Comparison of Phytolith Characteristics of Three Bamboo Species’ Cotyledon Organs
Abstract
1. Introduction
2. Results
2.1. Phytolith Content and Concentration Characteristics
2.2. Phytolith Particle Size Characteristics
2.3. Phytolith Morphology and Assemblages
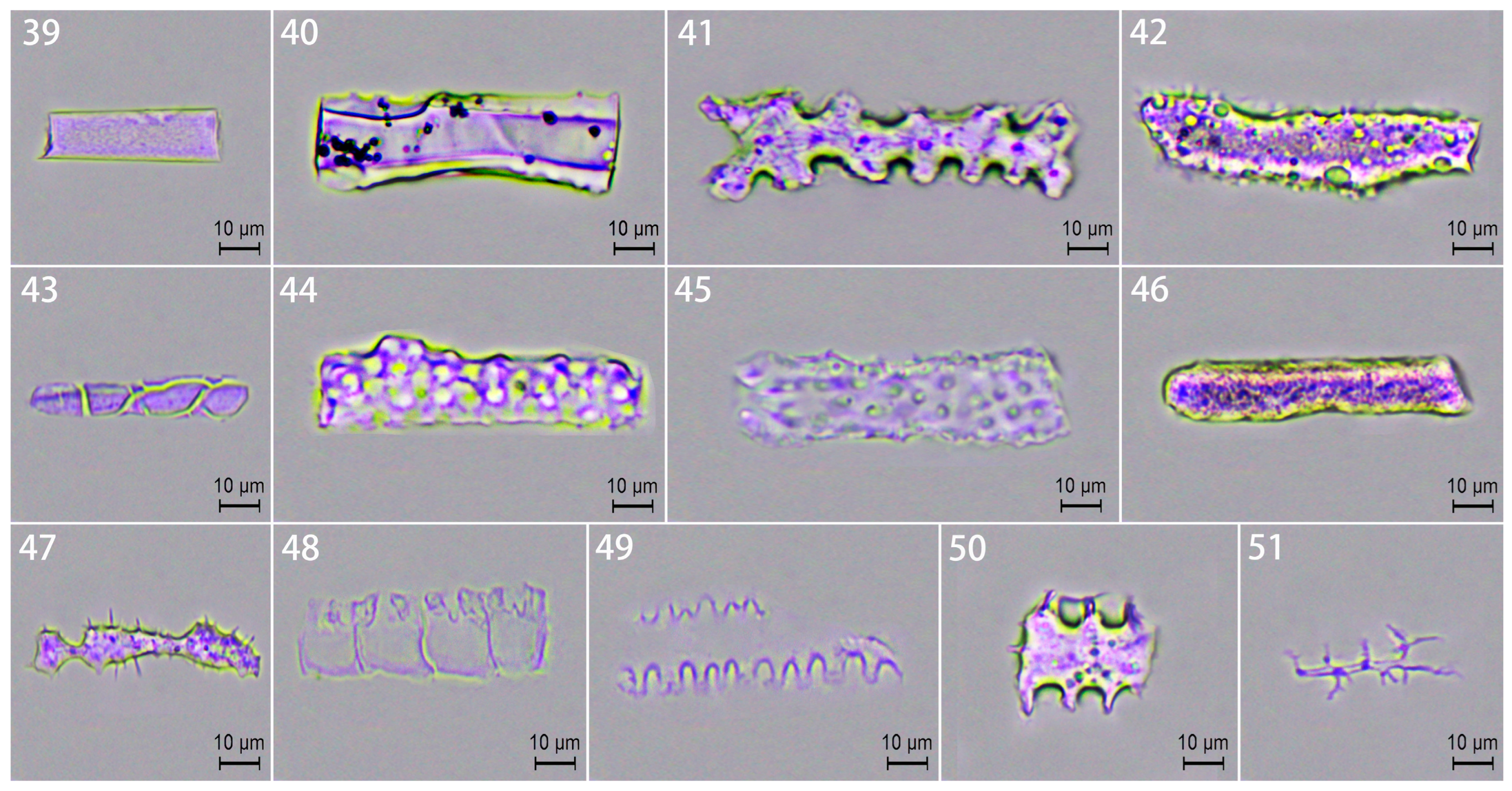
2.3.1. Phytolith Morphotypes
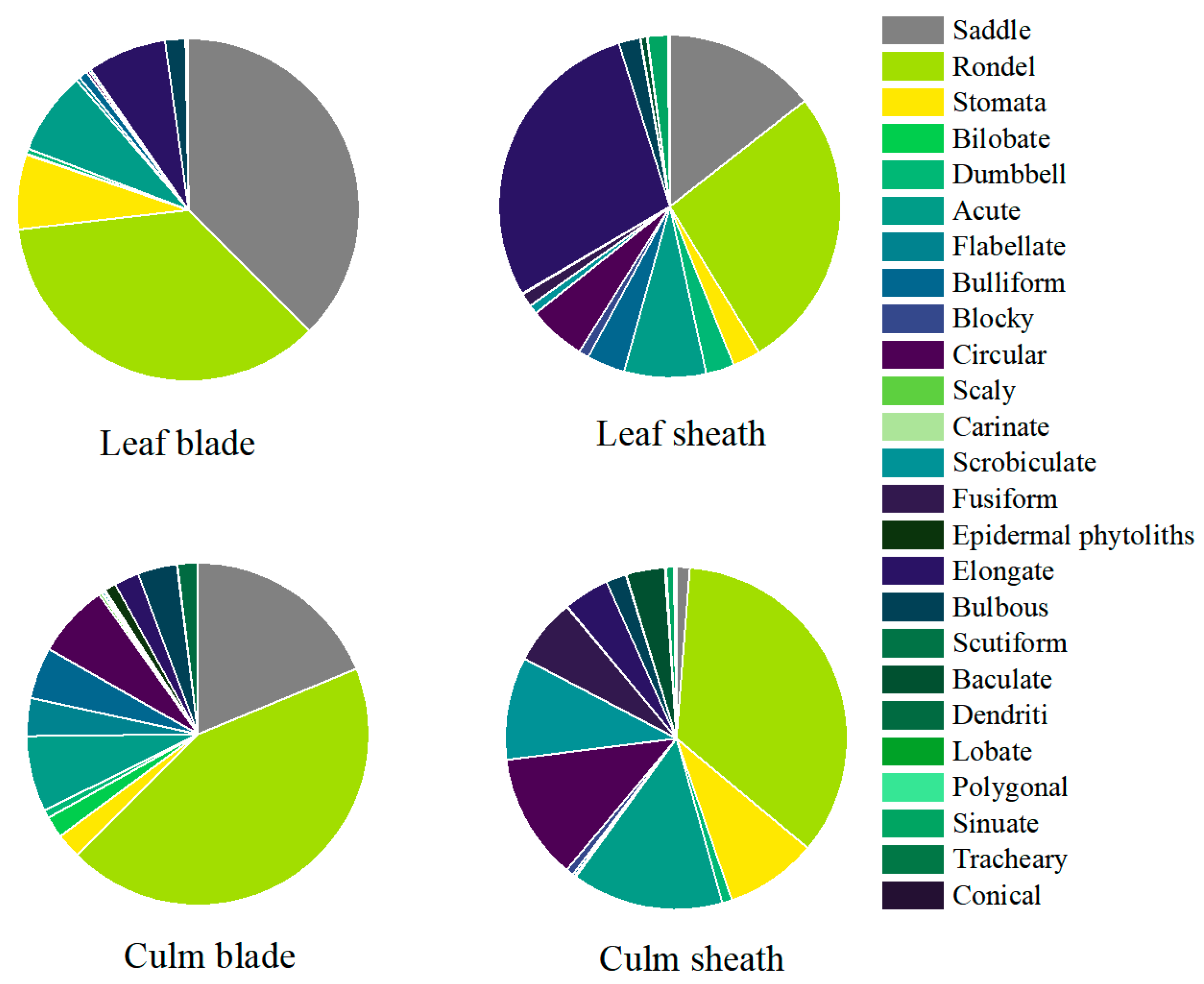
2.3.2. Phytolith Assemblages
2.3.3. Long-Saddle Phytolith Parameters
3. Discussion
3.1. Variation in Phytolith Content and Concentration in Different Organs of Different Bamboo Species
3.2. Functional Relationships Between Phytolith Morphology and Proportion in Bamboo Leaf Organs
3.3. Ecological Significance of Significant Differences in Phytoliths Among Different Bamboo Species and Organs
4. Materials and Methods
4.1. Regional Setting
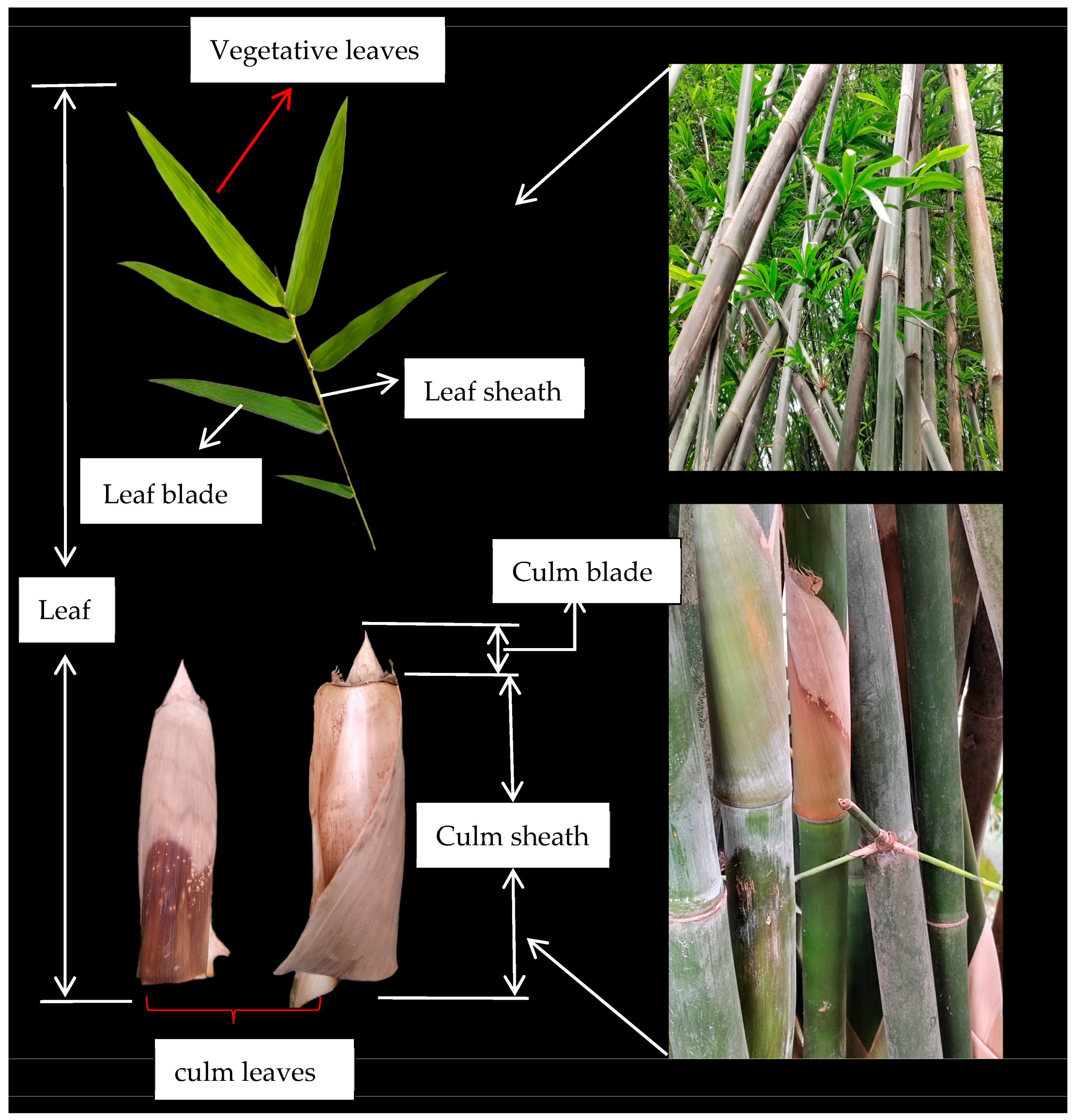
4.2. Sampling
4.3. Phytolith Extraction
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Minden, V.; Schaller, J.; Olde, V.H. Plants increase silicon content as a response to nitrogen or phosphorus limitation: A case study with Holcus lanatus. Plant Soil 2021, 462, 95–108. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W. Schiavon Physiological functions of beneficial elements. Curr. Planbiol. 2009, 12, 267–274. [Google Scholar]
- Wang, L.; Tao, L.; Xie, M. Dehydrogenation and aromatization of methane under non-oxidizing condition. Catal. Lett. 1993, 21, 35–41. [Google Scholar] [CrossRef]
- Piperno, D.R. Phytolith Analysis: An Archaeological and Geological Perspective; Academic Press: San Diego, CA, USA, 2006. [Google Scholar]
- Hyland, E. Phytolith as Tracers of Recent Environmental Change; Academic Press: San Diego, CA, USA, 2006. [Google Scholar]
- Madella, M.; Alexandre, A.; Ball, T. International Code for Phytolith Nomenclature 1.0. Ann. Bot. 2005, 96, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Han, J. Phytoliths in modern plants from Amazonia and the Neotropics: A reference collection for paleoecological and archaeological reconstructions. Exp. App Under Foss. Organi. Lessons Liv. 2014, 41, 207. [Google Scholar]
