.; Echevarria, G.; Pazzagli, L.; Bani, A.; Gonnelli, C.; Selvi, F. The Genus Odontarrhena (Brassicaceae) in Albania: Taxonomy and Nickel Accumulation in a Critical Group of Metallophytes from a Major Serpentine Hot-Spot. Phytotaxa 2018, 35, 1–28. [Google Scholar] [CrossRef]
- Mengoni, A.; Cecchi, L.; Gonnelli, C. Nickel Hyperaccumulating Plants and Alyssum bertolonii: Model Systems for Studying Biogeochemical Interactions in Serpentine Soils. In Bio-Geo Interactions in Metal-Contaminated Soils; Springer: Berlin/Heidelberg, Germany, 2011; pp. 279–296. [Google Scholar]
- Thompson, J.D. Plant Evolution in the Mediterranean: Insights for Conservation; Oxford University Press: Cary, NC, USA, 2020. [Google Scholar]
- Calvia, G.; Ruggero, A. The vascular flora of Mount Limbara (northern Sardinia): From a troubled past towards an uncertain future. Flora Medit. 2020, 30, 293–313. [Google Scholar]
- Kirk, D.A.; Goldsmith, F.B. Grazing pressure versus environmental covariates: Effects on woody and herbaceous plant biodiversity on a limestone mountain in northern Tunisia. PeerJ 2018, 7, e7296. [Google Scholar] [CrossRef]
- Mherzi, N.; Lamchouri, F.; Khabbach, A.; Boulfia, M.; Zalaghi, A.; Toufik, H. Ecological types and bioindicator macrophyte species of pollution of riparian vegetation of Oued Lârbaa in Taza City of Morocco. Environ. Monit. Assess. 2020, 192, 256. [Google Scholar] [CrossRef]
- Cuena-Lombraña, A.; Fois, M.; Cogoni, A.; Bacchetta, G. Where we come from and where to go: Six decades of botanical studies in the Mediterranean wetlands, with Sardinia (Italy) as a case study. Wetlands 2021, 41, 69. [Google Scholar] [CrossRef]
- Cannucci, S.; Angiolini, C.; Anselmi, B.; Banfi, E.; Biagioli, M.; Castagnini, P.; Centi, C.; Fiaschi, T.; Foggi, B.; Gabellini, A.; et al. Contribution to the knowledge of the vascular flora of Miniera di Murlo area (southern Tuscany, Italy). Ital. Bot. 2017, 7, 51–67. [Google Scholar] [CrossRef]
- Cherchi, A.; Montadert, L. Oligo-Miocene rift of Sardinia and the early history of the western Mediterranean basin. Nature 1982, 298, 736–739. [Google Scholar] [CrossRef]
- Mansion, G.; Rosenbaum, G.; Schoenenberger, N.; Bacchetta, G.; Rosselló, J.A.; Conti, E. Phylogenetic analysis informed by geological history supports multiple, sequential invasions of the Mediterranean Basin by the angiosperm family Araceae. Syst. Biol. 2008, 57, 269–285. [Google Scholar] [CrossRef]
- Médail, F. The specific vulnerability of plant biodiversity and vegetation on Mediterranean islands in the face of global change. Reg. Environ. Change 2017, 17, 1775–1790. [Google Scholar] [CrossRef]
- Caković, D.; Frajman, B. An integrative approach supports the taxonomic distinction of the Sardo-Corsican endemic Euphorbia semiperfoliata from the widespread E. amygdaloides (Euphorbiaceae). Plant Biosyst. 2023, 157, 958–969. [Google Scholar] [CrossRef]
- Acunto, S.; Bacchetta, G.; Bordigoni, A.; Cadoni, N.; Cinti, M.F.; Duràn Navarro, M.; Sanna, A. The LIFE+ project “RES MARIS-Recovering Endangered habitatS in the Capo Carbonara MARIne area, Sardinia”: First results. Plant Soc. 2017, 54, 85–95. [Google Scholar]
- Burges, A.; Alkorta, I.; Epelde, L.; Garbisu, C. From phytoremediation of soil contaminants to phytomanagement of ecosystem services in metal contaminated sites. Int. J. Phytoremediation 2018, 20, 384–397. [Google Scholar] [CrossRef]
- Canu, S.; Rosati, L.; Fiori, M.; Motroni, A.; Filigheddu, R.; Farris, E. Bioclimate map of Sardinia (Italy). J. Maps 2015, 11, 711–718. [Google Scholar] [CrossRef]
- Cidu, R.; Biagini, C.; Fanfani, L.; La Ruffa, G.; Marras, I. Mine closure at Monteponi (Italy): Effect of the cessation of dewatering on the quality of shallow groundwater. Appl. Geochem. 2001, 16, 489–502. [Google Scholar] [CrossRef]
- Cidu, R.; Biddau, R. Transport of trace elements under different seasonal conditions: Effects on the quality of river water in a Mediterranean area. Appl. Geochem. 2007, 22, 2777–2794. [Google Scholar] [CrossRef]
- Carmignani, L.; Oggiano, G.; Barca, S.; Conti, P.; Salvadori, I.; Eltrudis, A.; Pasci, S. Geologia della Sardegna: Note Illustrative della Carta Geologica della Sardegna in scala 1: 200.000. Mem. Descr. Carta Geol. D’it. 2001, 60, 1–283. [Google Scholar]
- Barbafieri, M.; Dadea, C.; Tassi, E.; Bretzel, F.; Fanfani, L. Uptake of heavy metals by native species growing in a mining area in Sardinia, Italy: Discovering native flora for phytoremediation. Int. J. Phytoremediation 2011, 13, 985–997. [Google Scholar] [CrossRef]
- Da Pelo, S. L’area mineraria dismessa di Montevecchio: Il Bacino di Levante. Interazione Acqua-Roccia: Aspetti Mineralogici, Petrologici, Geochimici e Ambientali. In Gruppo Nazionale di Mineralogia; Note All’escursione: Montevecchio e Furtei; Scuola di Mineralogia Torre dei Corsari: Cagliari, Italy, 1999. [Google Scholar]
