Integrating Rock Dust and Organic Amendments to Enhance Soil Quality and Microbial Activity for Sustainable Crop Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Collection of Samples, and Preparation
2.2. Media Formulation with Rock Dust (RD) Amendment
2.3. Media Preparation
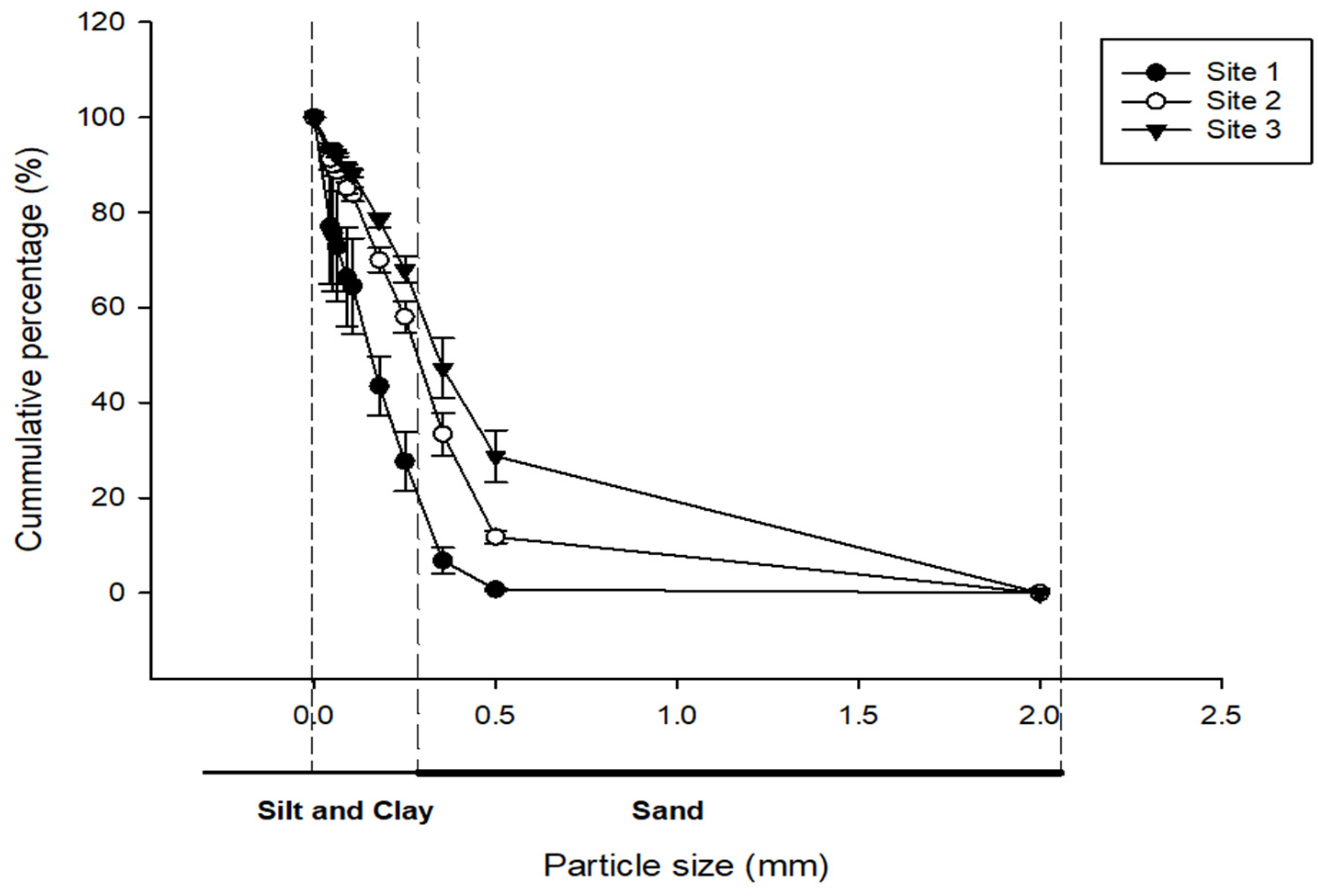
2.4. Particle Size Analysis
2.5. Physical Soil Properties Analysis
2.6. pH Analysis
2.7. Mineral Composition Analysis
2.8. Active Microbial Community Analysis
2.9. Statistical Analyses
3. Results and Discussion
3.1. Particle Size Distribution of Rock Dust
3.2. Physical Characteristics of Formulated RD-Based Amendments
3.3. Nutrient Composition of RD and the Formulated Amendments
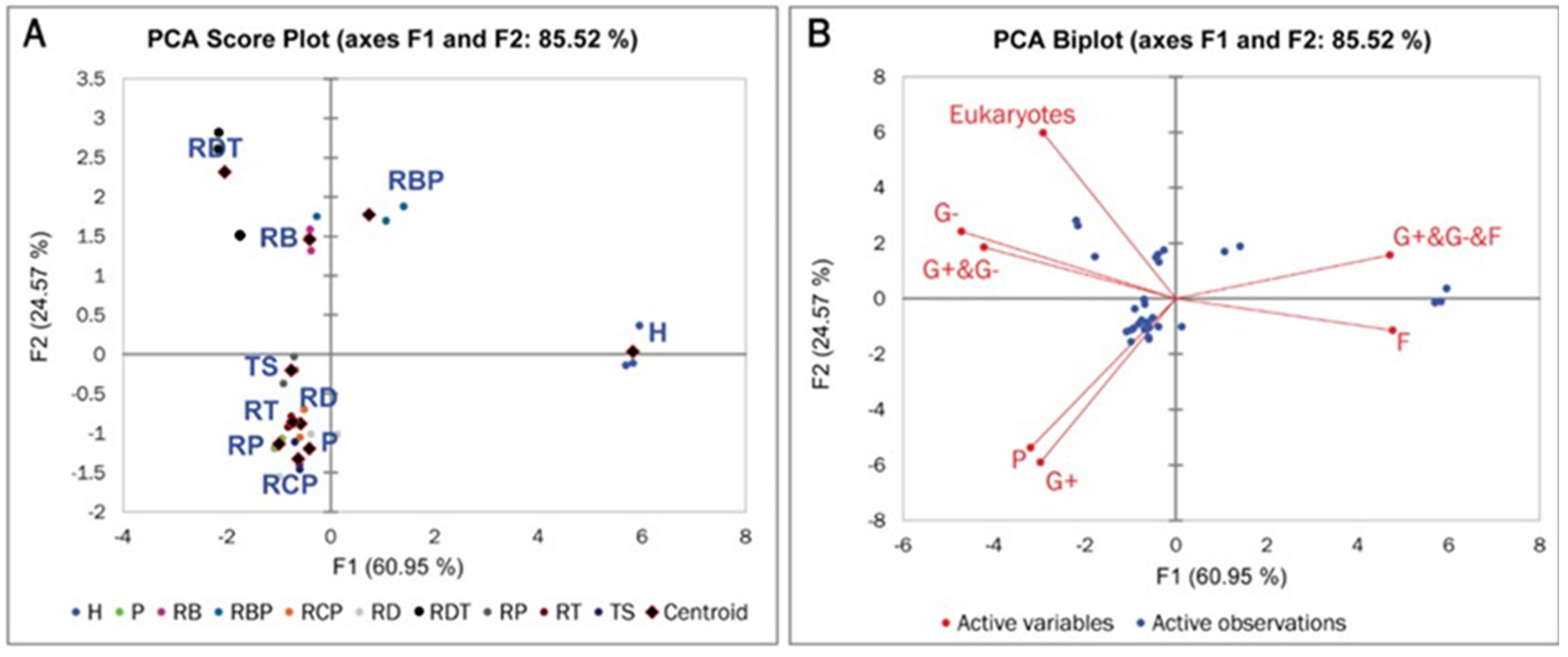
3.4. Active Microbial Community in RD and RD-Based Amendments
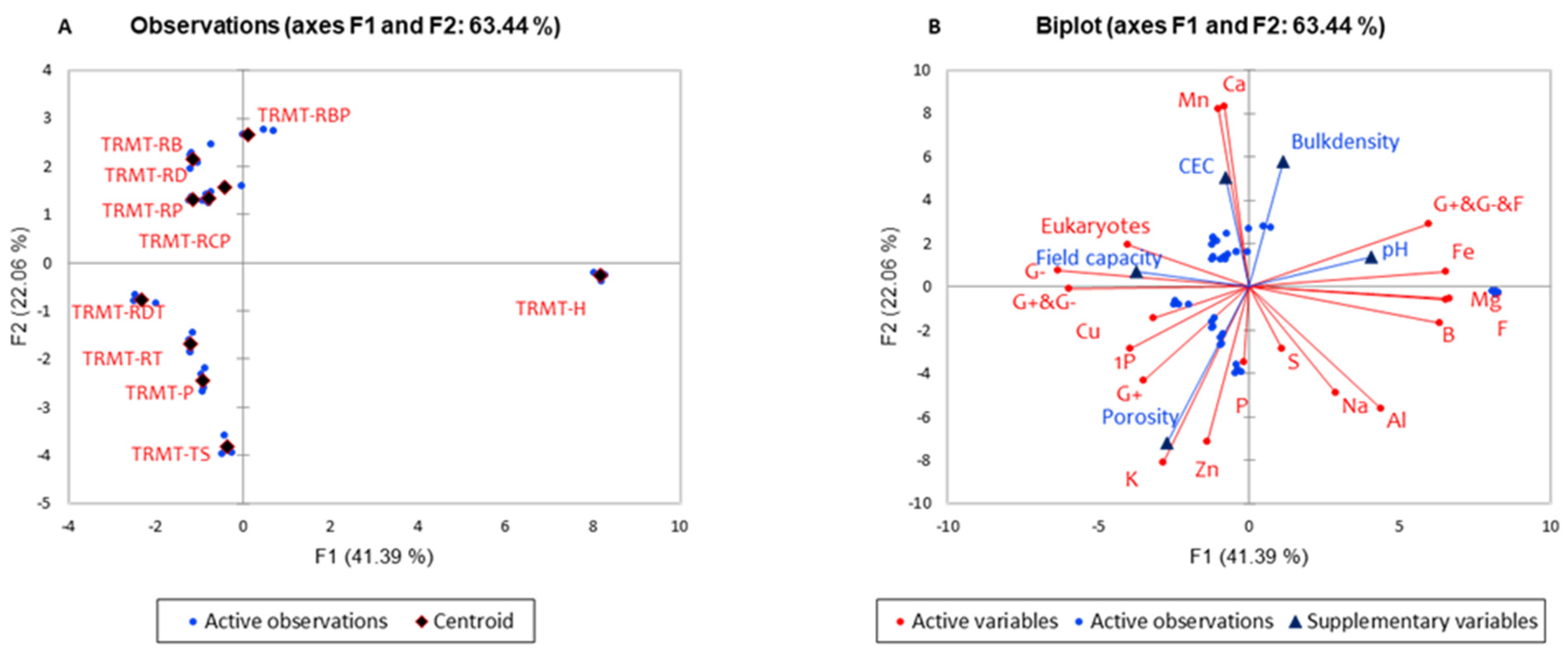
3.5. Relationship Between RD Media Amendments, Physical Properties, Nutrient Composition, and Microbial Biomass
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basak, B.B.; Sarkar, B.; Naidu, R. Environmentally safe release of plant available potassium and micronutrients from organically amended rock mineral powder. Environ. Geochem. Health 2020, 43, 3273–3286. [Google Scholar] [CrossRef]
- Sonter, L.J.; Herrera, D.; Barrett, D.J.; Galford, G.L.; Moran, C.J.; Soares-Filho, B.S. Mining drives extensive deforestation in the Brazilian Amazon. Nat. Commun. 2017, 8, 1013. [Google Scholar] [CrossRef] [PubMed]
- Ramezanian, A.; Dahlin, A.S.; Campbell, C.D.; Hillier, S.; Mannerstedt-Fogelfors, B.; Öborn, I. Addition of a volcanic rockdust to soils has no observable effects on plant yield and nutrient status or on soil microbial activity. Plant Soil 2013, 367, 419–436. [Google Scholar] [CrossRef]
