Divergent Mechanisms of Internode Elongation in Response to Far-Red in Two Rose Genotypes
Abstract
1. Introduction
2. Results
2.1. Effects of Far-Red (FR) on Internode Elongation in ‘The Fairy’ (TF) and Knock Out® Radrazz (KO)
2.2. Hormonal Changes Associated with FR-Induced Internode Elongation in TF and KO
2.3. Gene Expression Changes in the Internode in Response to FR in TF and KO
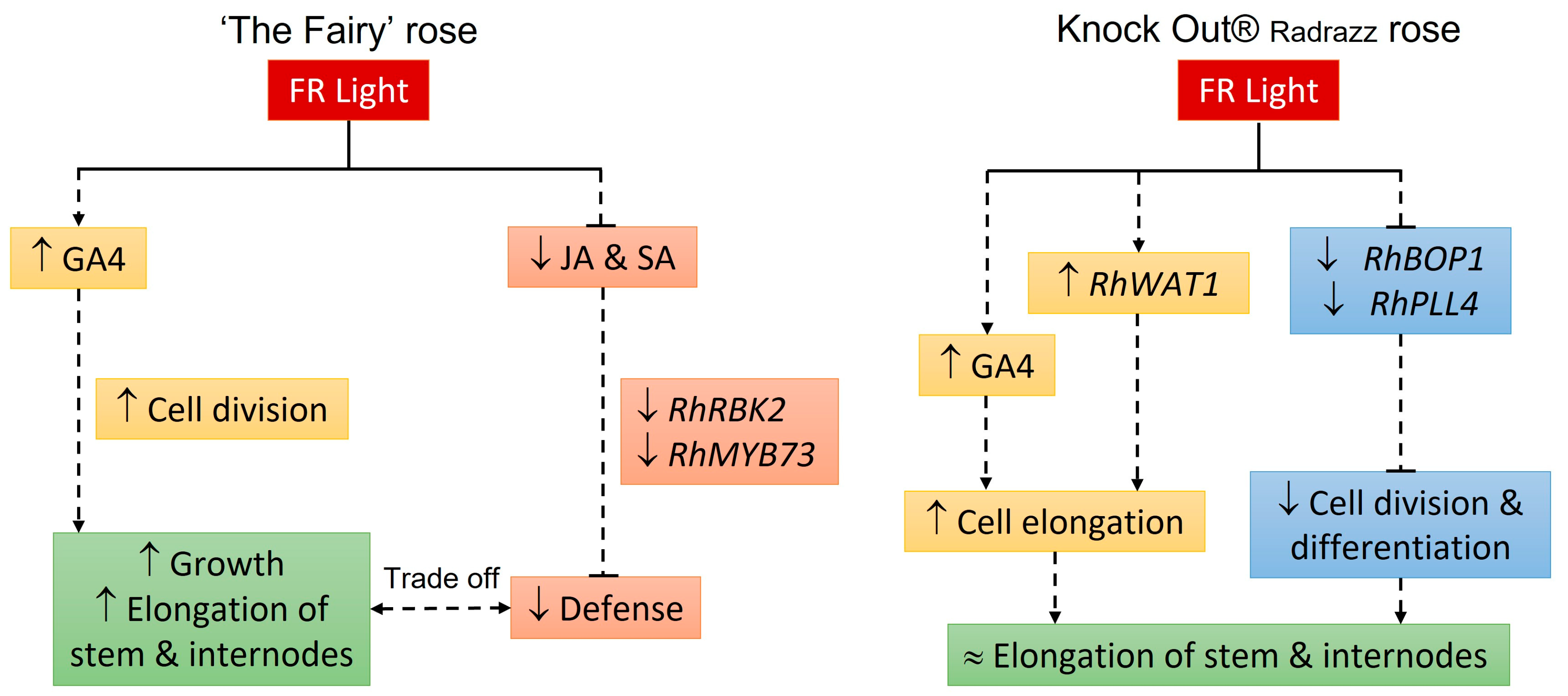
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Conditions
4.3. Kinetics of Construction of the Order 1 Axis
4.4. Measurement of the Length of Parenchyma Cells in the Fourth Internode Underlying the Apex of the Order 1 Axis
4.5. Hormone Concentration Measurements in the Internode Underlying the Apex of the Order 1 Axis
4.6. RNA Extraction, Illumina Sequencing, and RNA-Seq Data Analysis
4.7. Real-Time Quantitative Polymerase Chain Reaction (qRTPCR)
4.8. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boumaza, R.; Demotes-Mainard, S.; Huché-Thélier, L.; Guérin, V. Visual characterization of the esthetic quality of the rosebush. J. Sens. Stud. 2009, 24, 774–796. [Google Scholar] [CrossRef]
- Garbez, M.; Galopin, G.; Sigogne, M.; Favre, P.; Demotes-Mainard, S.; Symoneaux, R. Assessing the visual aspect of rotating virtual rose bushes by a labeled sorting task. Food Qual. Prefer. 2015, 40, 287–295. [Google Scholar] [CrossRef]
- Morel, P.; Galopin, G.; Donès, N. Using architectural analysis to compare the shape of two hybrid tea rose genotypes. Sci. Hortic. 2009, 120, 391–398. [Google Scholar] [CrossRef]
- Galopin, G.; Morel, P.; Crespel, L.; Darmet, P.; Fillatre, J.; Mary, L.; Edelin, C. The influence of pruning on morphological and architectural characteristics of Camellia japonica L. in a tropical climate. Eur. J. Hortic. Sci. 2011, 76, 182–187. [Google Scholar] [CrossRef]
- Crespel, L.; Morel, P.; Galopin, G. Architectural and genetic characterization in Hydrangea aspera subsp. aspera Kawakami group, H. aspera subsp. sargentiana and their hybrids. Euphytica 2012, 184, 289–299. [Google Scholar] [CrossRef]
- Crespel, L.; Le Bras, C.; Relion, D.; Morel, P. Genotype x year interaction and broad-sense heritability of architectural characteristics in rose bush. Plant Breed. 2014, 133, 412–418. [Google Scholar] [CrossRef]
- Demotes-Mainard, S.; Huché-Thélier, L.; Morel, P.; Boumaza, R.; Guérin, V.; Sakr, S. Temporary water restriction or light intensity limitation promotes branching in rose bush. Sci. Hortic. 2013, 150, 432–440. [Google Scholar] [CrossRef]
- Li-Marchetti, C.; Le Bras, C.; Relion, D.; Citerne, S.; Huché-Thélier, L.; Sakr, S.; Morel, P.; Crespel, L. Genotypic differences in architectural and physiological responses to water restriction in rose bush. Front. Plant Sci. 2015, 6, 355. [Google Scholar] [CrossRef]
- Li-Marchetti, C.; Le Bras, C.; Chastellier, A.; Relion, D.; Morel, P.; Sakr, S.; Hibrand-Saint Oyant, L.; Crespel, L. 3D Phenotyping and QTL analysis of a complex character: Rose bush architecture. Tree Genet. Genomes 2017, 13, 112. [Google Scholar] [CrossRef]
- Ye, Y.J.; Wu, J.Y.; Feng, L.; Ju, Y.Q.; Cai, M.; Cheng, T.R.; Pan, H.T.; Zhang, Q.X. Heritability and gene effects for plant architecture traits of crape myrtle using major gene plus polygene inheritance analysis. Sci. Hortic. 2017, 225, 335–342. [Google Scholar] [CrossRef]
- Jiji Allen, J.; Kannan, M.; Thamaraiselvi, S.P.; Kumar, M. Genetic variability, heritability and correlation studies in Hibiscus rosa-sinensis. J. Pharmacog. Phytochem. 2019, 8, 1001–1004. [Google Scholar]
- Demotes-Mainard, S.; Péron, T.; Corot, A.; Bertheloot, J.; Le Gourrierec, J.; Pelleschi-Travier, S.; Crespel, L.; Morel, P.; Huché-Thélier, L.; Boumaza, R.; et al. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. [Google Scholar] [CrossRef]
- Huché-Thélier, L.; Crespel, L.; Le Gourrierec, J.; Morel, P.; Sakr, S.; Leduc, N. Light signaling and plant responses to blue and UV radiations-Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. [Google Scholar] [CrossRef]