- Fanfani, L.; Caboi, R.; Cidu, R.; Cristini, A.; Frau, F.; Lattanzi, P.; Zuddas, P. Impatto ambientale dell’attività mineraria in Sardegna: Studi mineralogici e geochimica. Rend. Sem. Fac. Sc. Univ. Cagliari 2000, 70, 249–264. [Google Scholar]
- Marcello, A.; Pretti, S.; Valera, P. Metallogeny in Sardinia (Italy): From the Cambrian to the Tertiary. In Proceedings of the 32nd International Geological Congress, Florence, Italy, 20–28 August 2004. [Google Scholar]
- Cidu, R.; Mereu, L. The Abandoned Copper-Mine of Funtana Raminosa (Sardinia): Preliminary Evaluation of Its Impact on the Aquatic System. In Water in Mining Environments; Cidu, R., Frau, F., Eds.; IMWA Symposium: Cagliari, Italy, 2007. [Google Scholar]
- Biggeri, A.; Lagazio, C.; Catelan, D.; Pirastu, R.; Casson, F.; Terracini, B. Report on health status of residents in areas with industrial, mining or military sites in Sardinia, Italy. Epidemiol. Prev. 2006, 30, 5–95. [Google Scholar]
- Frau, F.; Ardau, C.; Fanfani, L. Environmental geochemistry and mineralogy of lead at the old mine area of Baccu Locci (South-East Sardinia, Italy). J. Geochem. Explor. 2008, 100, 105–115. [Google Scholar] [CrossRef]
- Sanna, E.; Floris, G.; Vallascas, E. Town and gender effects on hair lead levels in children from three sardinian towns (Italy) with different environmental backgrounds. Biol. Trace Elem. Res. 2008, 124, 52–59. [Google Scholar] [CrossRef]
- Bartolucci, F.; Peruzzi, L.; Galasso, G.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora native to Italy. Plant Biosyst. 2024, 158, 219–296. [Google Scholar] [CrossRef]
- Galasso, G.; Conti, F.; Peruzzi, L.; Alessandrini, A.; Ardenghi, N.M.G.; Bacchetta, G.; Banfi, E.; Barberis, G.; Bernardo, L.; Bouvet, D.; et al. A second update to the checklist of the vascular flora alien to Italy. Plant Biosyst. 2024, 158, 297–340. [Google Scholar] [CrossRef]
- PPG, I. A community-derived classification for extant lycophytes and ferns. J. Syst. Evol. 2016, 54, 563–603. [Google Scholar] [CrossRef]
- Pignatti, S.; Guarino, R.; La Rosa, M. Flora d’Italia, 2nd ed.; Edagricole Calderini: Bologna, Italy, 2017. [Google Scholar]
- APG IV. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar]
- Raunkiaer, C. The Life Forms of Plants and Statistical Plant Geography; Clarendon Press: Oxford, UK, 1934. [Google Scholar]
- Pyšek, P.; Richardson, D.M.; Rejmánek, M.; Webster, G.L.; Williamson, M.; Kirschner, J. Alien plants in checklists and floras: Towards better communication between taxonomists and ecologists. Taxon 2004, 53, 131–143. [Google Scholar] [CrossRef]
- Orsenigo, S.; Fenu, G.; Gargano, D.; Montagnani, C.; Abeli, T.; Alessandrini, A.; Bacchetta, G.; Bartolucci, F.; Carta, A.; Castello, M.; et al. Red list of threatened vascular plants in Italy. Plant Biosyst. 2021, 155, 310–335. [Google Scholar] [CrossRef]
- Baker, A.J.M. Accumulators and Excluders Strategies in Response of Plants to Heavy Metals. J. Plant Nutr. 1981, 3, 643–654. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Floridasite. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Brooks, R.R. Plants that Hyperaccumulate Heavy Metals: Their Role in Phytoremediation, Microbiology, Archaeology, Mineral Exploration and Phyto-Mining; CAB International: Oxford, UK, 1998. [Google Scholar]
- Bacchetta, G.; Casti, M.; Zavattero, L. Analisi della vegetazione del distretto minerario di Montevecchio (Sardegna sud-occidentale). Fitosociologia 2007, 44, 83–108. [Google Scholar]
- Angius, R.; Bacchetta, G.; Pontecorvo, C. Floristic and vegetational features of Monte Marganai (SW Sardinia). In Biodiversity of Marganai and Montimannu (Sardinia). Research in the Framework of the ICP Forests Network; Nardi, G., Whitmore, D., Bardiani, M., Birtele, D., Mason, F., Spada, L., Cerretti, P., Eds.; Centro Nazionale per lo Studio e la Conservazione: Veron, Italy, 2011; Volume 1, pp. 57–132. [Google Scholar]
- Zavattero, L.; Casti, M.; Di Pietro, R.; Rosati, L.; Bacchetta, G. Analisi vegetazionale e geo-topologica dell’area mineraria di Monteponi (Iglesiente, Sardegna sud-occidentale). Inf. Bot. Ital. 2005, 37, 296–297. [Google Scholar]
- Bacchetta, G.; Brullo, S.; Cusma Velari, T.; Feoli Campiella, L.; Kosovel, V. Taxonomic Notes on the Genista ephedroides Group (Fabaceae) from the Mediterranean Area. Novon 2011, 21, 4–19. [Google Scholar] [CrossRef]
- Fois, M.; Cuena-Lombraña, A.; Boi, M.E.; McInnes, R.J.; Bacchetta, G. Changes to biodiversity and ecosystem services over time in post-mining and quarry ponds. Hydrobiologia, submitted.