- Li, J.G.; Dong, Y.H. Effect of a rock dust amendment on disease severity of tomato bacterial wilt. Antonie Leeuwenhoek 2013, 103, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Arma, A.; Alrayes, L.; Pham, T.H.; Nadeem, M.; Manful, C.; Bartlett, O.; Thomas, R. Rock dust-based potting media enhances agronomic performance and nutritional quality of horticultural crops. Curr. Plant Biol. 2024, 40, 100419. [Google Scholar] [CrossRef]
- Ramos, C.G.; dos Santos de Medeiros, D.; Gomez, L.; Oliveira, L.F.S.; Schneider, I.A.H.; Kautzmann, R.M. Evaluation of Soil Re-mineralizer from By-Product of Volcanic Rock Mining: Experimental Proof Using Black Oats and Maize Crops. Nat. Resour. Res. 2020, 29, 1583–1600. [Google Scholar] [CrossRef]
- Altdorff, D.; Borchard, N.; Young, E.H.; Galagedara, L.; Sorvali, J.; Quideau, S.; Unc, A. Agriculture in boreal and Arctic regions requires an integrated global approach for research and policy. Agron. Sustain. Dev. 2021, 41, 23. [Google Scholar] [CrossRef]
- Arnott, A.; Galagedara, L.; Thomas, R.; Cheema, M.; Sobze, J.M. The potential of rock dust nanoparticles to improve seed germination and seedling vigor of native species: A review. Sci. Total Environ. 2021, 775, 145139. [Google Scholar] [CrossRef]
- Ufot, V. How Effective Are Current Regulations and Management Strategies in Reducing Heavy Metal Pollution in the Canadian Arctic; University of Ottawa: Ottawa, ON, Canada, 2023. [Google Scholar]
- Blaud, A.; Lerch, T.; Chevallier, T.; Nunan, N.; Chenu, C.; Brauman, A. Dynamics of bacterial communities in relation to soil aggregate formation during the decomposition of 13C-labelled rice straw. Appl. Soil Ecol. 2012, 53, 1–9. [Google Scholar] [CrossRef]
- Kukal, S.; Saha, D.; Bhowmik, A.; Dubey, R. Water retention characteristics of soil bio-amendments used as growing media in pot culture. J. Appl. Hortic. 2012, 14, 92–97. [Google Scholar] [CrossRef]
- Zaeem, M.; Nadeem, M.; Pham, T.H.; Ashiq, W.; Ali, W.; Gilani, S.S.M.; Elavarthi, S.; Kavanagh, V.; Cheema, M.; Galagedara, L. The potential of corn-soybean intercropping to improve the soil health status and biomass production in cool climate boreal ecosystems. Sci. Rep. 2019, 9, 13148. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils 2013, 49, 723–733. [Google Scholar] [CrossRef]