- Morel, P. Growth control of Hydrangea macrophylla through water restriction. Acta Hortic. 2001, 548, 51–58. [Google Scholar] [CrossRef]
- Morel, P.; Crespel, L.; Galopin, G. Effect of mechanical stimulation on the growth and branching of garden rose. Sci. Hortic. 2012, 135, 59–64. [Google Scholar] [CrossRef]
- Ley-Ngardigal, B.; Guérin, V.; Huché-Thélier, L.; Brouard, N.; Eveleens, T.; Roman, H.; Leduc, N. Impact of mechanical stimulation on Hydrangea macrophylla. Acta Hortic. 2023, 1372, 275–282. [Google Scholar] [CrossRef]
- Paradiso, R.; Proietti, S. Light quality manipulation to control plant growth and photomorphogenesis in greenhouse horticulture: The state of the art and the opportunities of modern LED systems. J. Plant Growth Reg. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Islam, M.A.; Kuwar, G.; Clarke, J.L.; Blystad, D.-R.; Gislerød, H.R.; Olsen, J.E.; Torre, S. Artificial light from light emitting diodes (LEDs) with a high portion of blue light results in shorter poinsettias compared to high pressure sodium (HPS) lamps. Sci. Hortic. 2012, 147, 136–143. [Google Scholar] [CrossRef]
- Ouzounis, T.; Fretté, X.; Rosenqvist, E.; Ottosen, C.O. Spectral effects of supplementary lighting on the secondary metabolites in roses, chrysanthemums, and campanulas. J. Plant Physiol. 2014, 171, 1491–1499. [Google Scholar] [CrossRef]
- Yuan, C.Q.; Ahmad, S.; Cheng, T.R.; Wang, J.; Pan, H.T.; Zhao, L.J.; Zhang, Q. Red to far-red light ratio modulates hormonal and genetic control of axillary bud outgrowth in chrysanthemum (Dendranthema grandiflorum ‘Jinba’). Int. J. Mol. Sci. 2018, 19, 1590. [Google Scholar] [CrossRef]
- Crespel, L.; Le Bras, C.; Amoroso, T.; Unda Ulloa, M.G.; Morel, P.; Sakr, S. Genotype × light quality interaction on rose architecture. Agronomy 2020, 10, 913. [Google Scholar] [CrossRef]
- Crespel, L.; Le Bras, C.; Amoroso, T.; Dubuc, B.; Citerne, S.; Perez-Garcia, M.D.; Sakr, S. Involvement of sugar and abscisic acid in the genotype-specific response of rose to far-red light. Front. Plant Sci. 2022, 13, 929029. [Google Scholar] [CrossRef] [PubMed]
- Beall, F.D.; Yeung, E.C.; Pharis, R.P. Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Can. J. Bot. 1996, 74, 743–752. [Google Scholar] [CrossRef]
- Martinez-Garcia, J.F.; Santes, C.M.; Garcia-Martinez, J.L. The end-of-day far-red irradiation increases gibberellin A1 content in cowpea (Vigna sinensis) epicotyls by reducing inactivation. Physiol. Plant 2000, 108, 426–434. [Google Scholar] [CrossRef]
- Hisamatsu, T.; King, R.W.; Helliwell, C.A.; Koshioka, M. The involvement of gibberellin 20-oxidase genes in phytochrome-regulated petiole elongation of Arabidopsis. Plant Physiol. 2005, 138, 1106–1116. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Emery, R.J.N.; Pharis, R.P.; Reid, D.M. Uncoupling light quality from light irradiance effects in Helianthus annuus shoots: Putative roles for plant hormones in leaf and internode growth. J. Exp. Bot. 2007, 58, 2145–2157. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Walton, L.J.; Hayward, A.; Emery, R.J.N.; Pharis, R.P.; Reid, D.M. Interactions between plant hormones and light quality signaling in regulating the shoot growth of Arabidopsis thaliana seedlings. Botany 2012, 90, 237–246. [Google Scholar] [CrossRef]
- Takemura, Y.; Kuroki, K.; Katou, M.; Kishimoto, M.; Tsuji, W.; Nishihara, E.; Tamura, F. Gene expression changes triggered by end-of-day far-red light treatment on early developmental stages of Eustoma grandiflorum (Raf.) Shinn. Sci. Rep. 2015, 5, 17864. [Google Scholar] [CrossRef]
- Li, W.; Liu, S.W.; Ma, J.J.; Liu, H.M.; Han, F.X.; Li, Y.; Niu, S.H. Gibberellin signaling is required for far-red light-induced shoot elongation in Pinus tabuliformis seedlings. Plant Physiol. 2020, 182, 658–668. [Google Scholar] [CrossRef]
