From Ordinary to Extraordinary: The Crucial Role of Common Species in Desert Plant Community Stability with Arbuscular Mycorrhizal (AM) Fungi Under Increased Precipitation
Abstract
1. Introduction
2. Results
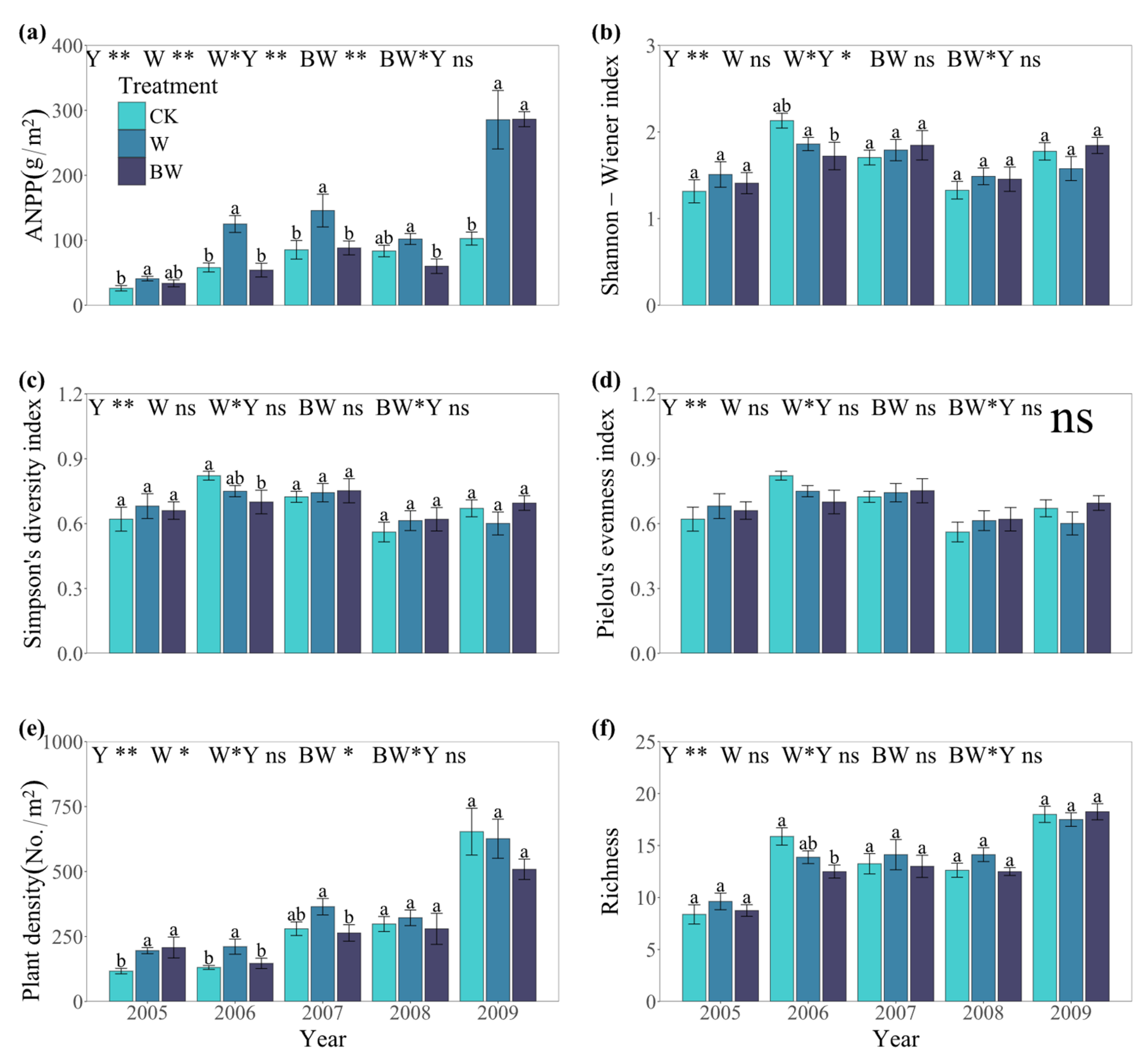
2.1. Responses of Plant Community to Increased Precipitation and Suppression of AM Fungi
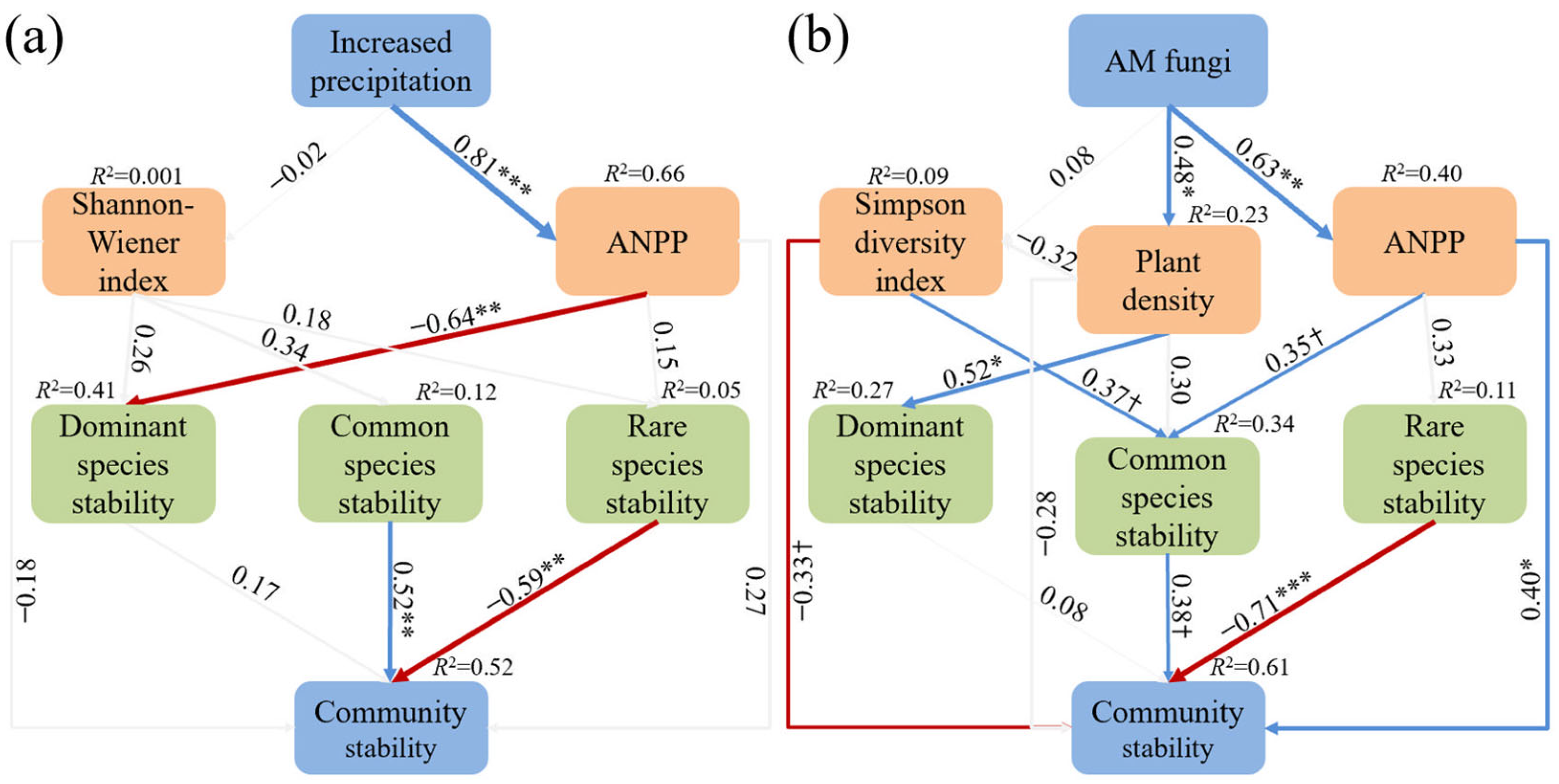
2.2. The Influence Pattern of Increased Precipitation and AM Fungi on Plant Community Stability
3. Discussion