- Bacchetta, G.; Cambria, S.; De Castro, O.; Fenu, G.; Brullo, S. Centranthus pontecorvi (Valerianaceae) a new species from Sardinia. Phytotaxa 2024, 661, 253–266. [Google Scholar] [CrossRef]
- Nirola, R.; Megharaj, M.; Aryal, R.; Naidu, R. Screening of metal uptake by plant colonizers growing on abandoned copper mine in Kapunda, South Australia. Int. J. Phytoremediation 2016, 18, 399–405. [Google Scholar] [CrossRef]
- Sinam, G.; Behera, S.K.; Mishra, R.K.; Sinha, S.; Mallick, S.; Khare, P.B. Comparison of two ferns (Adiantum capillus-veneris Linn. and Microsorium punctatum (Linn.) Copel) for their Cr accumulation potential and antioxidant responses. Int. J. Phytoremediation 2012, 14, 629–642. [Google Scholar] [CrossRef]
- Ramana, S.; Tripathi, A.K.; Kumar, A.; Dey, P.; Saha, J.K.; Patra, A.K. Phytoremediation of Soils Contaminated with Cadmium by Agave americana. J. Nat. Fibers 2021, 19, 4984–4992. [Google Scholar] [CrossRef]
- Pérez-de-Mora, A.; Madejón, E.; Burgos, P.; Cabrera, F. Trace element availability and plant growth in a mine-spill contaminated soil under assisted natural remediation. Sci. Total Environ. 2006, 363, 28–37. [Google Scholar] [CrossRef]
- Mohebzadeh, F.; Motesharezadeh, B.; Jafari, M.; Zare, S.; Aman, M.S. Remediation of heavy metal polluted soil by utilizing organic amendments and two plant species (Ailanthus altissima and Melia azedarach). Arab. J. Geosci. 2021, 14, 1211. [Google Scholar] [CrossRef]
- Widmer, J.; Norgrove, L. Identifying candidates for the phytoremediation of copper in viticultural soils: A systematic review. Environ. Res. 2023, 216, 114518. [Google Scholar] [CrossRef] [PubMed]
- Chaabani, S.; Abdelmalek-Babbou, C.; Ben Ahmed, H.; Chaabani, A.; Sebei, A. Phytoremediation Assessment of Native Plants Growing on Pb-Zn Mine Site in Northern Tunisia. Environ. Earth Sci. 2017, 76, 585–600. [Google Scholar] [CrossRef]
- Nahvi, H.; Torabian, S.; Hashemi, S.; Payam, H. Alnus glutinosa (Alder) sapling as a phytoremediator for cadmium in contaminated soil of industrial Park. Results Eng. 2024, 22, 102317. [Google Scholar] [CrossRef]
- Sipos, B.; Bibi, D.; Magura, T.; Tóthmérész, B.; Simon, E. High phytoremediation and translocation potential of an invasive weed species (Amaranthus retroflexus) in Europe in metal-contaminated areas. Environ. Monit. Assess. 2023, 195, 790. [Google Scholar] [CrossRef]
- Braglia, R.; Rugnini, L.; Malizia, S.; Scuderi, F.; Redi, E.L.; Canini, A.; Bruno, L. Exploiting the Potential in Water Cleanup from Metals and Nutrients of Desmodesmus sp. and Ampelodesmos mauritanicus. Plants 2021, 10, 1461. [Google Scholar] [CrossRef]
- Pratas, J.; Favas, P.J.; D’Souza, R.; Varun, M.; Paul, M.S. Phytoremedial assessment of flora tolerant to heavy metals in the contaminated soils of an abandoned Pb mine in Central Portugal. Chemosphere 2013, 90, 2216–2225. [Google Scholar] [CrossRef] [PubMed]
- Punetha, D.; Tewari, G.; Pande, C.; Kharkwal, G.C.; Tewari, K. Investigation on heavy metal content in common grown vegetables from polluted sites of Moradabad district, India. Indian Chem. Soc. 2015, 92, 97–103. [Google Scholar]
- Arsenov, D.; Župunski, M.; Pajević, S.; Borišev, M.; Nikolić, N.; Mimica-Dukić, N. Health assessment of medicinal herbs, celery and parsley related to cadmium soil pollution-potentially toxic elements (PTEs) accumulation, tolerance capacity and antioxidative response. Environ. Geochem. Health 2021, 43, 2927–2943. [Google Scholar] [CrossRef]
- Ning, W.; Yang, Y.; Chen, W.; Li, R.; Cao, M.; Luo, J. Effect of light combination on the characteristics of dissolved organic matter and chemical forms of Cd in the rhizosphere of Arabidopsis thaliana involved in phytoremediation. Ecotoxicol. Environ. Saf. 2022, 231, 113212. [Google Scholar] [CrossRef] [PubMed]
- Solis-Hernández, A.P.; Chávez-Vergar, B.M.; Aída, V.; Rodríguez-Tovar, A.V.; Beltrán-Paz, O.I.; Santillán, J.; Rivera-Becerril, F. Effect of the natural establishment of two plant species on microbial activity, on the composition of the fungal community, and on the mitigation of potentially toxic elements in an abandoned mine tailing. Sci. Total Environ. 2022, 802, 149788. [Google Scholar] [CrossRef]