- Robson, F.; Okamoto, H.; Patrick, E.; Harris, S.R.; Wasternack, C.; Brearley, C.; Turner, J.G. Jasmonate and phytochrome A signaling in Arabidopsis wound and shade responses are integrated through JAZ1 stability. Plant Cell 2010, 22, 1143–1160. [Google Scholar] [CrossRef]
- Leone, M.; Keller, M.M.; Cerrudo, I.; Ballare, C.L. To grow or defend? Low red: Far-red ratios reduce jasmonate sensitivity in Arabidopsis seedlings by promoting DELLA degradation and increasing JAZ10 stability. New Phytol. 2014, 204, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Dierck, R.; Dhooghe, E.; Van Huylenbroeck, J.; Van Der Straeten, D.; De Keyser, E. Light quality regulates plant architecture in different genotypes of Chrysanthemum morifolium Ramat. Sci. Hortic. 2017, 218, 177–186. [Google Scholar] [CrossRef]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Song, X.; Gu, X.; Chen, S.; Qi, Z.; Yu, J.; Zhou, Y.; Xia, X. Far-red light inhibits lateral bud growth mainly through enhancing apical dominance independently of strigolactone synthesis in tomato. Plant Cell Environ. 2024, 47, 429–441. [Google Scholar] [CrossRef]
- Doidy, J.; Wang, Y.; Gouaille, L.; Goma-Louamba, I.; Jiang, Z.; Pourtau, N.; Le Gourrierec, J.; Sakr, S. Sugar transport and signaling in shoot branching. Int. J. Mol. Sci. 2024, 25, 13214. [Google Scholar] [CrossRef]
- Matsubara, S.; Koshimizu, K.; Nakahira, R. Cytokinin activities of dihydrozeatin in several bioassays. Si. Rep. Kyoto Pref. Univ. 1968, 19, 19–24. [Google Scholar]
- Courbier, S.; Pierik, R. Canopy light quality modulates stress responses in plants. iScience 2019, 22, 441–452. [Google Scholar] [CrossRef]
- Pierik, R.; Ballaré, C.L. Control of plant growth and defense by photoreceptors: From mechanisms to opportunities in agriculture. Mol. Plant 2021, 14, 61–76. [Google Scholar] [CrossRef]
- Jia, J.; Xing, J.H.; Dong, J.G.; Han, J.M.; Liu, J.S. Functional analysis of MYB73 of Arabidopsis thaliana against Bipolaris oryzae. Agric. Sci. China 2011, 10, 721–727. [Google Scholar] [CrossRef]
- Gu, K.D.; Zhang, Q.Y.; Yu, J.Q.; Wang, J.H.; Zhang, F.J.; Wang, C.K.; Zhao, Y.W.; Sun, C.H.; You, C.X.; Hu, D.G.; et al. R2R3-MYB transcription factor MdMYB73 confers increased resistance to the fungal pathogen Botryosphaeria dothidea in apples via the salicylic acid pathway. J. Agric. Food Chem. 2020, 69, 447–458. [Google Scholar] [CrossRef]
- Molendijk, A.J.; Ruperti, B.; Singh, M.K.; Dovzhenko, A.; Ditengou, F.A.; Milia, M.; Westphal, L.; Rosahl, S.; Soellick, T.R.; Uhrig, J.; et al. A cysteine-rich receptor-like kinase NCRK and a pathogen-induced protein kinase RBK1 are Rop GTPase interactors. Plant J. 2008, 53, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, J.; Zhai, Q.; Zhou, W.; Qi, L.; Xu, L.; Wang, B.; Chen, R.; Jiang, H.; Qi, J.; et al. The basic helix-loop-helix transcription factor MYC2 directly represses PLETHORA expression during jasmonate-mediated modulation of the root stem cell niche in Arabidopsis. Plant Cell 2011, 23, 3335–3352. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.L.; Yao, J.; Mei, C.S.; Tong, X.H.; Zeng, L.J.; Li, Q.; Xiao, L.T.; Sun, T.P.; Li, J.; Deng, X.W.; et al. Plant hormone jasmonate prioritizes defense over growth by interfering with gibberellin signaling cascade. Proc. Natl. Acad. Sci. USA 2012, 109, E1192–E1200. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Chen, C.L.; Hsieh, H.L. Far-red light-mediated seedling development in Arabidopsis involves FAR-RED INSENSITIVE 219/JASMONATE RESISTANT 1-dependent and -independent pathways. PLoS ONE 2015, 10, e0132723. [Google Scholar] [CrossRef]
- Havko, N.; Major, I.; Jewell, J.; Attaran, E.; Howe, G. Control of carbon assimilation and partitioning by jasmonate: An accounting of growth–defense tradeoffs. Plants 2016, 5, 7. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Song, C.; Cao, Y.; Dai, J.; Li, G.; Manzoor, M.A.; Chen, C.; Deng, H. The multifaceted roles of MYC2 in plants: Toward transcriptional reprogramming and stress tolerance by jasmonate signaling. Front. Plant Sci. 2022, 13, 868874. [Google Scholar] [CrossRef]