- Liu, H.Y.; Liu, L.D.; Liu, H.Y. Soil phytolith assemblages and its preservation characteristics under typical plant communities in the midsubtropical zone of China. Quat. Res. 2024, 44, 563–578. [Google Scholar]
- Li, Q.; Xu, D.K.; Lü, H.Y. Morphology of phytolith in bambusoideae (gramineae) and its ecological sign if icance. Quat. Sci. 2005, 25, 777–784. [Google Scholar]
- Liang, Y.C.; Zhang, Y.C.; Ma, T.S. Silicon Nutrition in plants. Adv. Soil Sci. 1993, 21, 7–14. [Google Scholar]
- Gao, D.; Chen, J.L.; Chai, K.Z. Distribution and absorption of silicon in plant and its role in piant disease resistance under environmental stress. Acta Ecol. Sin. 2010, 30, 2745–2755. [Google Scholar]
- Wang, Y.J. A introduction of study on plant opal and its uses. J. Oceanogr. Huanghai Bohai Seas 1989, 7, 66–68. [Google Scholar]
- Li, Z.M.; Song, Z.L.; Jiang, P.K. The production and accumulation of phytoliths in rice ecosystems: A case study to Jiaxing paddy field. Acta Ecol. Sin. 2013, 33, 7197–7203. [Google Scholar]
- Jiang, P.K.; Meng, C.F.; Zhou, G.M. Comparative study of carbon storage in different forest stands in subtropical China. Bot. Rev. 2011, 77, 242–251. [Google Scholar] [CrossRef]
- Metcalfe, C.R. Gramineae. In Anatomy of the Monocotyledons; OUP: Oxford, UK, 1960. [Google Scholar]
- Chatterji, R.; Raizada, M.B. Culm-sheaths as aid to identification of bamboos. Phys. Med. Biol. 1963, 57, 6519–6540. [Google Scholar]
- Wang, K.M. Bamboo, the Amazing Grass, an Introduction to the Diversity and Study of Southeast Asian Bamboos; UPM Post Office: Kuala Lumpur, Malaysia, 2004. [Google Scholar]
- Wang, S.J. Bamboo heath: A modified branch based on the anatomical observations. Sci. Rep. 2017, 7, 16132–16470. [Google Scholar] [CrossRef]
- Strömberg, C.A.; Di Stilio, V.S.; Song, Z. Functions of phytoliths in vascular plants: An evolutionary perspective. Function. Ecolo. 2016, 30, 1286–1297. [Google Scholar] [CrossRef]
- Liese, W.; Kohl, M. Bamboo: The plant and its uses. J. Am. Bamboo Soc. 2015, 40, 1–30. [Google Scholar]
- Gu, H.J.; Zhang, S.S.; Wang, J.S. Comparison of basic morphological and functional traits of bamboos in China. Biodiversity 2019, 27, 10. [Google Scholar]