- Vicente, C.S.; Pérez-Fernández, M.A. Broad environmental tolerance of native root-nodule bacteria of Biserrula pelecinus indicate potential for soil fertility restoration. Plant Ecol. Divers. 2016, 9, 299–307. [Google Scholar] [CrossRef]
- Lombini, A.; Poschenrieder, C.; Llugany, M.; Dinelli, E.; Barceló, J. Copper resistance in Silene armeria ecotypes: Does co-tolerance play a role? In Plant Nutrition; Springer: Dordrecht, The Netherland, 2001; pp. 456–457. [Google Scholar]
- Papazoglou, E.G.; Fernando, A.L. Preliminary studies on the growth, tolerance and phytoremediation ability of sugarbeet (Beta vulgaris L.) grown on heavy metal contaminated soil. Ind. Crop Prod. 2017, 107, 463–471. [Google Scholar] [CrossRef]
- Pistelli, L.; D’Angiolillo, F.; Morelli, E.; Basso, B.; Rosellini, I.; Posarelli, M.; Barbafieri, M. Response of Spontaneous Plants from an Ex-Mining Site of Elba Island (Tuscany, Italy) to Metal(loid) Contamination. Environ. Sci. Pollut. Res. 2017, 24, 7809–7820. [Google Scholar] [CrossRef]
- Evangelou, M.W.; Kutschinski-Klöss, S.; Ebel, M.; Schaeffer, A. Potential of Borago officinalis, Sinapis alba L. and Phacelia boratus for phytoextraction of Cd and Pb from soil. Water Air Soil Pollut. 2007, 182, 407–416. [Google Scholar] [CrossRef]
- Sajad, M.A.; Khan, M.S.; Ali, H. Lead phytoremediation potential of sixty-one plant species: An open field survey. Pure Appl. Biol. 2019, 8, 405–419. [Google Scholar] [CrossRef]
- Lin, L.; Shi, J.; Liu, Q.; Liao, M.A.; Mei, L. Cadmium accumulation characteristics of the winter farmland weeds Cardamine hirsuta Linn. and Gnaphalium affine D. Don. Environ. Monit. Assess. 2014, 186, 4051–4056. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ginés, M.J.; Pastor, J.; Hernández, A.J. Heavy metals in native mediterranean grassland species growing at abandoned mine sites: Ecotoxicological assessment and phytoremediation of polluted soils. In Heavy Metal Contamination of Soils. Soil Biology; Springer: Cham, Switzerland, 2015; pp. 159–178. [Google Scholar]
- Fernández, S.; Poschenrieder, C.; Marcenò, C.; Gallego, J.R.; Jiménez-Gámez, D.; Bueno, A.; Afif, E. Phytoremediation capability of native plant species living on Pb-Zn and Hg-As mining wastes in the Cantabrian range, north of Spain. Geochem. Explor. 2017, 174, 10–20. [Google Scholar] [CrossRef]
- Ebrahimi, M. Enhanced phytoremediation capacity of Chenopodium album L. grown on Pb-contaminated soils using EDTA and reduction of leaching risk. Soil Sediment. Contam. 2016, 25, 652–667. [Google Scholar] [CrossRef]
- Wu, S.; Yang, Y.; Qin, Y.; Deng, X.; Zhang, Q.; Zou, D.; Zeng, Q. Cichorium intybus L. is a potential Cd-accumulator for phytoremediation of agricultural soil with strong tolerance and detoxification to Cd. J. Hazard. Mater. 2023, 451, 131182. [Google Scholar] [CrossRef]
- El Mamoun, I.; Mouna, F.; Mohammed, A.; Najib, B.; Zine-El Abidine, T.; Abdelkarim, G.; Didier, B.; Laurent, L.; Abdelaziz, S. Zinc, Lead, and Cadmium Tolerance and Accumulation in Cistus libanotis, Cistus albidus, and Cistus salviifolius: Perspectives on Phytoremediation. Remediation 2020, 30, 73–80. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Santos, E.S.; Carvalho, L.C.; Abreu, M.M.; Andrade, M.L. Cistus monspeliensis L. as a potential species for rehabilitation of soils with multielemental contamination under Mediterranean conditions. ESPR 2018, 25, 6443–6455. [Google Scholar] [CrossRef]
- Abreu, M.M.; Santos, E.S.; Magalhães, M.C.F.; Fernandes, E. Trace Elements Tolerance, Accumulation and Translocation in Cistus populifolius, Cistus salviifolius and Their Hybrid Growing in Polymetallic Contaminated Mine Areas. J. Geochem. Explor. 2012, 123, 52–60. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Santos, E.S.; Abreu, M.M. Accumulation of Mn and Fe in Aromatic Plant Species from the Abandoned Rosalgar Mine and Their Potential Risk to Human Health. Appl. Geochem. 2019, 104, 42–50. [Google Scholar]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; Montes, M.; De la Rosa, G.; Corral-Diaz, B. Bioaccumulation of cadmium, chromium and copper by Convolvulus arvensis L.: Impact on plant growth and uptake of nutritional elements. Bioresour. Technol. 2004, 92, 229–235. [Google Scholar] [CrossRef]