- Yu, K.M.J.; McKinley, B.; Rooney, W.L.; Mullet, J.E. High planting density induces the expression of GA3-Oxidase in leaves and GA mediated stem elongation in bioenergy sorghum. Sci. Rep. 2021, 11, 46. [Google Scholar] [CrossRef]
- Pandey, M.; Singh, A.K.; DePauw, R.M.; Bokore, F.E.; Ellouze, W.; Knox, R.E.; Cuthbert, R.D. Coleoptile length, gibberellin sensitivity, and plant height variation of durum wheat in Canada. Can. J. Plant Sci. 2015, 95, 1259–1264. [Google Scholar] [CrossRef]
- Ranocha, P.; Denancé, N.; Vanholme, R.; Freydier, A.; Martinez, Y.; Hoffmann, L.; Köhler, L.; Pouzet, C.; Renou, J.P.; Sundberg, B.; et al. Walls are thin 1 (WAT1), an Arabidopsis homolog of Medicago truncatula NODULIN21, is a tonoplast-localized protein required for secondary wall formation in fibers. Plant J. 2010, 63, 469–483. [Google Scholar] [CrossRef]
- Ju, F.; Liu, S.; Zhang, S.; Ma, H.; Chen, J.; Ge, C.; Shen, Q.; Zhang, X.; Zhao, X.; Zhang, Y.; et al. Transcriptome analysis and identification of genes associated with fruiting branch internode elongation in upland cotton. BMC Plant Biol. 2019, 19, 415. [Google Scholar] [CrossRef]
- Cho, H.K.; Ahn, C.S.; Lee, H.S.; Kim, J.K.; Pai, H.S. Pescadillo plays an essential role in plant cell growth and survival by modulating ribosome biogenesis. Plant J. 2013, 76, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.S.; Cho, H.K.; Lee, D.H.; Sim, H.J.; Kim, S.G.; Pai, H.S. Functional characterization of the ribosome biogenesis factors PES, BOP1, and WDR12 (PeBoW), and mechanisms of defective cell growth and proliferation caused by PeBoW deficiency in Arabidopsis. J. Exp. Bot. 2016, 67, 5217–5232. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.D.; Chatterjee, M.; Coleman, L.; Clancy, M.A.; Folta, K.M. Analysis of Block of cell proliferation 1 (BOP1) activity in strawberry and Arabidopsis. Plant Sci. 2016, 245, 84–93. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Z.; Wang, L.; Chen, L.; Ma, L.; Lv, J.; Qiao, K.; Fan, S.; Ma, Q. GhBOP1 as a key factor of ribosomal biogenesis: Development of wrinkled leaves in upland cotton. Int. J. Mol. Sci. 2022, 23, 9942. [Google Scholar] [CrossRef]
- Song, S.K.; Lee, M.M.; Clark, S.E. POL and PLL1 phosphatases are CLAVATA1 signaling intermediates required for Arabidopsis shoot and floral stem cells. Development 2006, 133, 4691–4698. [Google Scholar] [CrossRef]
- Gagne, J.M.; Clark, S.E. The Arabidopsis stem cell factor POLTERGEIST is membrane localized and phospholipid stimulated. Plant Cell 2010, 22, 729–743. [Google Scholar] [CrossRef]
- Song, S.K.; Clark, S.E. POL and related phosphatases are dosage-sensitive regulators of meristem and organ development in Arabidopsis. Dev. Biol. 2005, 285, 272–284. [Google Scholar] [CrossRef]
- White, J. The plant as a metapopulation. Annu. Rev. Ecol. Evol. Syst. 1979, 10, 109–145. [Google Scholar] [CrossRef]
- Seo, M.; Jikumaru, Y.; Kamiya, Y. Profiling of hormones and related metabolites in seed dormancy and germination studies. In Seed Dormancy. Methods in Molecular Biology; Kermode, A.R., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 773, pp. 99–111. [Google Scholar] [CrossRef]
- Cao, D.; Barbier, F.; Yoneyama, K.; Beveridge, C.A. A rapid method for quantifying RNA and phytohormones from a small amount of plant tissue. Front. Plant Sci. 2020, 11, 605069. [Google Scholar] [CrossRef]
- Hibrand Saint-Oyant, L.; Ruttink, T.; Hamama, L.; Kirov, I.; Lakhwani, D.; Zhou, N.N.; Bourke, P.M.; Daccord, N.; Leus, L.; Schulz, D.; et al. A high-quality genome sequence of Rosa chinensis to elucidate ornamental traits. Nat. Plants 2018, 4, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S. AnaDiff: A tool for differential analysis of microarrays and RNAseq (v4.3). Zenodo. 2022. Available online: https://zenodo.org/records/6477918 (accessed on 20 February 2025).