- Zelles, L. Fatty acid patterns of phospholipids and lipopolysaccharides in the characterisation of microbial communities in soil: A review. Biol. Fertil. Soils 1999, 29, 111–129. [Google Scholar] [CrossRef]
- Plata, L.G.; Ramos, C.G.; Oliveira, M.L.S.; Oliveira, L.F.S. Release kinetics of multi-nutrients from volcanic rock mining by-products: Evidences for their use as a soil remineralizer. J. Clean. Prod. 2021, 279, 123668. [Google Scholar] [CrossRef]
- Kelland, M.E.; Wade, P.W.; Lewis, A.L.; Taylor, L.L.; Sarkar, B.; Andrews, M.G.; Lomas, M.R.; Cotton, T.A.; Kemp, S.J.; James, R.H.; et al. Increased yield and CO2 sequestration potential with the C4 cereal Sorghum bicolor cultivated in basaltic rock dust—amended agricultural soil. Glob. Change Biol. 2020, 26, 3658–3676. [Google Scholar] [CrossRef]
- Ni, J.J.; Bordoloi, S.; Shao, W.; Garg, A.; Xu, G.; Sarmah, A.K. Two-year evaluation of hydraulic properties of biochar-amended vegetated soil for application in landfill cover system. Sci. Total Environ. 2020, 712, 136486. [Google Scholar] [CrossRef]
- Luna, L.; Vignozzi, N.; Miralles, I.; Solé-Benet, A. Organic amendments and mulches modify soil porosity and infiltration in semiarid mine soils. Land Degrad. Dev. 2018, 29, 1019–1030. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Masto, R.; Tripathi, R.; Srivastava, N. Application of soil quality indicators for the phytorestoration of mine spoil dumps. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Netherlands, 2019; pp. 361–388. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- de Oliveira, R.A.; Ramos, M.M.; de Aquino, L.A. Irrigation management. In Sugarcane; Elsevier: Amsterdam, The Netherlands, 2015; pp. 161–183. [Google Scholar] [CrossRef]