- Zhou, H.M.; Hu, X.B.; Yuan, C.H.; Yang, L.; Liu, W. Investigation on Fargesia dracocephala in changing nature reserve dedicating togiant panda habitat. Shaanxi For. Sci. Tech. 2015, 210, 33–35. [Google Scholar]
- Tan, H.C.; Li, R.; Tan, R.Q. Study on tissue culture and rapid propagation technology of Bambusa vulgaris schrader extend land. World Bamboo Ratt. 2017, 15, 23–26+36. [Google Scholar]
- Huang, H. Preliminary study on the age structure of Fargesia Dracocephala population in Bai shui jiang nature reserve. Sci. Silvae Sin. 1991, 27, 268–273. [Google Scholar]
- Shi, J.Y.; Zhou, D.Q.; Zhang, Y.X.; Ma, L.S.; Yao, J. On the bamboo cultivars from Bambusa around the world. World Bamboo Ratt. 2019, 17, 57–63. [Google Scholar]
- He, R.; Qiu, J.; Luo, B.; Deng, Y. Developmental changes and morphology of phytolith in Bambusa emeiensis. J. Northwest A F Univ. 2018, 46, 69–84. [Google Scholar]
- International Committee for Phytolith Taxonomy (ICPT). International code for phytolith nomenclature (ICPN)2.0. Ann. Bot. 2019, 124, 189–199. [Google Scholar] [CrossRef]
- Rashid, I.; Mir, S.H.; Zurro, D. Phytolith as proxies of the past. Earth-Sci. Rev. 2019, 194, 234–250. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.F.; Huang, Z.T.; Jiang, P.K.; Xiang, T.T.; Yin, Y.Q. Determination of Phytolith-occluded Carbon Content Using Alkali Dissolution-Spectrophotometry. Chin. J. Anal. Chem. 2014, 42, 1389–1390. [Google Scholar]
- Yang, J.; Wu, J.; Jiang, P.; Xu, Q.; Zhao, P.; He, S. A study of phytolith-occluded carbon stock in monopodial bamboo in China. Sci. Rep. 2015, 5, 13292. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Song, Z.L.; Jiang, P.K.; Zhou, G.M.; Li, Z.M. Phytolith distribution and carbon sequestration in China with Phyllostachys edulis. J. Zhejiang A F Univ. 2014, 31, 547–553. [Google Scholar]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phylogenetic variation in the silicon composition of plants. Ann. Bot. 2005, 96, 1027–1046. [Google Scholar] [CrossRef]