- Barbier, F.; Péron, T.; Lecerf, M.; Perez-Garcia, M.D.; Barrière, Q.; Rolčík, J.; Boutet-Mercey, S.; Citerne, S.; Lemoine, R.; Porcheron, B.; et al. Sucrose is an early modulator of the key hormonal mechanisms controlling bud outgrowth in Rosa hybrida. J. Exp. Bot. 2015, 66, 2569–2582. [Google Scholar] [CrossRef]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef]
- Chua, S.L.; Too, W.C.S.; Khoo, B.Y.; Few, L.L. UBC and YWHAZ as suitable reference genes for accurate normalisation of gene expression using MCF7, HCT116 and HepG2 cell lines. Cytotechnology 2011, 63, 645–654. [Google Scholar] [CrossRef]
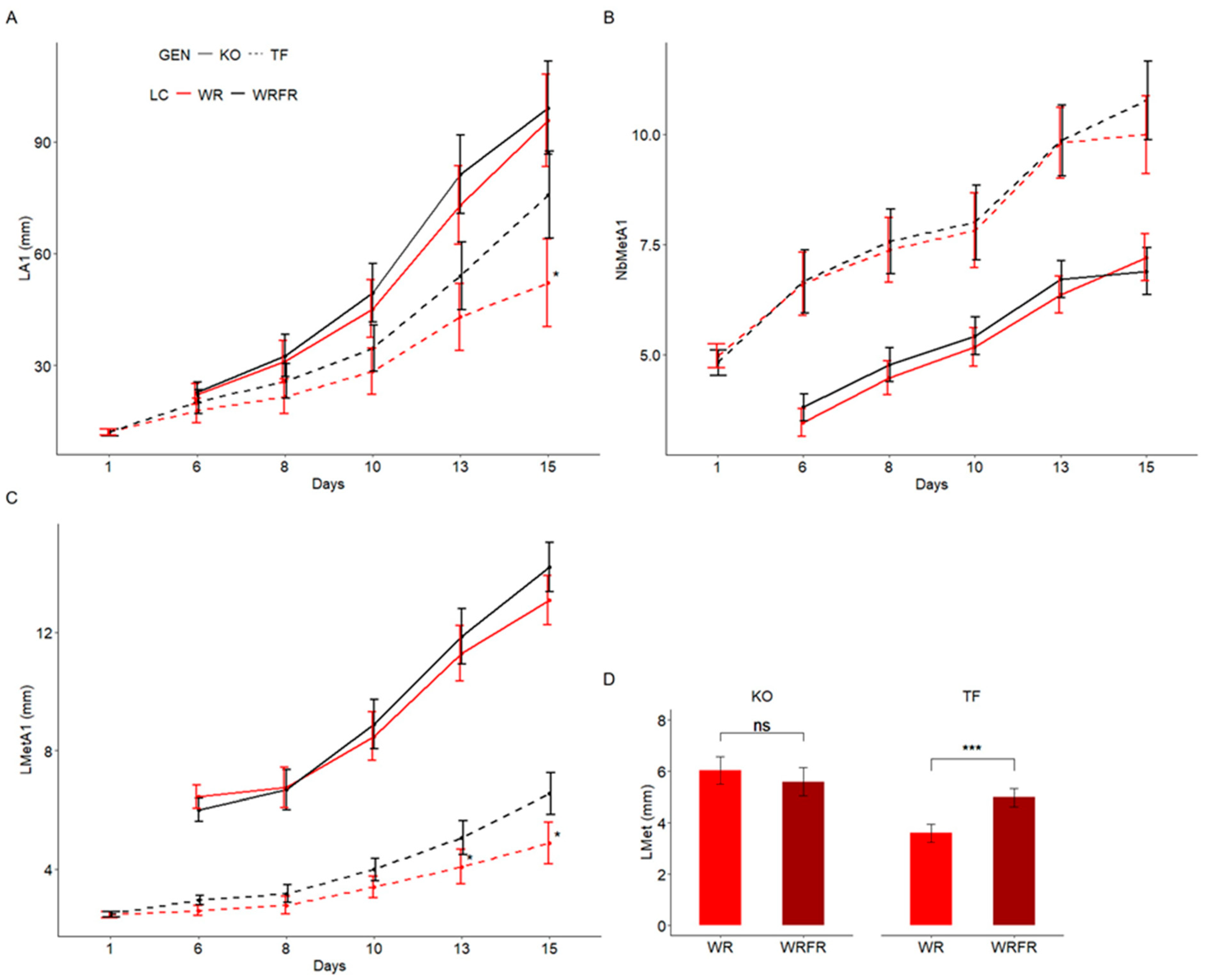
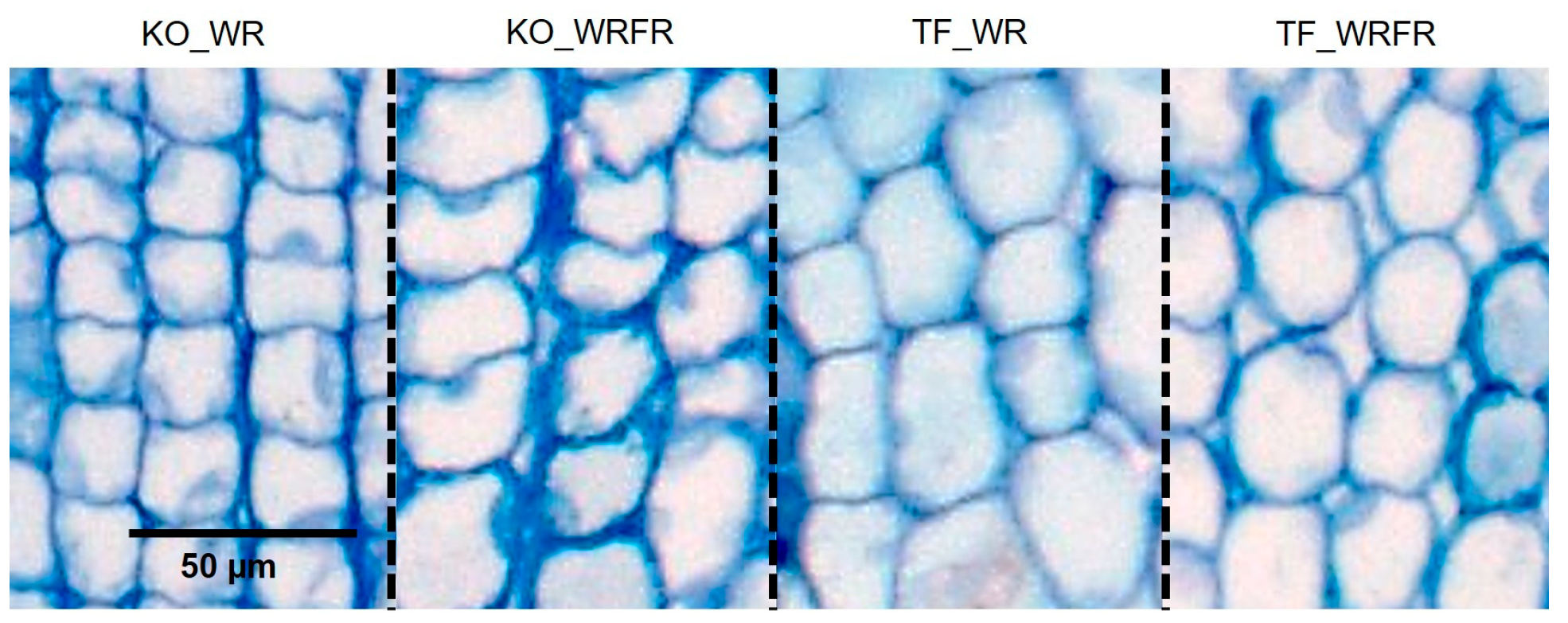
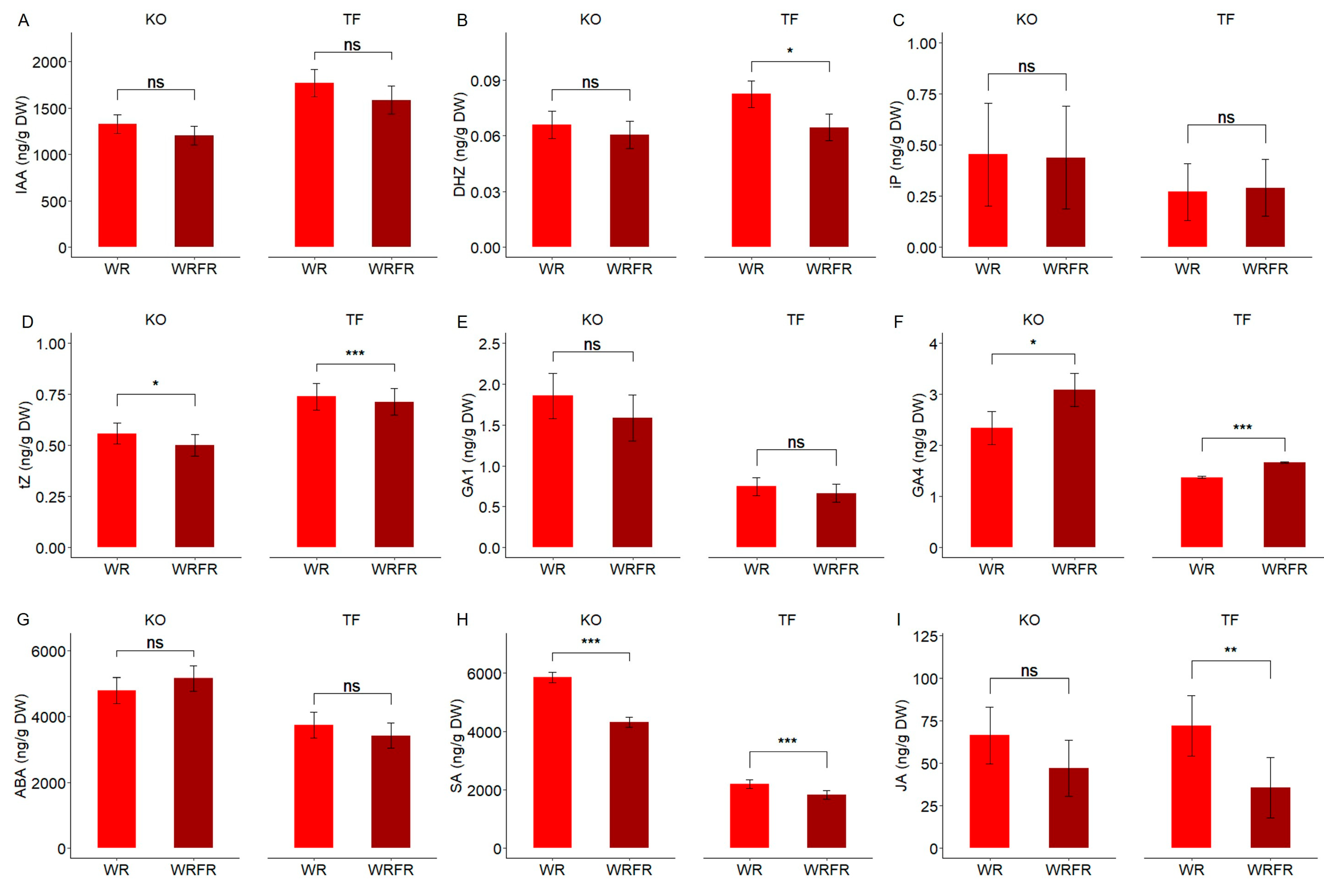
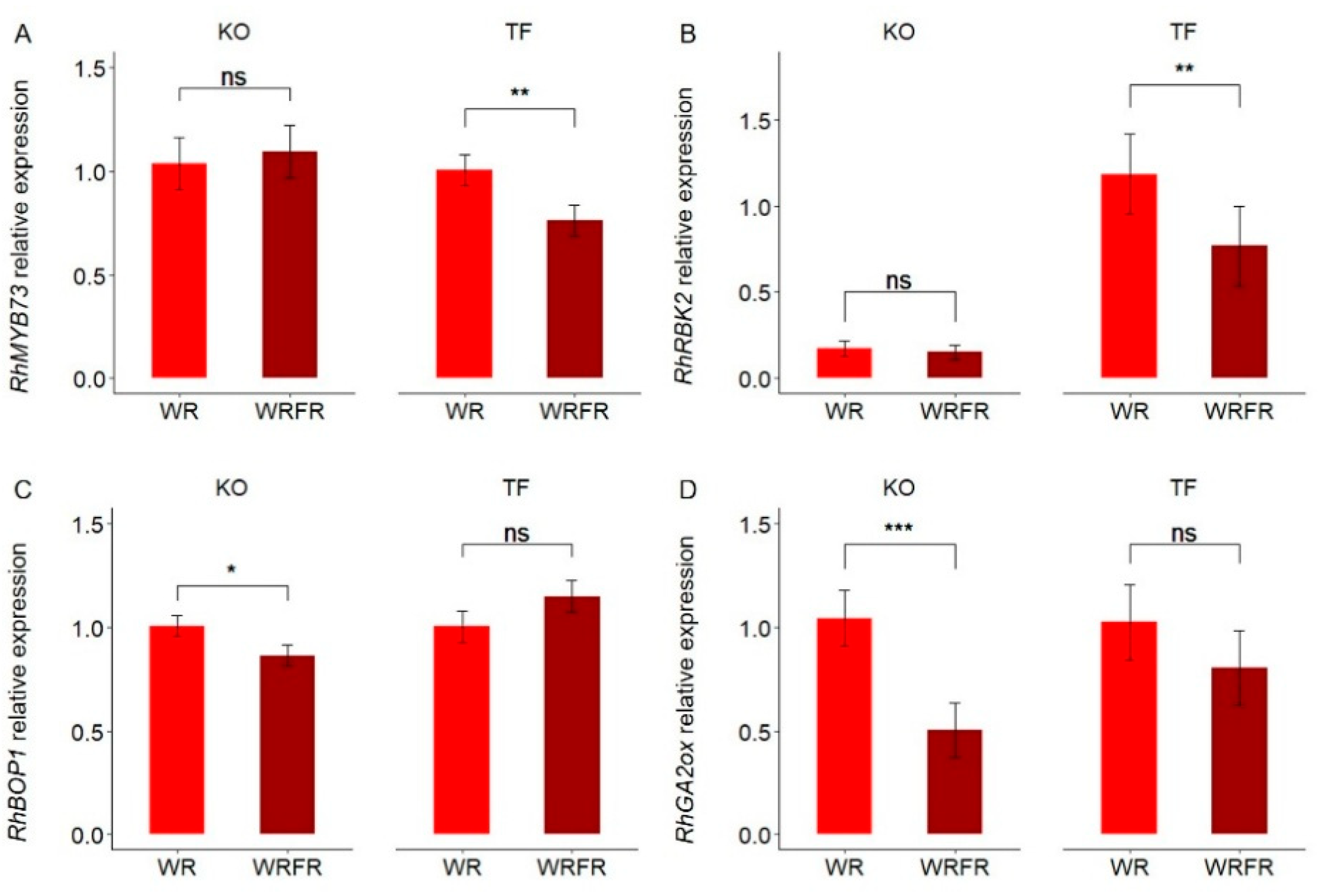
| Genotype | Fourth Internode Underlying the Apex on the 14th Day of Cultivation | Internode Underlying the Apex on the 13th Day of Cultivation | ||
|---|---|---|---|---|
| Cell Length (µm) | Estimated Vertical Cell Number | |||
