Abstract
The physical properties of plants affect the transmission of plant-borne vibrational signals, which many herbivorous insects use for communication. Male calling signals (MCaSs, with sections S0, S1, and S2) and courtship signals (MCoSs, with sections S1 and S2) of Empoasca onukii Matsuda (Hemiptera: Cicadellidae), a major pest of tea plant, have a multicomponent structure. The same MCaS was repeatedly played back on different leaves of a tea branch, and parameters of the transmitted signal and female responses were measured on the leaf inhabited by females. We also measured the signal parameters and behaviors of E. onukii on single leaves of different ages. The intensity of MCaSs from other leaves attenuated after they propagated to leaves on which females were located, which decreased the duration of MCaS-S2. Higher leaf thickness, leaf hardness, and leaf area were associated with an increased pulse repetition time (PRT) of MCaSs, number of pulses in MCaS-S2, and duration of MCaS-S2, respectively. MCoS-S1 had a higher dominant frequency (Df) in leaves with a long main vein and high hardness, and the PRT of MCoS-S2 was longer on thicker leaves. In the initial stage of courtship, the signal excitation of males was affected by leaf traits, especially the temporal parameters of MCaS-S2, which was the most significantly affected section after host transmission; it also had an important effect on the response delay of females. In the location stage, the signal excitation of males was not only affected by leaf traits but also interacted with the signal excitation of females. These results facilitate exploration of the interaction between leafhoppers and host plants during courtship communication and have implications for the breeding of E. onukii-resistant varieties.
1. Introduction
Many insects use plant-borne vibrational signals in their courtship communication, which includes signal excitation, transmission, and perception [1,2]. Plants are highly complex structures and exhibit high variability in traits that can affect the transmission of vibrational signals [3], which has important consequences for the insects that inhabit them [4,5].
In recent years, the effect of variation of plant traits on the transmission of vibrational signals or cues has received increased attention [6]. The physical constraints of host plants affect vibrational signals, including the loss of energy, filtering, or distortion of information in signal transmission [7]. Passive variation in characteristic parameters of vibrational signals (intensity, frequency spectrum, and time pattern) potentially affects the detection and assessment of signals or cues by the receiver, such as decreasing mate attraction [8]. Some physical properties of substrates are inherently fixed, whereas other properties and parameters can be selected by animals. For example, organisms on plant stems can influence bending waves by selecting plants with particular dimensions, material properties, and generated vibration frequencies [7]. The behavioral adaptations of animals for generating vibrations can promote information transfer and thus mitigate physical constraints. For example, the treehopper Enchenopa binotata Say (Hemiptera: Membracidae) and the jumping spider Habronattus dossenus Griswold (Aranea: Salticidae) can alter their behaviors to tailor generated signals to substrate properties, which can be modulated by feedback during signal excitation [9,10]. An understanding of the physical mechanisms is important for addressing biological questions relating to communication and information gathering.
Our previous studies have shown that the tea leafhopper Empoasca onukii, a major monophagous sucking pest of tea plants, prefers to feed on tender stems and leaves [11]; however, leafhoppers prefer to send mating signals during courtship on mature leaves [12]. The standard shape and canopy structure of tea trees, a perennial and ligneous plant, have been affected by artificial cultivation [13]. As leaf age increases, some physical traits of the leaves on the branches of the canopy vary significantly, including leaf area, thickness, hardness, and color. Plant-borne vibrational signals are the key cues used in the courtship communication of E. onukii [12]. The courtship of E. onukii comprises five conserved stages: (1) call-fly: males broadcast specific calling signals (MCaSs), which serve as calling signals for potential females on tea plants; (2) female identification: females perceive MCaSs on the host and send signals (FS1) to respond to males; (3) male location: the male perceives FS1 and sends specific courtship signals (MCoSs), which form a closed-loop pattern with female signals (FS2s); (4) courtship: when the male is close enough to females, it sends continuous MCoSs, ignoring the FS2s; and (5) copulation: the male jumps up and copulates with the female.
To enhance our understanding of the interaction between E. onukii and host plants in courtship communication, we designed two experiments to quantify the variability in mating signals and behaviors caused by changes in leaf traits/age. We speculate that variability in leaf traits affects the excitation and transmission of mating signals of male E. onukii and indirectly affects female signal perception and behavior. Different sections of the male signals may carry different information and play special roles in mating communication. When an important section of the male signal is changed due to leaf traits, it may cause the female to respond differently.
2. Results
2.1. Female Responses to Signals from Other Leaves
2.1.1. Variation in MCaS Parameters from Leaves of Different Ages
The signal parameters of the same MCaSs excited in leaves of different ages and transmitted to female leaves (i = 7) through stems (Figure 1A) varied significantly (N = 120; Figure 2), including the signal intensity (I) of MCaS-S0, MCaS-S1, and MCaS-S2 (p < 0.001; Table S1; Figure 2A,B) and the duration (D) of MCaS-S2 (F5,114 = 5.09, p < 0.001). Greater transmitted distances were associated with greater intensity attenuation in each section of the MCaS. Each section of the MCaS decreased in duration due to intensity attenuation (Figure 2D). Changes observed in other signal parameters were not significant (Table S1).
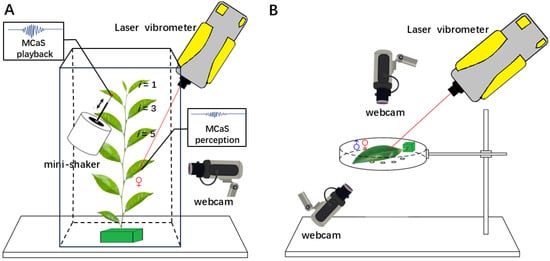
Figure 1.