- Zhu, F.W.; Niu, Z.H.; Li, J.; Yu, L.X.; Wang, S.G.; Wang, C.M.; Zhan, H. Variation of the Phytolith Content and Morphology of Dendrocalamus giganteus at Different Phenological Periods. J. Southwest For. Univ. 2021, 42, 71–77. [Google Scholar]
- Liu, L.D.; Je, D.M.; Liu, H.Y.; Guo, M.E.; Li, N.N. Change characters of Phragmites australis phytolith in Northeast China. Chin. J. Plant Ecolo. 2013, 37, 861–871. [Google Scholar] [CrossRef]
- Bian, Y.; Pan, X.L. Observation of plant spit water phenomenon. Plants. J. 1995, 5, 38. [Google Scholar]
- Yan, J.P.; Wei, Y.C. Research to the law of summer corn guttation. J. Henan Sci. Tech. Agri. Coll. 1985, 13, 58–65. [Google Scholar]
- Xiao, Q.M.; Ma, X.Q.; Lou, C.R.; Sun, W.T.; Fu, Z.X. The research to relationship between the stage nutrition of silicon and effective. Soil. Sci. 1999, 30, 185–188. [Google Scholar]
- Li, Z.M.; Song, Z.L.; Liu, H.Y. Distribution of phytolith in bamboos and its environmental implications. Env. Earth Sci. 2013, 68, 2159–2167. [Google Scholar]
- Liese, W. The Anatomy of Bamboo Culms; Brill: Leiden, The Netherlands, 1998. [Google Scholar]
- Hodson, M.J.; White, P.J.; Mead, A.; Broadley, M.R. Phytoliths in grasses and their potential for studying plant-based processes. New Phytol. 2005, 167, 725–745. [Google Scholar]
- Gu, X.; Ding, Y.; Du, F. Morphological and anatomical adaptations of bamboo culm sheaths to their protective function. J. Plant Res. 2012, 125, 207–216. [Google Scholar]
- Gu, Y.S.; Jie, Y.B.; Guan, S.; Liu, H.Y.; Mi, Y.C.; Wang, H.L.; Li, R.C. An introduction to the research and application of phytolith morphometrics. Quat. Res. 2019, 39, 12–23. [Google Scholar]
- Zhang, H. Silicon Distribution and Phytolith Morphology in the Karst Critical Zone of Southwestern China. Ph.D. Thesis, Tianjin University, Tianjing, China, 2020. [Google Scholar]
- Li, R.C.; Fan, J.; Gao, C.G. Advances in Modern Phytolith Research. Adv. Earth Sci. 2013, 28, 1287–1295. [Google Scholar]
- Baker, S. The ecological significance of phytoliths in plants. Plant Ecol. 2006, 183, 99–110. [Google Scholar]
- Madella, M.; Jones, M.K.; Echlin, P. Plant water availability and analytical microscopy of phytoliths: Implications for ancient irrigation in arid zones. Quatern Int. 2009, 193, 32–40. [Google Scholar] [CrossRef]