| WR | WRFR | WR | WRFR | |
| KO | 21.4 a | 23.7 b | 283.0 | 236.0 |
| TF | 20.9 a | 21.4 a | 173.0 | 233.0 |
| DEGs | Gene ID | Description | Name | KO_WRFR vs. KO_WR | ||
|---|---|---|---|---|---|---|
| log2 Fold Change | p-Value | Adjusted p-Value (FDR) 1 | ||||
| Down-regulated | RC7G0576300 | Block of cell proliferation 1 | BOP1 | −1.02 | 4.4 × 10−7 | 7.0 × 10−3 |
| RC5G0246800 | Gibberellin 2-beta-dioxygenase 1 | GA2ox1 | −1.0 | 2.5 × 10−6 | 2.0 × 10−2 | |
| RC5G0037300 | Gibberellin 2-beta-dioxygenase 8 | GA2ox8 | −1.6 | 1.9 × 10−5 | 9.0 × 10−2 | |
| RC3G0259000 | Poltergeist-like | POL-like; PLL4 | −1.8 | 2.5 × 10−5 | 9.0 × 10−2 | |
| RC6G0422400 | S-adenosyl-L-methionine-dependent tRNA 4-demethylwyosine synthase | TYW1 | −2.5 | 2.1 × 10−5 | 9.0 × 10−2 | |
| Up-regulated | novel,2696 | Prolyl-tRNA synthetase | PRORS1 | 2.5 | 7.5 × 10−7 | 8.0 × 10−3 |
| RC0G0173400 | Walls are thin 1 related protein | WAT1 | 2.5 | 2.6 × 10−5 | 9.0 × 10−2 | |