- Onyia, P.C.; Ozoko, D.C.; Ifediegwu, S.I. Phytoremediation of arsenic-contaminated soils by arsenic hyperaccumulating plants in selected areas of Enugu State, Southeastern, Nigeria. Geol. Geogr. Ecol. 2021, 5, 308–319. [Google Scholar] [CrossRef]
- Millán, R.; Gamarra, R.; Schmid, T.; Sierra, M.J.; Quejido, A.J.; Sánchez, D.M.; Vera, R. Mercury content in vegetation and soils of the Almadén mining area (Spain). Sci. Total Environ. 2006, 368, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayaydeh, R.S.; Al-Hawadi, J.S.; Al-Habahbeh, K.A.; Al-Nawaiseh, M.B.; Albdaiwi, R.N.; Ayad, J.Y. Phytoremediation potential of selected ornamental woody species to heavy metal accumulation in response to long-term irrigation with treated wastewater. Water 2022, 14, 2086. [Google Scholar] [CrossRef]
- Lago-Vila, M.; Arenas-Lago, D.; Rodríguez-Seijo, A.; Andrade, M.L.; Vega, F.A. Ability of Cytisus scoparius for phytoremediation of soils from a Pb/Zn mine: Assessment of metal bioavailability and bioaccumulation. JEM 2019, 235, 152–160. [Google Scholar] [CrossRef]
- Brunetti, G.; Soler-Rovira, P.; Farrag, K.; Senesi, N. Tolerance and accumulation of heavy metals by wild plant species grown in contaminated soils in Apulia region, Southern Italy. Plant Soil 2009, 318, 285–298. [Google Scholar] [CrossRef]
- Mahdavian, K.; Ghaderian, S.M.; Torkzadeh-Mahani, M. Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. JSS 2017, 17, 1310–1320. [Google Scholar] [CrossRef]
- Baycu, G.; Tolunay, D.; Ozden, H.; Csatari, I.; Karadag, S.; Agba, T.; Rognes, S.E. An Abandoned Copper Mining Site in Cyprus and Assessment of Metal Concentrations in Plants and Soil. Int. J. Phytoremediation 2015, 17, 622–631. [Google Scholar] [CrossRef]
- González, H.; Fernández-Fuego, D.; Bertrand, A.; González, A. Effect of pH and citric acid on the growth, arsenic accumulation, and phytochelatin synthesis in Eupatorium cannabinum L., a promising plant for phytostabilization. Environ. Sci. Pollut. Res. 2019, 26, 26242–26253. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Carucci, A.; Lai, T.; Bacchetta, G.; Casti, M. Use of Native Species and Biodegradable Chelating Agent in Phytoremediation of Abandoned Mining Area. J. Chem. Technol. Biotechnol. 2009, 84, 884–889. [Google Scholar] [CrossRef]
- Massa, N.; Andreucci, F.; Poli, M.; Aceto, M.; Barbato, R.; Berta, G. Screening for heavy metal accumulators amongst autochtonous plants in a polluted site in Italy. Ecotoxicol. Environ. Saf. 2010, 73, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, M.J.; García-Lorenzo, M.L.; Pérez-Sirvent, C.; Bech, J. Trace element accumulation in plants from an aridic area affected by mining activities. J. Geochem. Explor. 2012, 123, 8–12. [Google Scholar] [CrossRef]
- Sinha, S.; Mishra, R.K.; Sinam, G.; Mallick, S.; Gupta, A.K. Comparative Evaluation of Metal Phytoremediation Potential of Trees, Grasses, and Flowering Plants from Tannery-Wastewater-Contaminated Soil in Relation with Physicochemical Properties. Soil Sediment Contam. 2013, 22, 958–983. [Google Scholar] [CrossRef]
- Bacchetta, G.; Boi, M.E.; Cappai, G.; Giudici, G.; Piredda, M.; Porceddu, M. Metal tolerance capability of Helichrysum microphyllum Cambess. subsp. tyrrhenicum Bacch., Brullo & Giusso: A candidate for phytostabilization in abandoned mine sites. Bull. Environ. Contam. Toxicol. 2018, 101, 758–765. [Google Scholar]
- Boi, M.E.; Medas, D.; Aquilanti, G.; Bacchetta, G.; Birarda, G.; Cappai, G.; Carlomagno, I.; Casu, M.A.; Gianoncelli, A.; Meneghini, C.; et al. Mineralogy and Zn Chemical Speciation in a Soil-Plant System from a Metal-Extreme Environment: A Study on Helichrysum microphyllum subsp. tyrrhenicum. Minerals 2020, 10, 259. [Google Scholar]
- Nematian, M.A.; Kazemeini, F. Accumulation of Pb, Zn, Cu and Fe in plants and hyperaccumulator choice in Galali iron mine area, Iran. Int. J. Agric. Crop Sci. 2013, 5, 426–432. [Google Scholar]