- Liu, Y. Silica bodies in bamboo: Distribution and environmental correlation. J. Plant Sci. 2015, 78, 235–245. [Google Scholar]
- Motomura, H.; Fujii, T.; Suzuki, M. Silica deposition in relation to ageing of leaf tissues in Sasav eitchii (Carrière) Rehder (Poace-ae: Bambusoideae). Ann. Bot. 2004, 93, 235–248. [Google Scholar] [CrossRef]
- Lux, A.; Luxová, M.; Abe, J.; Morita, S.; Inanaga, S. Silicification of bamboo (Phyllostachys heterocycla Mitf.) root and leaf. Plant Soil 2003, 255, 85–91. [Google Scholar] [CrossRef]
- Xu, R.; He, H.L.; Guo, J.H.; Zhu, F.W.; Wang, S.G.; Dai, C.F.; Zheng, X.F.; Xie, D.B.; Li, H.M.; Wang, C.M.; et al. Characteristics of silicon and phytolith distribution in bamboo (Ferrocalamus strictus): Variations between different organs and ages. Rev. Palaeobot. Palynol. 2023, 311, 104817. [Google Scholar] [CrossRef]
- Jin, D.K.; Lu, Z.; Wang, S.G.; Long, H.; Zhang, C.; Wang, S.H. Comparison of anatomical structure of six bamboo species cotyedon organs. J. Nanjing For. Univ. 2023, 47, 109–120. [Google Scholar]
- Obón, C.; Rivera, D. Phytoliths (a comprehensive guide for archaeologist and paleoecologists). Econ. Bot. 2006, 60, 391–392. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H. Phytolith Study and It’s Application. Ocean Press: Beijing, China, 1993. [Google Scholar]
- Li, R.; Fan, J.; Carter, J.; Jiang, N.; Gu, Y.S. Monthly variations of phytoliths in the leaves of the bamboo Dendrocalamus ronganensis (Poaceae: Bambusoideae). Rev. Palaeobot. Palyno. 2017, 24, 62–69. [Google Scholar] [CrossRef]
- Zhan, H. Morphological Characteristics of Phytoliths in 10 Clumping Bamboo Species and the Effects of Exogenous Silicon on Bamboo Seedling Cold Resistance. Ph.D. Thesis, Southwest Forestry University, Kunming, China, 2017. [Google Scholar]
- Zhang, T.; Li, P.; Liu, M. Functional analysis of silica bodies in bamboo leaves and their ecological implications. Ecol. Plant Biol. 2021, 29, 203–211. [Google Scholar]
- Wang, X.; Xu, R.; Zheng, X.F.; Wang, C.M.; Zhou, J.H.; Duan, S.Y. Phytolith Morphological Differences of Bamboo Culm Leaf in Phyllostachys edulis and Two Variants. J. West China For. Sci. 2024, 53, 46–52. [Google Scholar]