Experimental design. (A) The setup used for playback and monitoring male calling signals of Empoasca onukii from other leaves to the leaf inhabited by a female. The same male calling signals were played back via a mini-shaker from leaves of different ages (i, i = 1–10 from top to bottom) and propagated to the leaf that a female inhabited (i = 7). (B) The vibrational signals and behaviors of a pair of E. onukii adults on a single tea leaf were recorded simultaneously via a laser vibrometer and two webcams.
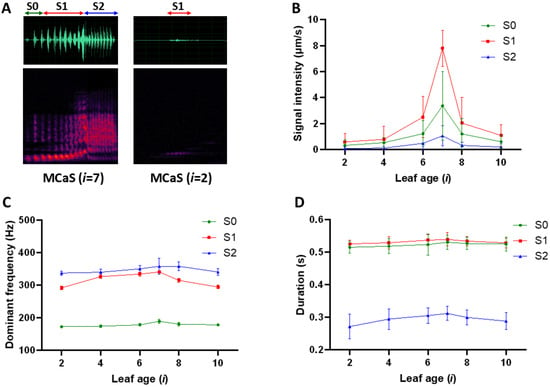
Figure 2.
The variation in parameters of the same male calling signal (MCaS) after transmission from different leaves to the female on the seventh leaf below the tea bud. (A) Oscillogram (above) and spectrogram (below) of the same MCaS that was played back on the seventh leaf (left) or on the second leaf (right) below the tea bud and was recorded on the position inhabited by a female on the seventh leaf. i is the leaf age, which indicates the leaf stage of maturity (i = 1–10 from top to bottom). MCaS has a multicomponent structure and three sections: S0, S1, and S2. The intensity (B), dominant frequency (C), and duration (D) values (mean ± SD) of each section in the transmitted MCaSs at the location of the female on the seventh leaf below the tea bud.
2.1.2. Effect of MCaS Parameters on Female Behavior
A total of 65 females responded to the MCaS playback out of a total of 120 tests performed. A comparison of the MCaS parameters from tests with female responses and non-female responses revealed that none of the MCaS parameters affected female responses (p > 0.05; Table S2), which indicated that whether the female responds to the MCaS was only related to the female’s physiological state and was independent of the intensity of the MCaS and the duration of MCaS-S2.
In the 65 tests with female responses, MCaS playback on each leaf stimulated female responses. After attenuation, the intensity of each section of the MCaS and DMCaS-S0 were used as independent variables, which were significantly correlated with NFS1 (all abbreviations are listed in Table 1; Figure 3). The multiple linear regression model of NFS1 was significant (F4,60 = 2.765, p = 0.035), and the effect of IMCaS-S0 was significant (t = 2.012, p = 0.049). In the multiple linear regression model (F1,63 = 4.976, p = 0.029), DMCaS-S1 had a significant effect on DelayID (t = 2.231, p = 0.029). Therefore, the intensity of MCaS-S0 might ensure the sustained attraction of the MCaS to the female; long MCaS-S1 durations resulted in long response delay of females to MCaSs (Figure 4A).

Table 1.
Abbreviations and data collected in the experiments.
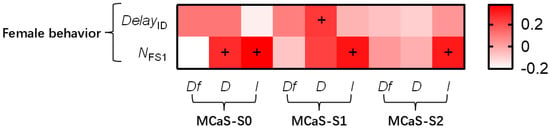
Figure 3.
The correlation analysis between the parameters of transmitted MCaSs and female behavior. + indicates that an MCaS parameter was significantly positively correlated with female behavior (Spearman’s correlation analysis, p < 0.05). The abbreviations of signal parameters and female behavior indexes are shown in Table 1.
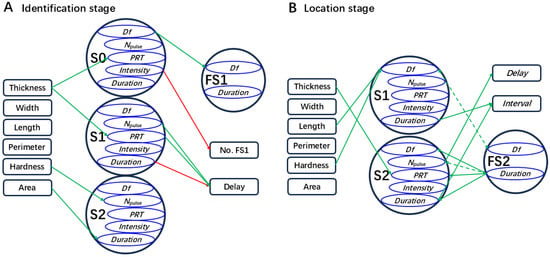
Figure 4.
Diagram describing potential relationships between leaf traits and the signals and behavior of Empoasca onukii. In the identification stage (A) or the localization stage (B), a parameter of the male calling signal (MCaS) pointed to by the arrow indicates that this parameter is significantly affected by the leaf traits in the multiple linear regression model (p < 0.05); a parameter of the female signal or a behavioral index pointed to by the arrow indicates that this parameter or index is significantly affected by the MCaS parameters in the multiple linear regression model (p < 0.05). Red arrows are derived from the MCaS playback test in Section 4.4.1. Green arrows are derived from the signal acquisition test in Section 4.4.2. The dashed lines indicate that the factors significantly affect each other (p < 0.05). The abbreviations of signal parameters and female behavioral indexes are shown in Table 1.
2.2. Female Responses to Male Signals from the Same Leaf
2.2.1. Effect of Leaf Age
As leaf age (i) increased, the leaf thickness (F8,171 = 29.308, p < 0.001; Figure 5A), leaf width (F8,171 = 4.381, p < 0.001; Figure 5B), main vein length (F8,171 = 4.063, p < 0.001; Figure 5C), leaf margin perimeter (F8,171 = 3.833, p < 0.001; Figure 5D), and leaf area (welch8,69.928 = 13.33, p < 0.001; Figure 5E) gradually increased, and it tended to stabilize after the third or fourth leaf under the bud; however, the leaf hardness gradually increased (F8,171 = 23.088, p < 0.001; Figure 5F), peaked at the sixth leaf (i = 6), and then decreased after the ninth leaf.
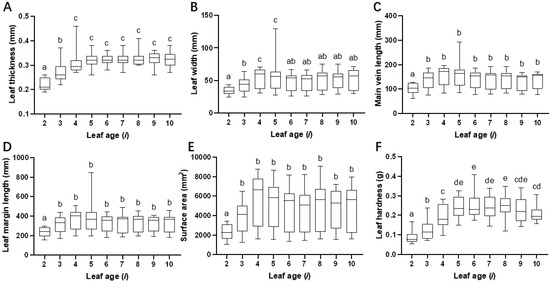
Figure 5.