- Nett, L.; Ruppel, S.; Ruehlmann, J.; George, E.; Fink, M. Influence of soil amendment history on decomposition of recently applied organic amendments. Soil Sci. Soc. Am. J. 2012, 76, 1290–1300. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-chemical properties and microbial responses in biochar-amended soils: Mechanisms and future directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- McCormack, S.A.; Ostle, N.; Bardgett, R.D.; Hopkins, D.W.; Vanbergen, A.J. Biochar in bioenergy cropping systems: Impacts on soil faunal communities and linked ecosystem processes. Gcb Bioenergy 2013, 5, 81–95. [Google Scholar] [CrossRef]
- Koyama, A.; Wallenstein, M.D.; Simpson, R.T.; Moore, J.C. Soil bacterial community composition altered by increased nutrient availability in Arctic tundra soils. Front. Microbiol. 2014, 5, 516. [Google Scholar] [CrossRef]
- Mathew, R.P.; Feng, Y.; Githinji, L.; Ankumah, R.; Balkcom, K.S. Impact of no-tillage and conventional tillage systems on soil microbial communities. Appl. Environ. Soil Sci. 2012, 2012, 548620. [Google Scholar] [CrossRef]
- Lauber, C.L.; Strickland, M.S.; Bradford, M.A.; Fierer, N. The influence of soil properties on the structure of bacterial and fungal communities across land-use types. Soil Biol. Biochem. 2008, 40, 2407–2415. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Xiao, Y.; Gu, Y.; Liu, H.; Liang, Y.; Liu, X.; Hu, J.; Meng, D.; Yin, H. An integrated insight into the relationship between soil microbial community and tobacco bacterial wilt disease. Front. Microbiol. 2017, 8, 2179. [Google Scholar] [CrossRef]
- Ribeiro, I.D.A.; Volpiano, C.G.; Vargas, L.K.; Granada, C.E.; Lisboa, B.B.; Passaglia, L.M.P. Use of mineral weathering bacteria to enhance nutrient availability in crops: A review. Front. Plant Sci. 2020, 11, 590774. [Google Scholar] [CrossRef]
| Treatment Code | Treatment | Combination Level |
|---|---|---|
| P | PromixTM | 100% |
| RBP | RD + biochar + PromixTM | 50% + 25% + 25% |