- Dai, C.F.; Xu, R.; Zhu, F.W.; Li, M.B.; Li, J.; Wang, S.G.; Wang, C.M.; Zhan, H. Silicon uptake and phytolith morphology in Dendrocalamus brandisii seedling leaf from different rearing methods. Forests 2023, 14, 1877. [Google Scholar] [CrossRef]
- Tao, X.; Wen, M.; Li, R.C.; Vachula, R.S.; Pang, L.N. Phytolith sizes and assemblages differentiate genera and ecotypes of woody bamboos in subtropical Southwest China. Rev. Palaeobot. Palynol. 2020, 272, 104129. [Google Scholar] [CrossRef]
- Samuels, G.J.; Gauthier, G.M. Silicon and its role in plant physiology and anatomy: A review. Ann. Bot. 2012, 90, 1093–1115. [Google Scholar]
- He, R.; Qiu, J.; Luo, B.; Deng, Y. Developmental changes and morphology of phytolith in Bambusa emeiensis. J. Northwest A F Univ. 2018, 46, 69–84. [Google Scholar]
- Chen, W.; Zhang, X.; Li, Y. The role of silica bodies in bamboo leaves and their ecological significance. Plant Sci. J. 2016, 118, 312–320. [Google Scholar]
- Li, X.; Wang, F.; Liu, J. Morphological variation of silica bodies in different bamboo species. J. Bamboo Res. 2019, 34, 45–52. [Google Scholar]
- Wang, Q.; Chen, Z.; Liu, P. Genetic factors influencing silica body formation in bamboo. Czech J. Genet. Plant Breed. 2020, 48, 143–152. [Google Scholar]
- Zhao, L.H.; Huang, C.P.; Wang, Y.Y.; Huang, Z.T. Stability of Phyllostachys edulis phytolith by scanning electron microscopy. J. Zhejiang For. Univ. 2018, 35, 1177–1181. [Google Scholar]




| Bamboo Species | Leaf Blade (g/kg) | Leaf Sheath (g/kg) | Culm Blade (g/kg) | Culm Sheath (g/kg) | Means (g/kg) |
|---|---|---|---|---|---|
| Bambusa vulgaris | 23.22 ± 0.46 Ab | 43.13 ± 5.15 Ab | 37.06 ± 4.37 Ab | 13.17 ± 3.09 Cc | 29.15 ± 12.64 b |
| Bambusa tulda | 70.54 ± 10.44 Aa | 81.99 ± 11.55 Aa | 74.92 ± 4.17 Aa | 37.07 ± 4.19 Ba | 66.13 ± 19.38 a |
| Bambusa dolichoclada | 63.08 ± 23.71 Aab | 79.69 ± 9.58 Aa | 96.35 ± 19.29 Aa | 29.96 ± 0.60 Cb | 67.27 ± 29.05 a |
| Means (g/kg) | 52.28 ± 19.99 A | 68.27 ± 20.48 A | 64.83 ± 34.09 A | 26.73 ± 10.94 B | / |