The effect of leaf age on six leaf traits. The effect of variation in leaf age (i) on leaf thickness (A), leaf width (B), main vein length (C), leaf margin perimeter (D), surface area (E), and leaf hardness (F). Each trait of leaves of different ages was compared by analysis of variance. One-way ANOVA and LSD tests were used when the data met assumptions of normality and homoscedasticity; otherwise, Welch’s ANOVA and Tamhane’s T2 (M) tests were performed (p < 0.05). Mean values with different letters indicate significant differences within the histogram.
Significant differences in seven MCaS parameters—including the Df and Npulse of MCaS-S0 and -S1, DMCaS-S1, PRTMCaS-S2, DFS1, and female DelayID—were observed for pairs of leafhoppers that established identification duets (N = 70) on single leaves of different ages (p < 0.05; Table S3). Significant differences in five MCaS parameters, Df, DMCoS-S1, Npulse, PRT, and DMCoS-S2, and two behavioral indexes, female DelayLO and male IntervalLO, were observed for pairs of leafhoppers that established localization duets (N = 70) on leaves of different ages (p < 0.05; Table S4). Leaf age had no significant effect on the whole time spent in courtship (tcourtship; welch9,25.76 = 0.95, p = 0.5) and the number of localization cycles (Ncycle; welch9,25.17 = 1.15, p = 0.37).
2.2.2. Effect of Leaf Traits on the Identification Stage
The first MCaS parameters of males, the signal senders, should be affected only by leaf traits in the identification stage. There was a positive correlation between leaf thickness and PRTMCaS-S2 (p < 0.05, Spearman’s; Figure 6A), but the multiple linear regression model of PRTMCaS-S2 was not significant (F1,68 = 3.16, p = 0.078). In the multiple linear regression model of PRTMCaS-S0 (F1,68 = 5.13, p = 0.025), the effect of leaf thickness was significant (t = 2.265, p = 0.025). In the regression model of DMCaS-S2 (F1,68 = 5.59, p = 0.019), two leaf traits with multicollinearity [VIF (main vein and margin perimeter) > 5] were eliminated, and the effect of leaf area was significant (t = 2.36, p = 0.019). Leaf hardness (t = 2.624, p = 0.01) significantly affected the Npulse of MCaS-S2 in the regression model (F1,68 = 6.88, p = 0.01). Eliminating leaf hardness (VIF > 5), PRTMCaS-S1 was significantly affected by leaf thickness (t = 1.987, p = 0.049) in the regression model (F1,68 = 3.95, p = 0.049). Therefore, high leaf thickness resulted in a long PRT of each section of the MCaS; high leaf hardness resulted in more pulses in the MCaS-S2, and large leaf area resulted in long MCaS-S2 durations (Figure 4A).
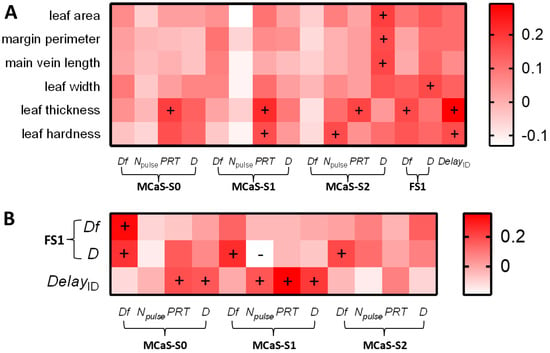
Figure 6.
The correlation analysis between leaf traits, signal parameters, and female behavioral indexes in the identification stage of Empoasca onukii. (A) The correlations between six leaf traits and signal parameters or the female delay after the MCaS. (B) The correlation between the MCaS parameters and the FS1 parameters or the female delay after the MCaS. + and −, respectively, indicated that a factor was significantly positively and negatively correlated with another factor (Spearman’s correlation analysis, p < 0.05). The abbreviations of signal parameters and female behavioral indexes are shown in Table 1.
Signal parameters of females, the signal receivers, should be affected not only by leaf traits, but also by MCaS parameters in the identification stage. DfFS1 was significantly affected by DfMCaS-S0 (t = 4.32, p < 0.001) in the multiple linear regression model (F2,67 = 11.4, p < 0.001). DFS1 was significantly positively correlated with leaf width and four MCaS parameters (p < 0.05; Figure 6), but the multiple linear regression model of DFS1 was not significant (F5,64 = 1.81, p = 0.12). Leaf thickness (t = 3.26, p = 0.001), Npulse of MCaS-S1 (t = 2.21, p = 0.029), and PRTMCaS-S1 (t = 2.4, p = 0.018) significantly affected female DelayID in the multiple linear regression model (F7,62 = 5.61, p < 0.001). Therefore, the dominant frequencies of male and female signals were affected by each other. A higher leaf thickness, longer PRT, and higher Npulse of MCaS-S1 led to increases in the response delay of females to the MCaS (Figure 4A).