- Poschenrieder, P.; Bech, J.; Llugany, M.; Pace, A.; Fenés, E. Copper in Plant Species in a Copper Gradient in Catalonia (North East Spain) and Their Potential for Phytoremediation. Plant Soil 2001, 2, 247–256. [Google Scholar] [CrossRef]
- Moreira, H.; Marques, A.P.; Rangel, A.O.; Castro, P.M. Heavy Metal Accumulation in Plant Species Indigenous to a Contaminated Portuguese Site: Prospects for Phytoremediation. Water Air Soil Pollut. 2011, 221, 377–389. [Google Scholar] [CrossRef]
- Branković, S.; Glišić, R.; Topuzović, M.; Simić, Z.; Đekić, V.; Nenadović, N. The Bioacumulation Potential of Species Juncus articulatus L. in the Mine Drainage Water Basin and Flotation “Rudnik”, DOO, Serbia. Water Res. Manag. 2020, 10, 3–7. [Google Scholar]
- Pérez-Sirvent, C.; Hernández-Pérez, C.; Martínez-Sánchez, M.J.; García-Lorenzo, M.L.; Bech, J. Metal uptake by wetland plants: Implications for phytoremediation and restoration. JSS 2017, 17, 1384–1393. [Google Scholar] [CrossRef]
- Paniagua-López, M.; García-Robles, H.; Aguilar-Garrido, A.; Romero-Freire, A.; Lorite, J.; Sierra-Aragón, M. Vegetation establishment in soils polluted by heavy metal(loid)s after assisted natural remediation. Plant Soil 2024, 497, 257–275. [Google Scholar] [CrossRef]
- Ben Jeddau, K.; Vogiatzi, C.; Stamatakis, A.; Grigorakis, S.; Lydakis Simantiris, N. Heavy Metals Accumulation in Plant Tissues of Satureja cretica and Lathyrus ochrus Grown in Contaminated Soils. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017. [Google Scholar]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S.; et al. Flax (Linum usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [Google Scholar] [CrossRef]
- Akacha, B.B.; Michalak, M.; Romdhane, W.B.; Kačániová, M.; Saad, R.B.; Mnif, W.; Kukula-Koch, W.; Stefania Garzoli, S.; Hsouna, A.B. Recent advances in phytochemistry, pharmaceutical, biomedical, phytoremediation, and bio-preservative applications of Lobularia maritima. S. Afr. J. Bot. 2024, 165, 202–216. [Google Scholar] [CrossRef]
- Desjardins, D.; Brereton, N.J.; Marchand, L.; Brisson, J.; Pitre, F.E.; Labrecque, M. Complementarity of three distinctive phytoremediation crops for multiple-trace element contaminated soil. Sci. Total Environ. 2018, 610, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Ginés, M.J.; Hernández, A.J.; Pastor, J. Impacts of soil-soluble anions on wild cultivated herbaceous species: Implications for soil phytoremediation. J. Soil Sci. Plant Nutr. 2016, 16, 423–437. [Google Scholar] [CrossRef]
- Safronova, V.I.; Piluzza, G.; Zinovkina, N.Y.; Kimeklis, A.K.; Belimov, A.A.; Bullitta, S. Relationships between pasture legumes, rhizobacteria and nodule bacteria in heavy metal polluted mine waste of SW Sardinia. Symbiosis 2012, 58, 149–159. [Google Scholar] [CrossRef]
- Bashir, S.K.; Irshad, M.; Bacha, A.U.R.; An, P.; Faridullah, F.; Ullah, Z. Investigation of heavy metals uptake in root-shoot of native plant species adjoining wastewater channels. Environ. Monit. Assess. 2024, 196, 6. [Google Scholar] [CrossRef]
- Aloud, S.S.; Alotaibi, K.D.; Almutairi, K.F.; Albarakah, F.N. Assessment of Heavy Metals Accumulation in Soil and Native Plants in an Industrial Environment, Saudi Arabia. Sustainability 2022, 14, 5993. [Google Scholar] [CrossRef]
- Perlein, A.; Bert, V.; de Souza, M.F.; Papin, A.; Meers, E. Field evaluation of industrial non-food crops for phytomanaging a metal-contaminated dredged sediment. Environ. Sci. Pollut. Res. 2023, 30, 44963–44984. [Google Scholar] [CrossRef]
- Midhat, L.; Ouazzani, N.; Aziz, F.; Esshaimi, M.; Hejjaj, A.; Mandi, L. Screening of new native metallophytes from copper abandoned mining site: Promising tool for phytoremediation. Land Degrad. Dev. 2023, 34, 3700–3711. [Google Scholar] [CrossRef]
- Manousaki, E.; Kalogerakis, N. Halophytes present new opportunities in phytoremediation of heavy metals and saline soils. Ind. Eng. Chem. Res. 2011, 50, 656–660. [Google Scholar] [CrossRef]
- Márquez-García, B.; Córdoba, F. Antioxidative System in Wild Populations of Erica andevalensis. Environ. Exp. Bot. 2010, 68, 58–65. [Google Scholar] [CrossRef]