| Bamboo Species | Leaf Blade (×106 Particles g−1) | Leaf Sheath (×106 Particles g−1) | Culm Blade (×106 Particles g−1) | Culm Sheath (×106 Particles g−1) | Means (×106 Particles g−1) |
|---|---|---|---|---|---|
| Bambusa vulgaris | 18.73 | 32.26 | 13.86 | 5.33 | 17.56 |
| Bambusa tulda | 19.19 | 19.45 | 10.56 | 9.93 | 13.28 |
| Bambusa dolichoclada | 11.06 | 14.49 | 23.61 | 3.29 | 13.11 |
| Means (×106 particles g−1) | 16.33 | 22.07 | 16.01 | 6.18 | / |
| Phytolith Assemblage | Bambusa vulgaris | Bambusa tulda | Bambusa dolichoclada | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf Sheath (%) | Leaf Blade (%) | Culm Blade (%) | Culm Sheath (%) | Leaf Sheath (%) | Leaf Blade (%) | Culm Blade (%) | Culm Sheath (%) | Leaf Sheath (%) | Leaf Blade (%) | Culm Blade (%) | Culm Sheath (%) | |
| Saddle | 9.53 | 32.31 | 16.85 | / | 14.33 | 46.78 | 6.92 | 1.00 | 20.19 | 33.25 | 31.03 | 2.48 |
| Rondel | 23.52 | 37.91 | 43.11 | 25.00 | 35.91 | 30.81 | 60.67 | 36.16 | 17.27 | 38.62 | 28.9 | 41.9 |
| Stomata | 1.69 | 14.08 | 3.72 | 10.55 | 4.28 | 2.35 | 2.77 | 4.99 | 1.22 | 2.94 | 0.89 | 9.9 |
| Bilobate | / | / | / | / | / | / | 0.59 | / | / | 0.38 | 4.96 | / |
| Dumbbell | 1.06 | 0.36 | 0.44 | 0.69 | 4.12 | 0.72 | 1.38 | 1.75 | 2.43 | 0.38 | 0.53 | 0.57 |
| Acute | 6.78 | 6.59 | 14.00 | 11.01 | 6.75 | 2.97 | 0.99 | 4.99 | 10.22 | 15.73 | 7.09 | 24.19 |
| Flabellate | / | 0.09 | 0.22 | 0.23 | / | 0.82 | 0.59 | 0.25 | / | 0.26 | 9.04 | / |
| Bulliform | 11.23 | 0.81 | 7.22 | 0.69 | / | 0.92 | 5.14 | / | 0.24 | 0.64 | 2.66 | / |
| Blocky | 1.06 | / | / | 1.61 | 1.48 | / | / | 0.75 | / | / | / | / |
| Circular | 7.63 | 0.36 | 1.53 | 18.35 | 4.28 | 0.31 | 8.1 | 1.00 | 4.62 | / | 10.28 | 14.86 |
| Scaly | / | / | / | / | / | / | / | / | / | / | 0.71 | / |
| Carinate | / | / | / | / | / | / | / | / | / | / | 0.18 | / |
| Scrobiculate | 2.54 | 0.27 | 0.44 | 24.31 | / | 0.2 | 0.2 | 6.23 | 0.24 | 0.13 | / | 0.19 |
| Fusiform | 4.03 | / | 0.22 | / | / | / | / | 21.2 | / | / | 0.35 | / |
| Epidermal phytoliths | / | 0.09 | 3.5 | 0.23 | / | / | / | / | 0.24 | / | / | / |
| Elongate | 29.03 | 3.79 | 3.5 | 1.38 | 22.08 | 13.1 | 1.19 | 7.48 | 37.96 | 5.63 | 2.48 | 4.38 |