2.2.3. Effect of Leaf Traits on Localization Stage
In the localization stage, MCoSs and FS2s alternated in a closed-loop mode. The second MCoS was affected not only by leaf traits but also by FS2 parameters. The multiple linear regression model (F5,64 = 4.62, p = 0.001) showed that DfMCoS-S1 was significantly affected by the main vein length (t = −2.28, p = 0.024), leaf hardness (t = 2.48, p = 0.014), and DfFS2 (t = 2.5, p = 0.013). When DFS2 was used as the independent variable (Figure 7A), it had a significant effect on the Df (t = −2.37, p = 0.019), Npulse (t = −4.49, p < 0.001), and duration (t = −3.5, p = 0.001) of MCoS-S2 in the linear regression model (Df, F1,68 = 5.6, p = 0.019; Npulse, F1,68 = 20.19, p < 0.001; duration, F1,68 = 12.24, p = 0.001). PRTMCoS-S2 was significantly affected by leaf thickness (t = 2.04, p = 0.043) and DFS2 (t = 1.98, p = 0.049) in the regression model (F2,67 = 3.46, p = 0.034). Similarly, FS2 parameters might be affected by leaf traits and MCoS parameters. DfMCoS-S1 (t = 2.401, p = 0.017) significantly affected DfFS2 in the regression model (F4,65 = 3.43, p = 0.01). DFS2 was significantly affected by the Npulse of MCoS-S2 (t = −3.757, p < 0.001) in the multiple linear regression model (F7,62 = 5.07, p < 0.001). Longer main veins and harder leaves led to increases in the Df of MCoS-S1, and thicker leaves resulted in increases in PRTMCoS-S2. The signal parameters of females and males affected each other (Figure 4B).
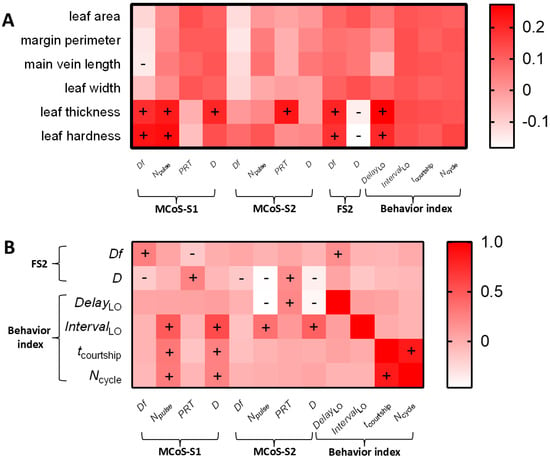
Figure 7.
The correlation analysis between leaf traits, signal parameters, and behavioral indexes in the localization stage of Empoasca onukii. (A) The correlations of six leaf traits with signal parameters and behavioral indexes. (B) The correlations of the MCoS parameters with FS2 parameters and behavioral indexes. + and −, respectively, indicated that a factor was significantly positively and negatively correlated with another factor (Spearman’s correlation analysis, p < 0.05). The abbreviations of signal parameters and female behavioral indexes are shown in Table 1.
DelayLO indicated the response time of a female to an MCoS. When the leaf thickness, hardness, and Npulse of MCoS-S2, PRTMCoS-S2, and DMCoS-S2 (VIF > 5) were used as the independent variables (Figure 7B), only the Npulse of MCoS-S2 (t = −6.43, p < 0.001) had a significant effect on DelayLO in the multiple linear regression model (F4,65 = 15.44, p < 0.001). IntervalLO indicated the feedback speed of males to FS2. When the Npulse of MCoS-S1, Npulse of MCoS-S2, DMCoS-S1, and DMCoS-S2 were used as the independent variables (Figure 7B), DMCoS-S1 (t = 5.32, p < 0.001) and DMCoS-S2 (t = 5.03, p < 0.001) had a significant effect on IntervalLO in the multiple linear regression model (F4,65 = 13.62, p < 0.001). When the Npulse and DMCoS-S1 were used as independent variables, significant regression models were observed for tcourtship (F2,67 = 3.744, p = 0.029) or Ncycle (F2,67 = 4.25, p = 0.018), but the independent variables did not reach significance. Therefore, leaf traits had no effect on the time (tcourtship) and efficiency (Ncycle) required by males to locate females on a single leaf. More pulses in MCoS-S2 resulted in a longer response delay of females to MCoSs, and longer MCoS durations resulted in a slower feedback speed of males to FS2s (Figure 4B).
3. Discussion
Clarifying host–pest interactions during the courtship communication of E. onukii, an obligate pest of tea plants, has important implications for understanding the mechanisms of adaptation of E. onukii to its host. During the whole courtship process, parameters of mating signals of male or female E. onukii changed within a certain range (Figure S1). Some parameter variations may be affected by host filtering of signals, but the intraindividual variability in parameters indicated that individuals might actively alter the parameters of their emitted signals. Our findings revealed that tea leaf traits affected the signal excitation and transmission of E. onukii, which indirectly affected mating behaviors. In addition, the results indicated that each section of male signals may have different functions.
3.1. Effects of Leaf Traits on Mating Behavior of Leafhoppers
Under natural conditions, female and male E. onukii are not located on the same leaves in most cases, and mating signals need to be transmitted through the host plant (male leaf–stem–female leaf). According to the review of Mortimer (2017) [7], the filtering of frequency is the first physical constraint acting on information propagation. As the low-pass filter of vibrational signals, plant tissue is not conducive to the transmission of high-frequency (>5 kHz) signals [14]. The frequency range of E. onukii mating signals is less than 2 kHz, and the pulse in MCaS-S0 has the lowest frequency (<0.4 kHz) [12,15], which is conducive to transmission via the plant. After the mating signal propagates from one leaf to another, the intensity inevitably decreases as the transmission distance increases. The wave speed distorts the temporal pattern of the propagating vibrational signal because the wave speed is not always the same for all frequency components of a signal [7]. MCaS playback on each leaf stimulated female responses, indicating that signal transmission on the same branch did not cause the distortion; that is, the whole branch was within the “active space” of the MCaS if the female was located on the seventh leaf below the tea bud [16]. The physical constraints of host plants appear to have no effect on the transmission of mating signals of E. onukii if the male and female are in the same branch, which indicates that E. onukii is highly adapted to its host.