- Stavi, I. Ecosystem services related with Opuntia ficus-indica (prickly pear cactus): A review of challenges and opportunities. ASFS 2022, 46, 815–841. [Google Scholar] [CrossRef]
- Benhabylès, L.; Djebbar, R.; Miard, F.; Nandillon, R.; Morabito, D.; Bourgerie, S. Biochar and compost effects on the remediative capacities of Oxalis pes-caprae L. growing on mining technosol polluted by Pb and As. ESPR 2020, 27, 30133–30144. [Google Scholar] [CrossRef]
- Shu, W.S.; Ye, Z.H.; Lan, C.Y.; Zhang, Z.Q.; Wong, M.H. Lead, zinc and copper accumulation and tolerance in populations of Paspalum distichum and Cynodon dactylon. Environ. Pollut. 2002, 120, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Kharazian, P.; Bacchetta, G.; Cappai, G.; Piredda, M.; Giudici, G. An Integrated Geochemical and Mineralogical Investigation on Soil-Plant System of Pinus halepensis Pioneer Tree Growing on Heavy Metal Polluted Mine Tailing. Plant Biosyst. 2022, 157, 272–285. [Google Scholar] [CrossRef]
- Kharazian, P.; Cappai, G.; Boi, M.E.; Porceddu, M.; Piredda, M.; De Giudici, G.; Bacchetta, G. Greenhouse investigation on the phytoremediation potential of pioneer tree Pinus halepensis Mill. in abandoned mine site. Int. J. Phytoremediation 2023, 26, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Heckenroth, A.; Rabier, J.; Dutoit, T.; Torre, F.; Prudent, P.; Laffont-Schwob, I. Selection of native plants with phytoremediation potential for highly contaminated Mediterranean soil restoration: Tools for a non-destructive and integrative approach. JEM 2016, 183, 850–863. [Google Scholar] [CrossRef]
- González, R.C.; González-Chávez, M.C.A. Metal accumulation in wild plants surrounding mining wastes. Environ. Pollut. 2006, 144, 84–92. [Google Scholar] [CrossRef]
- Saghi, A.; Rashed Mohassel, M.H.; Parsa, M.; Hammami, H. Phytoremediation of lead-contaminated soil by Sinapis arvensis and Rapistrum rugosum. Int. J. Phytoremediation 2016, 18, 387–392. [Google Scholar] [CrossRef]
- Hasnaoui, S.E.; Fahr, M.; Keller, C.; Levard, C.; Angeletti, B.; Chaurand, P.; Triqui, Z.E.A.; Guedira, A.; Rhazi, L.; Colin, F.; et al. Screening of Native Plants Growing on a Pb/Zn Mining Area in Eastern Morocco: Perspectives for Phytoremediation. Plants 2020, 9, 1458. [Google Scholar] [CrossRef]
- Chirilă Băbău, A.M.; Micle, V.; Damian, G.E.; Sur, I.M. Lead and copper removal from sterile dumps by phytoremediation with Robinia pseudoacacia. Sci. Rep. 2024, 14, 9842. [Google Scholar] [CrossRef]
- Afonso, T.F.; Demarco, C.F.; Pieniz, S.; Quadro, M.S.; Camargo, F.A.; Andreazza, R. Bioprospection of indigenous flora grown in copper mining tailing area for phytoremediation of metals. JEM 2020, 256, 109953. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Moreira, H.; Rangel, A.O.; Castro, P.M. Arsenic, lead and nickel accumulation in Rubus ulmifolius growing in contaminated soil in Portugal. J. Hazard. Mater. 2009, 165, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Barrutia, O.; Garbisu, C.; Hernández-Allica, J.; García-Plazaola, J.I.; Becerril, J.M. Differences in EDTA-assisted metal phytoextraction between metallicolous and non-metallicolous accessions of Rumex acetosa L. Environ. Pollut. 2010, 158, 1710–1715. [Google Scholar] [CrossRef]
- Zhuang, P.; Yang, Q.W.; Wang, H.B.; Shu, W.S. Phytoextraction of heavy metals by eight plant species in the field. Water Air Soil Pollut. 2007, 184, 235–242. [Google Scholar] [CrossRef]
- Ieviņa, S.; Karlsons, A.; Osvalde, A.; Andersone-Ozola, U.; Ievinsh, G. Coastal wetland species Rumex hydrolapathum: Tolerance against flooding, salinity, and heavy metals for its potential use in phytoremediation and environmental restoration technologies. Life 2023, 13, 1604. [Google Scholar] [CrossRef]
- Urošević, J.; Stanković, D.; Jokanović, D.; Trivan, G.; Rodzkin, A.; Jović, Đ.; Jovanović, F. Phytoremediation Potential of Different Genotypes of Salix alba and S. viminalis. Plants 2024, 13, 735. [Google Scholar] [CrossRef]
- Medina-Díaz, H.L.; López-Bellido, F.J.; Alonso-Azcárate, J.; Fernández-Morales, F.J.; Rodríguez, L. A new hyperaccumulator plant (Spergularia rubra) for the decontamination of mine tailings through electrokinetic-assisted phytoextraction. Sci. Total Environ. 2024, 912, 169543. [Google Scholar] [CrossRef]