| H | Huplaso | 100% |
| RT | RD + topsoil | 25% +75% |
| RP | RD + Promix | 50% + 50% |
| RCP | RD + compost + PromixTM | 50% + 25% + 25% |
| RD | RD | 100% |
| RDT | RD + topsoil | 50% + 50% |
| RB | RD + biochar | 50% + 50% |
| TS | Topsoil | 100% |
| Treatment | Bulk Density (g/cm3) | Porosity (%) | Field Capacity (%) |
|---|---|---|---|
| P | 0.24 ± 0.06 a | 60.69 ± 0.02 c | 30.52 ± 0.04 bc |
| RBP | 1.17 ± 0.01 g | 44.00 ± 0.01 a | 35.90 ± 0.01 c |
| H | 1.19 ± 0.01 f | 41.07 ± 0.01 a | 22.62 ± 0.01 a |
| RT | 0.85 ± 0.01 c | 56.44 ± 0.01 b | 35.15 ± 0.01 c |
| RP | 0.87 ± 0.01 c | 55.87 ± 0.01 b | 36.29 ± 0.01 c |
| RCP | 0.93 ± 0.01 e | 59.85 ± 0.02 bc | 36.09 ± 0.03 c |
| RD | 1.50 ± 0.01 h | 43.92 ± 0.02 a | 26.74 ± 0.03 c |
| RDT | 1.10 ± 0.01 f | 43.21 ± 0.01 a | 30.40 ± 0.01 bc |
| RB | 0.96 ± 0.01 d | 45.74 ± 0.01 a | 31.84 ± 0.01 a |
| TS | 0.57 ± 0.00 b | 71.66 ± 0.04 d | 32.11 ± 0.01 bc |
| Recommended level | <1.6 | 50–70 | 10–33 |
| Minerals (mg kg−1) | RD | TS | H | P | RP | RB | RT | RDT | RCP | RBP | Level Required for Crop Growth |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphorus | 1 a | 109 f | 4 ab | 89 e | 15 cd | 31 d | 86 e | 76 e | 25 cd | 15 bc | 12–14 |
| Potassium | 35 a | 201 c | 101 b | 387 e | 254 d | 13 a | 44 a | 29 a | 152 b | 116 b | 121–181 |
| Calcium | 9747 f | 3905 j | 4909 e | 2435 a | 11,102 j | 10,497 ij | 4102 d | 3029 bc | 9470 f | 10,276 h | >40–60 |
| Magnesium | 126 b | 175 e | 517 h | 217 g | 205 f | 132 c | 130 b | 104 a | 217 i | 169 d | 50–70 |
| Sulphur | 40 g | 40 g | 34 de | 37 f | 33 de | 22 a | 30 c | 26 b | 31 cd | 37 ef | 10–20 |
| Zinc | 0.4 a | 20.2 i | 1.6 d | 1.4 cd | 2.9 f | 2.1 ef | 12.6 h | 7.8 g | 1.3 c | 0.9 b | >1.5 |
| Copper | 1.6 c | 2.7 d | 0.9 ab | 0.6 a | 2.7 de | 2.7 de | 2.8 e | 3.2 f | 0.9 ab | 1.1 b | 1–1.8 |
| Sodium | 11 a | 110 f | 114 g | 86 e | 45 c | 78 d | 11 a | 75 d | 21 b | 21 b | - |