Leaf thickness is positively correlated with the attenuation speed of vibrational signals [6]. Energy consumption has important implications for insects [17], and the ability to locate mates rapidly can prevent predation [18]. Our results showed that increases in leaf thickness (1) lead to a longer PRT of male signals, which is less conducive to saving energy, and (2) prolong the response delay of females to male signals, which impedes the ability of males to rapidly locate females. Therefore, thick leaves may negatively affect the courtship communication of E. onukii, which is consistent with the conclusion that increases in leaf thickness improve resistance to leafhopper infestations [19]. The hardness of plant tissues affects the excitation and propagation of vibrational signals [20]. In the identification stage, E. onukii males need MCaSs to be transmitted sufficiently far so that females can detect them. Our results showed that males might increase the number of pulses in MCaS-S2 on leaves with high hardness, which tended to be the sixth or seventh mature leaves below the bud. The increase in leaf hardness might lead to more information being carried by MCaSs.
The duration of the localization stage is approximately equivalent to the entire courtship time; in this stage, male E. onukii continuously move and exchange signals with females. During this stage, the leaf hardness and main vein length affected the frequency of MCoS-S1, which is consistent with the model of de Langre (2019) [20]. The signals of E. onukii might be tuned on the basis of the physical properties of the substrate, as has been reported in various insects [10,21,22]. A coordinated reciprocal exchange of information is common in male–female arthropods relying on substrate-borne vibrational signals [23]. The frequencies of MCoS-S1 and FS2 interact with each other, and the length of FS2 affects many parameters of MCoS-S1, indicating that the MCoS excitation of males is affected by female signals, as well as leaf traits.
3.2. Potential Information Carried in Different Sections of Male Signals
Similar to information transfer in other modalities, vibrational signals provide information on the location, identity, and even state of the sender [24,25]. Insect mating signals often have a multicomponent structure, and different sections of the signal may carry different types of information [26,27]. For example, the low-frequency pulses in MCaS-S0 may be used to maintain the female’s attention, which is consistent with the finding that males frequently send pulses similar to MCaS-S0 during the identification stage.
The distance from the signal playback point to the recording point affected the peak frequency measurements, with lower peak frequencies recorded at larger distances [4,6]; thus, the Df of each section in the MCaS was slightly decreased after transmission, but this did not affect MCaS recognition by females. Npulse, PRT, and DMCaS-S1 affect the signal response delay of females; we speculate that MCaS-S1 transmits information on species identity. In the localization stage, the Df of MCoS-S1 was positively correlated with that of FS2, perhaps because the Df of MCoS-S1 was affected by leaf traits, which indirectly affected the Df of female signals. Similarly to the leafhopper Aphrodes makarovi Zachvatkin (Hemiptera: Cicadellidae), the duration of the last section of the male localization signal was positively correlated with the duration of the female signal and the response delay of females, which indicates that vibrational duetting may entail the exchange of more complex information than just temporal coordination [23]. Few studies have examined the functions of different sections of vibrational signals with a multicomponent structure, and hypothesized functions need to be verified by playback experiments.
4. Materials and Methods
4.1. Insect Rearing
Adult tea leafhoppers were collected from the Tea Research Institute, Chinese Academy of Agricultural Sciences (TRI, CAAS), Hangzhou, China (30.18° N, 120.09° E) from September to November (peak period of E. onukii occurrence in autumn) in 2023 and 2024. Following the method described by Shan et al. (2023) [12], the leafhoppers were reared on tea branches (Longjing 43 variety) in an insectary with a stable environment (L10:D14, 25 ± 2 °C, 75 ± 5% RH). The adults used for testing were from the second and subsequent generations. Sexually mature seven-day-old virgin E. onukii adults were used in subsequent experiments. Each leafhopper was tested only once.
4.2. Plants and Leaf Trait Measurements
After pruning in the spring, the buds in the tea trees formed under an apical dominant growth pattern, and new branches had one bud and several leaves in summer and autumn. The tea branches (Longjing 43 variety) used for feeding and tests were collected at the same time in autumn from TRI, CAAS. The branches were immediately planted in sponges full of water and then reared in an insectary without pests. Branches with 10 leaves were used for tests of mating signal playback. From the top to the bottom of a tea branch, leaves below the bud were numbered according to their age (i = 1, 2 … 10, Figure 1A). Six leaf traits were measured in the laboratory (Table 1)—leaf area (cm2), leaf thickness (mm), leaf width (mm), main vein length (mm), leaf margin perimeter (mm), and leaf hardness (g)—following the handbook for standardized measurements of plant leaf traits. The leaf area, leaf width, main vein length, and leaf margin perimeter were first measured using an Intelligent and High Accuracy Leaf Area Tester (YMJ-CHA3, Zhejiang Top Instrument Co., Ltd., Hangzhou, China). Leaf hardness was measured using a Texture Analyzer (Model TA.XT plusC, Stable Micro Systems Ltd., Surrey, UK) equipped with a needle probe (P/2N-2 mm). The pre-test, test, and post-test speeds were 1, 2, and 10 mm/s, respectively, and the trigger force was 0.05 g. Measurements were taken from eight points distributed on both sides of the main vein of the leaves. Leaf thickness was finally measured using calipers on the same eight locations on the leaf.
4.3. Signal Recording and Playback
Vibrational signals were recorded using a previously described method [12]. These signals included the playback MCaS and mating signals, MCaSs, FS1s, MCoSs, and FS2s (Table 1), which were recorded using a laser vibrometer (VGO-200, Polytec, Waldbronn, Germany); these signals were digitized at a 48 kHz sample rate and 16-bit depth, transmitted via the generator module (LAN-XI 3160, Brüel and Kjær, Virum, Denmark), and saved using BK Connect software 2019 (Brüel and Kjær). To maximize the signal-to-noise ratio, the laser beam was focused on a reflective sticker (5 mm2) on the tea leaf. Leafhopper behavior was recorded via webcams (C1000e, Logitech, Lausanne, Switzerland), which enabled us to simultaneously observe behaviors associated with the emission of vibrational signals on the computers (Figure 1).