| DEGs | Gene ID | Description | Name | TF_WRFR vs. TF_WR | ||
| log2 Fold Change | p-Value | Adjusted p-Value (FDR) | ||||
| Down-regulated | RC6G0094600 | Transcription factor | MYB73 | −1.0 | 6.4 × 10−6 | 3.0 × 10−2 |
| RC4G0261800 | Receptor-like cytosolic serine/ threonine-protein kinase | RBK2 | −1.2 | 9.4 × 10−6 | 4.0 × 10−2 | |
| RC6G0474700 | Uncharacterized protein | Y1015 | −3.6 | 5.8 × 10−8 | 9.0 × 10−4 | |
| Up-regulated | RC6G0451600 | Nucleoid-associated protein | STIC2 | 2.8 | 4.6 × 10−6 | 3.0 × 10−2 |
| Light Conditions | PPFD (µmol m−2 s−1) | TPFD (µmol m−2 s−1) | YPFD (µmol m−2 s−1) | B/R | B/G | R/FR |
|---|---|---|---|---|---|---|
| WR | 184.4 a 1 | 189.4 a | 165.8 a | 0.2 a | 0.7 a | 26.6 b |
| WRFR | 182.8 a | 204.8 b | 166.5 a | 0.2 a | 0.7 a | 6.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crespel, L.; Le Bras, C.; Dubuc, B.; Perez-Garcia, M.-D.; Carrera, E.; Rolland, A.; Gardet, R.; Sakr, S. Divergent Mechanisms of Internode Elongation in Response to Far-Red in Two Rose Genotypes. Plants 2025, 14, 1115. https://doi.org/10.3390/plants14071115
Crespel L, Le Bras C, Dubuc B, Perez-Garcia M-D, Carrera E, Rolland A, Gardet R, Sakr S. Divergent Mechanisms of Internode Elongation in Response to Far-Red in Two Rose Genotypes. Plants. 2025; 14(7):1115. https://doi.org/10.3390/plants14071115
Chicago/Turabian StyleCrespel, Laurent, Camille Le Bras, Bénédicte Dubuc, Maria-Dolores Perez-Garcia, Esther Carrera, Aurélia Rolland, Rémi Gardet, and Soulaiman Sakr. 2025. "Divergent Mechanisms of Internode Elongation in Response to Far-Red in Two Rose Genotypes" Plants 14, no. 7: 1115. https://doi.org/10.3390/plants14071115
APA StyleCrespel, L., Le Bras, C., Dubuc, B., Perez-Garcia, M.-D., Carrera, E., Rolland, A., Gardet, R., & Sakr, S. (2025). Divergent Mechanisms of Internode Elongation in Response to Far-Red in Two Rose Genotypes. Plants, 14(7), 1115. https://doi.org/10.3390/plants14071115