| Iron | 396 c | 236 b | 1175 d | 73 a | 259 b | 241 b | 248 b | 258 b | 249 b | 255 b | 6.0–1 × 106 |
| Boron | 1.2 c | 1.7 d | 4.3 e | 0.3 a | 0.5 ab | 0.4 ab | 0.9 bc | 0.6 ab | 0.3 a | 0.3 bc | 1–3 |
| Manganese | 147 f | 73 c | 77 d | 6 a | 174 j | 163 h | 72 b | 83 e | 148 g | 166 h | 20–30 |
| Aluminum | 38 b | 439 d | 626 e | 41 b | 7 a | 24 ab | 435 d | 228 c | 12 a | 17 ab | <2–5 |
| pH | 8.4 de | 7.2 f | 9.1 g | 4.9 a | 7.4 c | 8.0 de | 8.0 ef | 7.1 ef | 6.5 b | 7.4 cd | 5.5–7.0 |
| Treatment | Eukaryotes (%) | G− (%) | G+ (%) | F (%) | P (%) | G+ plus G− (%) | G+ plus G− plus F (%) |
|---|---|---|---|---|---|---|---|
| P | 3.52 ± 0.09 d | 21.72 ± 0.39 def | 26.40 ± 0.25 def | 9.33 ± 0.13 c | 3.30 ± 0.08 e | 26.25 ± 0.02 b | 9.49 ± 0.06 c |
| RBP | 4.94 ± 0.35 f | 18.45 ± 0.93 b | 9.71 ± 0.73 ab | 7.12 ± 0.57 b | 1.44 ± 0.11 ab | 29.97 ± 2.21 c | 28.36 ± 4.88 d |
| H | 0.00 ± 0.00 a | 2.99 ± 0.25 a | 7.51 ± 0.05 a | 40.16 ± 0.13 g | 0.77 ± 0.03 a | 18.33 ± 0.16 a | 30.49 ± 0.16 e |
| RT | 1.37 ± 0.03 b | 19.91 ± 0.38 bc | 22.48 ± 0.11 d | 11.76 ± 0.17 f | 2.55 ± 0.07 ab | 36.06 ± 0.26 e | 5.89 ± 0.17 ab |
| RP | 4.21 ± 0.10 e | 23.65 ± 1.15 d | 27.24 ± 0.36 ef | 10.01 ± 0.23 cde | 1.98 ± 0.09 bc | 25.43 ± 0.07 b | 7.49 ± 0.17 abc |
| RCP | 2.15 ± 0.08 c | 19.89 ± 0.23 bc | 23.28 ± 0.41 de | 12.67 ± 0.06 f | 2.68 ± 0.10 d | 31.81 ± 0.41 cd | 7.52 ± 0.07 ab |
| RD | 1.98 ± 0.11 bc | 22.00 ± 1.09 cd | 26.39 ± 0.39 def | 9.45 ± 0.32 cd | 2.79 ± 0.25 de | 24.49 ± 0.52 b | 12.91 ± 2.49 c |
| RDT | 8.27 ± 0.45 g | 31.16 ± 1.33 e | 17.00 ± 3.97 c | 5.21 ± 0.39 a | 1.42 ± 0.19 ab | 34.15 ± 1.55 de | 2.78 ± 0.20 a |
| RB | 3.97 ± 0.11 de | 23.70 ± 1.10 d | 12.01 ± 0.31 b | 10.38 ± 0.13 de | 1.41 ± 0.08 ab | 36.91 ± 0.46 e | 11.61 ± 0.12 bc |
| TS | 1.67 ± 0.04 bc | 17.78 ± 1.13 b | 30.19 ± 0.46 f | 10.44 ± 0.20 e | 2.30 ± 0.05 cd | 31.38 ± 0.49 cd | 6.25 ± 0.02 ab |
| QUADRANT 1 (RBP) | Variables | pH | G+ plus G− plus F | ||||
| pH | −0.982 | ||||||