In Section 4.4.1, a female was repeatedly stimulated with a pre-recorded MCaS from an E. onukii male. The playback signal (repeated MCaSs) was sent to the generator module by BK Connect and converted into electrical signals, which were then transmitted through a power amplifier (type 2718, Brüel and Kjær) to a mini-shaker (Type 4810, Brüel and Kjær). A conical rod attached to the mini-shaker was in contact with the lower lamina of the target leaf to transmit MCaSs (Figure 1). To ensure that the parameters of the playback MCaS on the leaf in contact with the conical rod were consistent for each test, we recorded the playback with the laser vibrometer and fine-tuned the sound file before the test until the MCaS had the desired properties [28]. Based on the mean intensity of MCaSs recorded in Section 4.4.2, the signal intensity was adjusted to 8 ± 2 μm/s using BK Connect and the power amplifier before each test. During each test, the vibrations in the tea leaves were monitored in real time. The playback stimulation and the signals emitted by E. onukii were distinguished by differences in the signal spectrograms.
4.4. Experimental Setup
All experiments were conducted at dawn, between 5:00 h and 8:30 h, or dusk, between 17:00 h and 21:30 h, to ensure optimal mating activity of E. onukii [12] in an anechoic and sound-insulated chamber at TRI, CAAS with the same environment as the insectary.
4.4.1. Female Responses to Signals from Other Leaves
Repeated units of a pre-recorded MCaS (duration = 1.491 s; Df = 340.8 Hz) were used to synthesize a 5 min playback signal at 2.5 s intervals by Adobe Audition 2021 (Adobe Systems Incorporated, San Jose, CA, USA). The synthesized signal was played back from leaves of different leaf ages and transmitted to female leaves (i = 7) (Figure 1A). We determined the parameters of transmitted MCaSs, as well as the responses of female E. onukii (Table 1). The key parameters of MCaSs affecting female responses were analyzed.
First, we inserted the tea branch into the flower mud and kept it vertical. The reflective stickers were attached to the middle of the main vein on the front lamina of each leaf on the branch. Next, the intensity of the playback signal on the target leaf that was in contact with the conical rod of the mini-shaker was pre-adjusted. The playback was paused. Next, a female leafhopper was guided to the seventh leaf under the bud, where females preferred to mate [12]. After a 5 min acclimation period, the synthesized signal was played back for 5 min; the vibrational signals on the leaf that the female inhabited were recorded (signals FS1). Finally, we removed the female and attached a reflective sticker to the location of the female, we then played back the MCaSs and recorded the signals (signals MCaS) again. To prevent the insects from escaping, the experiments were performed within a clear plexiglass cylinder (20 × 20 × 35 cm). In each test, we played back the synthesized signal on the second, fourth, sixth, seventh, eighth, and tenth leaves under the bud (i = 2, 4, 6, 7, 8, and 10, respectively; Figure 1). A new female was used for each playback test. The tests were repeated on a total of 20 tea branches.
A total of 120 signal samples were recorded, and each signal sample included a ‘signals FS1’ and a ‘signals MCaS’; the 120 trials were divided into two categories: female responses and female non-responses. We measured three signal parameters [N = 120; Df, D, and I] of the first MCaS in each ‘signals MCaS’. In the female response trials, we determined two female behavioral indexes in each ‘signals FS1’: NFS1 and DelayID (Table 1). NFS1 was the total number of FS1 in a signal sample, which indicated whether the transmitted MCaS had an enduring attraction for females. DelayID was the time interval from the end of the MCaS to the beginning of the subsequent FS1 and indicated the response delay of females to signals from males.
4.4.2. Female Responses to Signals from the Same Leaf
In this test, we recorded the mating signals of a male–female pair on a single leaf of different leaf ages. The petiole of a tea leaf was placed into flower mud filled with water to prevent withering and keep it horizontal. The reflective sticker was attached to the middle of the main vein of the tea leaf. A pair of leafhoppers was guided to the leaf, and the vibrational signals on the leaf were recorded at the same time until the male and female copulated. To prevent the leafhoppers from escaping, the setup was contained within a plastic 6 cm diameter Petri dish (Figure 1B). The first to tenth single leaves under the bud (i from 1 to 10) were used. A new pair of leafhoppers was used for each test, and tests with the leaves of each age were repeated seven times. A total of 113 pairs were tested, and 70 pairs mated. Pairs that failed to copulate within 20 min were regarded as failed tests.
After the tests, we measured six leaf traits: leaf area, leaf thickness, leaf width, main vein length, leaf margin perimeter, and leaf hardness (Table 1). The tests generated 70 signal samples. In each signal sample, considering the intraindividual variability in mating signal parameters of E. onukii (Figure S1), we collected signal parameters in the first identification duet and the second location duet (N = 70; Table 1) to determine the effects of leaf traits on signal parameters and the effects of signal parameters on leafhopper behaviors. In addition to the six leaf traits and signal parameters, the whole time (tcourtship) spent in the courtship process in each signal sample was recorded. We also measured the first DelayID in the identification stage, the second DelayLO and IntervalLO in the location stage, and the number (Ncycle) of localization cycles in each signal sample. IntervalLO was the interval between two MCoSs and indicated the feedback speed of males to FS2 in the localization stage (Table 1).
4.5. Terminology and Data Analyses
The terms and acronyms were designated on the basis of signal structure, behavioral context, and previous studies [12,29].
Intensity analysis of vibrational signals was performed using BK Connect software 2019 with the following Fast Fourier Transform (FFT) parameters: Hanning window, 75% overlap, 12.8 kHz frequency span, and 8 Hz frequency resolution. The mean vibration velocity at the dominant frequency component within the playback signal was regarded as the signal intensity. The temporal parameters of the signals recorded were analyzed using Adobe Audition 2021 with the following FFT parameters: Hanning window, window length of 8192 samples, and 75% overlap.