- Anishchenko, O.V.; Tolomeev, A.P.; Ivanova, E.A.; Drobotov, A.V.; Kolmakova, A.A.; Zuev, I.V.; Gribovskaya, I.V. Accumulation of elements by submerged (Stuckenia pectinata (L.) Börner) and emergent (Phragmites australis (Cav.) Trin. ex Steud.) macrophytes under different salinity levels. PPB 2020, 154, 328–340. [Google Scholar] [CrossRef]
- Marchiol, L.; Fellet, G.; Boscutti, F.; Montella, C.; Mozzi, R.; Guarino, C. Gentle remediation at the former “Pertusola Sud” zinc smelter: Evaluation of native species for phytoremediation purposes. Ecol. Eng. 2013, 53, 343–353. [Google Scholar] [CrossRef]
- Escarré, J.; Lefèbvre, C.; Raboyeau, S.; Dossantos, A.; Gruber, W.; Cleyet Marel, J.C.; Frérot, H.; Noret, N.; Mahieu, S.; Collin, C.; et al. Heavy metal concentration survey in soils and plants of the Les Malines mining district (Southern France): Implications for soil restoration. Water Air Soil Pollut. 2011, 216, 485–504. [Google Scholar] [CrossRef]
- Accogli, R.; Tomaselli, V.; Direnzo, P.; Perrino, E.V.; Albanese, G.; Urbano, M.; Laghetti, G. Edible halophytes and halo-tolerant species in Apulia region (Southeastern Italy): Biogeography, traditional food use and potential sustainable crops. Plants 2023, 12, 549. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Llugany, M.; Lombini, A.; Dinelli, E.; Bech, J.; Barceló, J. Smilax aspera L. an evergreen Mediterranean climber for phytoremediation. J. Geochem. Explor. 2012, 123, 41–44. [Google Scholar] [CrossRef]
- Zu, Y.; Li, Y.; Chen, J.; Chen, H.; Li, Q.; Schvartz, C. Hyperaccumulation of Pb, Zn and Cd in herbaceous grown on lead–zinc mining area in Yunnan, China. Environ. Int. 2005, 31, 755–762. [Google Scholar]
- Santos, E.S.; Abreu, M.M.; Peres, S.; Magalhães, M.C.F.; Leitão, S.; Santos Pereira, A.; Cerejeira, M.J. Potential of Tamarix africana and Other Halophyte Species for Phytostabilisation of Contaminated Salt Marsh Soils. J. Soils Sediments 2017, 17, 1459–1473. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.; Peñalosa, J.M.; Esteban, E.; Pilar Bernal, M. Feasibility of Arsenic Phytostabilisation Using Mediterranean Shrubs: Impact of Root Mineralisation on As Availability in Soils. J. Environ. Monit. 2009, 11, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Vural, A. Trace element accumulation behavior, ability, and propensity of Taraxacum officinale FH Wigg (Dandelion). Environ. Sci. Pollut. Res. 2024, 31, 16667–16684. [Google Scholar] [CrossRef]
- Mohamed, H.; Mohamed, H.; Mostefa, T.; Yasmina, B.; Ayoub, K. Phytoremediation potential of spontaneous plant species in soils contaminated by hexavalent chromium in Djelfa city (Algeria). Res. J. Chem. Environ. 2022, 26, 66–74. [Google Scholar]
- Pajuelo, E.; Carrasco, J.A.; Romero, L.C.; Chamber, M.A.; Gotor, C. Evaluation of the metal phytoextraction potential of crop legumes. Regulation of the expression of O-acetylserine (thiol) lyase under metal stress. Plant Biol. 2007, 9, 672–681. [Google Scholar] [CrossRef]
- Panich-Pat, T.; Upatham, S.; Pokethitiyook, P.; Kruatrachue, M.; Lanza, G.R. Phytoextraction of metal contaminants by Typha angustifolia: Interaction of lead and cadmium in soil-water microcosms. J. Environ. Prot. 2010, 1, 431. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boi, M.E.; Sarigu, M.; Fois, M.; Casti, M.; Bacchetta, G. The First Inventory of Sardinian Mining Vascular Flora. Plants 2025, 14, 1225. https://doi.org/10.3390/plants14081225
Boi ME, Sarigu M, Fois M, Casti M, Bacchetta G. The First Inventory of Sardinian Mining Vascular Flora. Plants. 2025; 14(8):1225. https://doi.org/10.3390/plants14081225
Chicago/Turabian StyleBoi, Maria Enrica, Marco Sarigu, Mauro Fois, Mauro Casti, and Gianluigi Bacchetta. 2025. "The First Inventory of Sardinian Mining Vascular Flora" Plants 14, no. 8: 1225. https://doi.org/10.3390/plants14081225
APA StyleBoi, M. E., Sarigu, M., Fois, M., Casti, M., & Bacchetta, G. (2025). The First Inventory of Sardinian Mining Vascular Flora. Plants, 14(8), 1225. https://doi.org/10.3390/plants14081225