| G+ plus G− plus F | −0.982 | ||||||
| Fe | 0.618 | ||||||
| QUADRANT 2 (H) | Variables | F | Na | ||||
| Na | −0.997 | ||||||
| Al | 0.958 | ||||||
| QUADRANT 3 (RDT, RT, P, TS) | Variables | Porosity | G+ | 1P | G+ plus G− | K | Zn |
| G+ | 0.715 | ||||||
| G+ plus G− | −0.574 | −0.575 | |||||
| P | 0.680 | ||||||
| K | 0.580 | 0.737 | −0.885 | ||||
| Zn | 0.634 | ||||||
| Cu | −0.822 | 0.876 | −0.902 | 0.558 | |||
| QUADRANT 4 (RB, RD, RP, RCP) | Variables | CEC | Eukaryotes | G− | |||
| G− | 0.585 | ||||||
| G+ | 0.949 | ||||||
| Ca | 0.847 | 0.579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armah, A.; Alrayes, L.; Pham, T.H.; Nadeem, M.; Bartlett, O.; Fordjour, E.; Cheema, M.; Galagedara, L.; Abbey, L.; Thomas, R. Integrating Rock Dust and Organic Amendments to Enhance Soil Quality and Microbial Activity for Sustainable Crop Production. Plants 2025, 14, 1163. https://doi.org/10.3390/plants14081163
Armah A, Alrayes L, Pham TH, Nadeem M, Bartlett O, Fordjour E, Cheema M, Galagedara L, Abbey L, Thomas R. Integrating Rock Dust and Organic Amendments to Enhance Soil Quality and Microbial Activity for Sustainable Crop Production. Plants. 2025; 14(8):1163. https://doi.org/10.3390/plants14081163
Chicago/Turabian StyleArmah, Abraham, Linda Alrayes, Thu Huong Pham, Muhammad Nadeem, Owen Bartlett, Eric Fordjour, Mumtaz Cheema, Lakshman Galagedara, Lord Abbey, and Raymond Thomas. 2025. "Integrating Rock Dust and Organic Amendments to Enhance Soil Quality and Microbial Activity for Sustainable Crop Production" Plants 14, no. 8: 1163. https://doi.org/10.3390/plants14081163
APA StyleArmah, A., Alrayes, L., Pham, T. H., Nadeem, M., Bartlett, O., Fordjour, E., Cheema, M., Galagedara, L., Abbey, L., & Thomas, R. (2025). Integrating Rock Dust and Organic Amendments to Enhance Soil Quality and Microbial Activity for Sustainable Crop Production. Plants, 14(8), 1163. https://doi.org/10.3390/plants14081163