4.5.1. Female Responses to Signals from Other Leaves
The test in Section 4.4.1 involved only the identification stage of E. onukii. Using leaf age (i) as the categorical variable, we determined the effect of leaf age on the signal parameters of transmitted MCaSs by analysis of variance. One-way ANOVA was used when the data met assumptions of normality and homoscedasticity; otherwise, Welch’s ANOVA was performed (p < 0.05; SPSS 25.0, IBM Corp., Armonk, NY, USA).
We used correlation analysis and multiple linear regression to identify potential factors that might contribute to explaining variance in signal parameters or mating behavior indexes. To determine which MCaS parameters contributed to female behaviors, we designated MCaS parameters that were significantly correlated with female behaviors (N = 120, Spearman’s correlation analysis, p < 0.05; SPSS 25.0) as independent variables; we then determined the MCaS parameters that significantly affected female behaviors using multiple linear regression (p < 0.05; SPSS 25.0). The independence between samples was then assessed by a Durbin–Watson test (p > 0.05). If the standardized residuals did not meet the assumptions of homoscedasticity and normality, the variables were ln-transformed to meet these requirements. The multicollinearity of the independent variable was determined by the variance inflation factor (VIF < 5), and independent variables with high linear relationships were eliminated; a secondary analysis was conducted to finally identify significant variables (p < 0.05).
In addition, MCaS parameters between the two categories (female response and non-response) were compared using an independent sample t-test (two-tailed, p < 0.05; SPSS 25.0) to determine the effects of these parameters on female behavior.
4.5.2. Female Responses to Signals from the Same Leaf
The test in Section 4.4.2 involved the identification and localization stages of E. onukii. Using leaf age (i) as the categorical variable, we determined the effect of leaf age on leaf traits and mating signals by analysis of variance, which was performed as described in Section 4.5.1.
To determine which leaf traits potentially affect signal parameters and which signal parameters contribute to variance in behavioral indexes, the correlations between leaf traits, signal parameters, and four behavioral indexes were first determined (N = 70, Spearman’s, p < 0.05; SPSS 25.0); next, we used the leaf traits that were significantly correlated with signal parameters as independent variables to determine the leaf traits that significantly affected signal parameters using multiple linear regression (p < 0.05; SPSS 25.0); finally, signal parameters correlated with behavioral indexes were used as independent variables to determine the potential signal parameters that significantly affected the behavioral indexes in the multiple linear regression (p < 0.05).
5. Conclusions
Herbivorous insects need to adapt to hosts to optimize their reproduction [10]. The significant correlation between host leaf traits and mating signal parameters, as well as between female and male signal parameters, indicated that leaf traits affect the excitation and transmission of mating signals of male E. onukii and indirectly affect female signal excitation and behavior. Host plant adaptations of E. onukii for generating vibrational signals promote information transfer and mitigate physical constraints. As a commercially significant crop [30], tea exhibits remarkable diversity in the physical properties of its leaves across different varieties. These variations manifest in characteristics such as color, size, shape, thickness, and hardness [13,31]. Our findings show that some leaf traits are not conducive to the mating communication of E. onukii and have implications for the breeding of varieties with resistance to this serious pest.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14071147/s1.
Author Contributions
Conceptualization, Y.S., Q.Y. and Q.J.; data curation, Y.S., Q.Y., Q.J. and J.L.; formal analysis, Y.S., Q.Y., Q.J. and J.L.; funding acquisition, L.B.; investigation, J.L.; methodology, Y.S., Q.Y. and Q.J.; project administration, Z.C.; resources, X.C., Z.C. and L.B.; software, X.C. and L.B.; supervision, Z.C.; validation, X.C. and L.B.; visualization, X.C. and L.B.; writing—original draft, Y.S., Q.Y. and Q.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the Youth Innovation Program of the Chinese Academy of Agricultural Sciences (Y2022QC25, China) and the Modern Agricultural Industry Technology System (CARS-19, China).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Cividini, S.; Giuseppe, M. Biotremology in arthropods. Learn. Behav. 2020, 48, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, V.; Anfora, G.; Cocroft, R.B.; Fatouros, N.E.; Groot, A.T.; Gross, J.; Hill, P.S.M.; Hoch, H.; Ioriatti, C.; Nieri, R.; et al. Bridging biotremology and chemical ecology: A new terminology. Trends Plant Sci. 2024, 29, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Cocroft, R.B.; Rodríguez, R.L. The behavioral ecology of insect vibrational communication. Bioscience 2005, 55, 323–334. [Google Scholar] [CrossRef]
- Cocroft, R.B.; Shugart, H.J.; Konrad, K.T.; Tibbs, K. Variation in plant substrates and its consequences for insect vibrational communication. Ethology 2006, 112, 779–789. [Google Scholar] [CrossRef]
- Joyce, A.L.; White, W.H.; Medina, R.F. Host plants impact courtship vibration transmission and mating success of a parasitoid wasp, Cotesia flavipes (Hymenoptera: Braconidae). Evol. Ecol. 2014, 28, 361–372. [Google Scholar] [CrossRef]