| Bulbous | 0.64 | 3.25 | 5.25 | 4.82 | 2.97 | 0.41 | 5.53 | 0.25 | 2.19 | 1.92 | 0.9 | 0.77 |
| Scutiform | / | / | / | / | 0.18 | / | / | / | / | 0 | / | 0.19 |
| Baculate | / | / | / | 0.69 | 1.48 | / | 0.2 | 11.47 | / | 0.12 | / | / |
| Dendriti | 0.21 | / | / | 0.44 | / | / | 5.73 | / | / | / | / | / |
| Lobate | / | 0.09 | / | / | / | / | / | / | / | / | / | / |
| Polygonal | / | / | / | / | / | 0.41 | / | / | / | / | / | / |
| Sinuate | 1.05 | / | / | / | 2.14 | 0.1 | / | 2.48 | 2.43 | / | / | / |
| Tracheary | / | / | / | / | / | 0.1 | / | / | / | / | / | 0.38 |
| Conical | / | / | / | / | / | / | / | / | 0.75 | / | / | 0.19 |
| Types | 15 | 13 | 13 | 14 | 12 | 14 | 13 | 13 | 13 | 12 | 14 | 12 |
| Bamboo Species | Distinction Type | Total | |||
|---|---|---|---|---|---|
| Bambusa vulgaris | Bambusa tulda | Bambusa dolichoclada | |||
| Discriminant number | Bambusa vulgaris | 3 | 0 | 0 | 3 |
| Bambusa tulda | 0 | 3 | 1 | 4 | |
| Bambusa dolichoclada | 1 | 1 | 2 | 4 | |
| Correct rate (%) | Bambusa vulgaris | 100.0 | 0.0 | 0 | 100.0 |
| Bambusa tulda | 0.0 | 75.0 | 25.0 | 100.0 | |
| Bambusa dolichoclada | 25.0 | 25.0 | 50.0 | 100.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, G.; Liu, C.; Xu, R.; Wang, C.; Zhao, T.; Duan, M.; Gao, K. Comparison of Phytolith Characteristics of Three Bamboo Species’ Cotyledon Organs. Plants 2025, 14, 1174. https://doi.org/10.3390/plants14081174
Luo G, Liu C, Xu R, Wang C, Zhao T, Duan M, Gao K. Comparison of Phytolith Characteristics of Three Bamboo Species’ Cotyledon Organs. Plants. 2025; 14(8):1174. https://doi.org/10.3390/plants14081174
Chicago/Turabian StyleLuo, Guomi, Chengyao Liu, Rui Xu, Changming Wang, Taiyang Zhao, Mengsi Duan, and Kemei Gao. 2025. "Comparison of Phytolith Characteristics of Three Bamboo Species’ Cotyledon Organs" Plants 14, no. 8: 1174. https://doi.org/10.3390/plants14081174
APA StyleLuo, G., Liu, C., Xu, R., Wang, C., Zhao, T., Duan, M., & Gao, K. (2025). Comparison of Phytolith Characteristics of Three Bamboo Species’ Cotyledon Organs. Plants, 14(8), 1174. https://doi.org/10.3390/plants14081174