- Velilla, E.; Polajnar, J.; Virant-Doberlet, M.; Commandeur, D.; Simon, R.; Cornelissen, J.H.; Ellers, J.; Halfwerk, W. Variation in plant leaf traits affects transmission and detectability of herbivore vibrational cues. Ecol. Evol. 2020, 10, 12277–12289. [Google Scholar] [CrossRef]
- Mortimer, B. Biotremology: Do physical constraints limit the propagation of vibrational information? Anim. Behav. 2017, 130, 165–174. [Google Scholar] [CrossRef]
- Rosenthal, M.F.; Hebets, E.A.; Kessler, B.; McGinley, R.; Elias, D.O. The effects of microhabitat specialization on mating communication in a wolf spider. Behav. Ecol. 2019, 30, 1398–1405. [Google Scholar] [CrossRef]
- Elias, D.O.; Mason, A.C.; Hoy, R.R. The effect of substrate on the efficacy of seismic courtship signal transmission in the jumping spider Habronattus dossenus (Araneae: Salticidae). J. Expe Biol. 2004, 207, 4105–4110. [Google Scholar] [CrossRef]
- McNett, G.D.; Cocroft, R.B. Host shifts favor vibrational signal divergence in Enchenopa binotata treehoppers. Behav. Ecol. 2008, 19, 650–656. [Google Scholar] [CrossRef]
- Bian, L.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Chen, Z.M. Foliage intensity is an important cue of habitat location for Empoasca onukii. Insects 2020, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Zhou, X.S.; Cai, X.M.; Luo, Z.X.; Li, Z.Q.; Xiu, C.L.; Chen, Z.M.; Bian, L. Mating and post-copulation behavior in the tea leafhopper, Empoasca onukii (Hemiptera: Cicadellidae). Front. Plant Sci. 2023, 14, 1273718. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, L.K.; Bhuyan, M.; Hazarika, B.N. Insect pests of tea and their management. Annu. Rev. Entomol. 2009, 54, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Virant-Doberlet, M.; Stritih-Peljhan, N.; Žunič-Kosi, A.; Polajnar, J. Functional diversity of vibrational signaling systems in insects. Annu. Rev. Entomol. 2023, 68, 191–210. [Google Scholar] [CrossRef]
- Zhang, H.; Bian, L.; Cai, X.M.; Yao, Q.; Fu, N.X.; Shan, Y.; Chen, Z.M. Vibrational signals are species-specific and sex-specific for sexual communication in the tea leafhopper, Empoasca onukii. Entomol. Exp. Appl. 2023, 171, 277–286. [Google Scholar] [CrossRef]
- Mazzoni, V.; Eriksson, A.; Anfora, G.; Lucchi, A.; Virant-Doberlet, M. Active space and the role of amplitude in plant-borne vibrational communication. In Studying Vibrational Communication. Animal Signals and Communication; Cocroft, R., Gogala, M., Hill, P., Wessel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 3, pp. 125–145. [Google Scholar] [CrossRef]
- Kuhelj, A.; De Groot, M.; Pajk, F.; Simčič, T.; Virant-Doberlet, M. Energetic cost of vibrational signalling in a leafhopper. Behav. Ecol. Sociobiol. 2015, 69, 815–828. [Google Scholar]
- Virant-doberlet, M.; King, R.A.; Polajnar, J.; Symondson, W.O. Molecular diagnostics reveal spiders that exploit prey vibrational signals used in sexual communication. Mole Ecol. 2011, 20, 2204–2216. [Google Scholar]
- Huang, Y.H.; Zhang, J.W.; Zhang, Y.L.; Yang, Y.; Wang, Y.J. Anatomical characteristics of leaf structure of tea plant resistant to leafhopper (Empoasca vitis Gothe). J. Tea Sci. 1998, 18, 35–38. [Google Scholar]
- De Langre, E. Plant vibrations at all scales: A review. J. Exp. Bot. 2019, 70, 3521–3531. [Google Scholar] [CrossRef]
- Polajnar, J.; Kavčič, A.; Kosi, A.; Čokl, A. Palomena prasina (Hemiptera: Pentatomidae) vibratory signals and their tuning with plant substrates. Open Life Sci. 2013, 8, 670–680. [Google Scholar]
- Čokl, A.; Zorović, M.; Millar, J.G. Vibrational communication along plants by the stink bugs Nezara viridula and Murgantia histrionica. Behav. Process. 2007, 75, 40–54. [Google Scholar]
- Kuhelj, A.; de Groot, M.; Blejec, A.; Virant-Doberlet, M. Sender-receiver dynamics in leafhopper vibrational duetting. Anim. Behav. 2016, 114, 139–146. [Google Scholar]
- Guilford, T.; Dawkins, M.S. Receiver psychology and the evolution of animal signals. Anim. Behav. 1991, 42, 1–14. [Google Scholar]
- Pollack, G. Who, what, where? Recognition and localization of acoustic signals by insects. Curr. Opin. Neurobiol. 2000, 10, 763–767. [Google Scholar] [PubMed]
- Čokl, A.; Dias, A.M.; Moraes, M.C.B.; Borges, M.; Laumann, R.A. Rivalry between stink bug females in a vibrational communication network. J. Insect Behav. 2017, 30, 741–758. [Google Scholar]
- Čokl, A.; Blassioli-Moraes, M.C.; Laumann, R.A.; Žunič, A.; Borges, M. Stinkbugs: Multisensory Communication with Chemical and Vibratory Signals Transmitted Through Different Media. In Biotremology: Studying Vibrational Behavior. Animal Signals and Communication; Hill, P., Lakes-Harlan, R., Mazzoni, V., Narins, P., Virant-Doberlet, M., Wessel, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 6, pp. 91–122. [Google Scholar]
- Cocroft, R.B.; Hamel, J.; Su, Q.; Gibson, J. Vibrational Playback Experiments: Challenges and Solutions. In Studying Vibrational Communication. Animal Signals and Communication; Cocroft, R., Gogala, M., Hill, P., Wessel, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 3, pp. 249–274. [Google Scholar]
- Nieri, R.; Anfora, G.; Mazzoni, V.; Stacconi, R. Semiochemicals, semiophysicals and their integration for the development of innovative multi-modal systems for agricultural pests’ monitoring and control. J. Mediterr. Ecol. 2021, 19, 42. [Google Scholar]
- Chen, Z.M.; Luo, Z.X. Management of insect pests on tea plantations: Safety, sustainability, and efficiency. Annu. Rev. Entomol. 2025, 70, 359–377. [Google Scholar]
- Awmack, C.S.; Leather, S.R. Host plant quality and fecundity in herbivorous insects. Annu. Rev. Entomol. 2002, 47, 817–844. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).