3.1. Underlying Mechanisms of Increased Precipitation and AM Fungi on Plant Community Stability
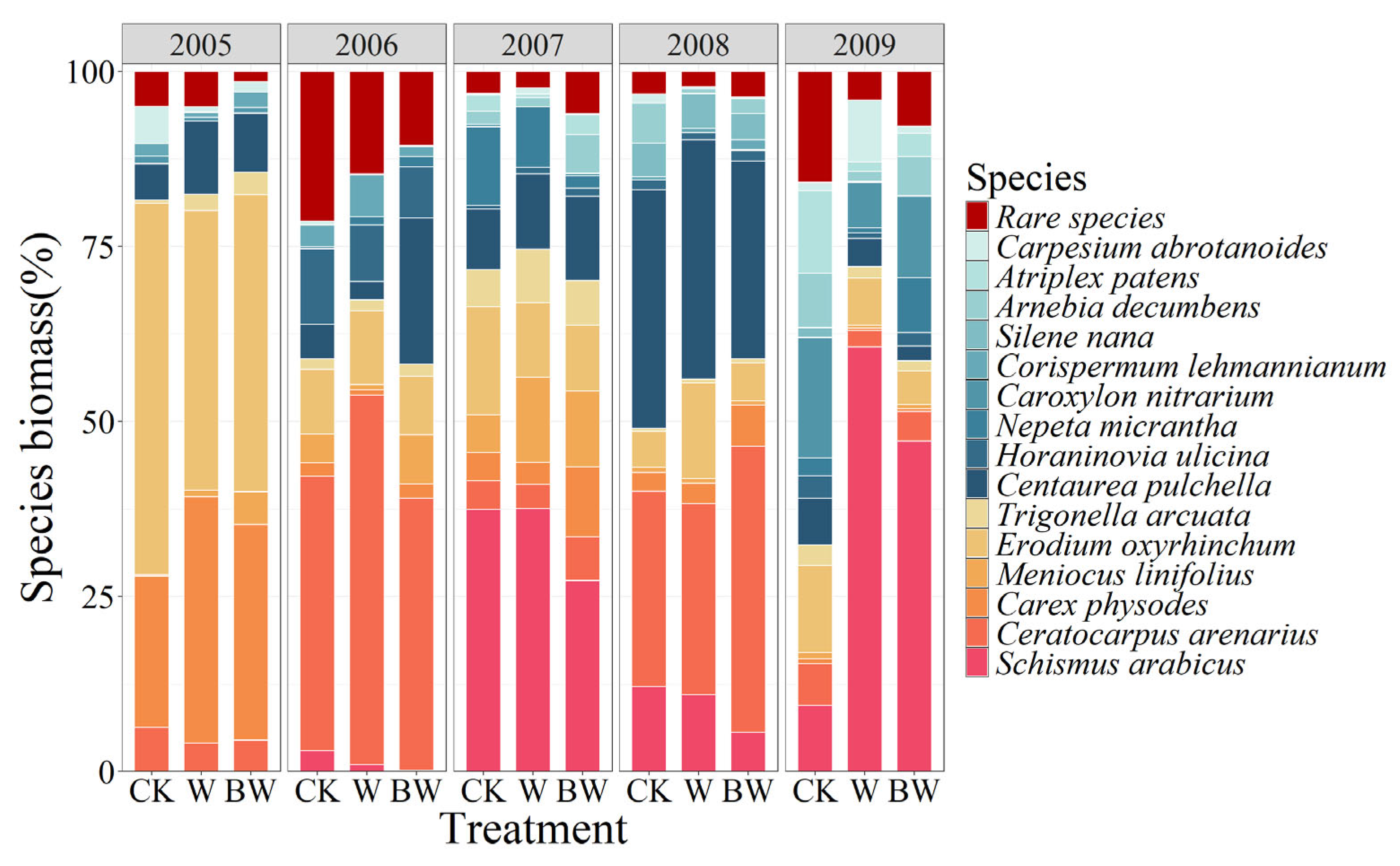
3.2. The Impacts of Increased Precipitation and AM Fungi on Plant Community Structure
4. Materials and Methods
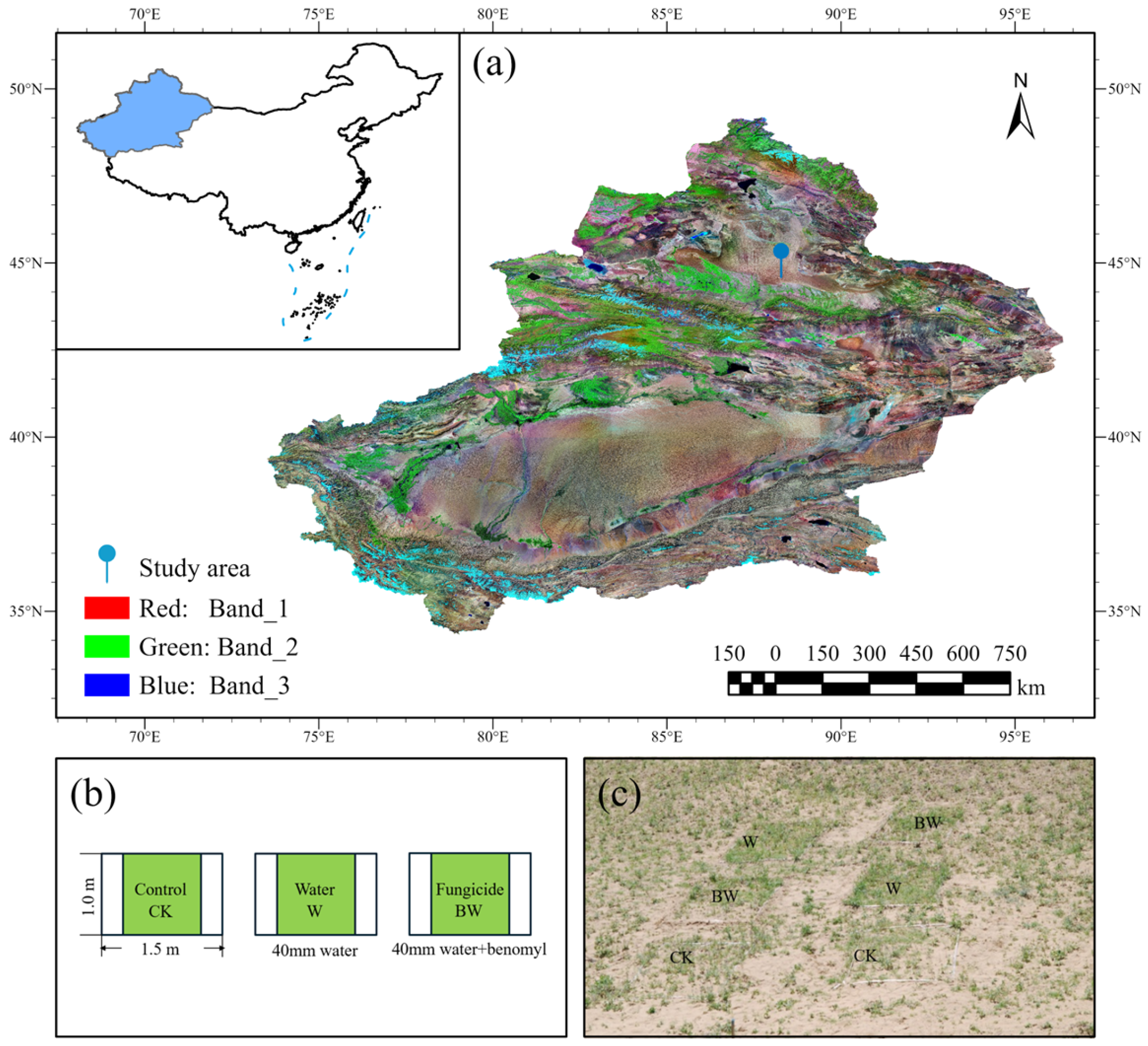
4.1. Study Site
4.2. Experimental Design
4.3. Sampling
4.4. Plant Community Diversity and Stability Analysis
4.5. Statistical Analysis
5. Conclusions
- (1)
- Dominant species drive community stability under increased precipitation. Increased precipitation enhanced ANPP and plant density and improved community stability. The dominant plant Meniocus linifolius was the primary contributor to community stability, supporting the mass ratio hypothesis. Dominant species dominated community dynamics under increased water availability due to their superior resource competitiveness and phenotypic plasticity.
- (2)
- AM fungi stabilize plant communities by enhancing ANPP and common species stability. AM fungi significantly increased the ANPP and plant density. By enhancing ANPP and stabilizing common species, AM fungi maintained plant community stability. Furthermore, the overall contribution of rare species to community stability was non-negligible, thereby validating the subordinate insurance hypothesis. AM fungi alleviated competitive pressure from dominant species through nutrient redistribution and improved the environmental adaptability of subordinate species.
- (3)
- ANPP, not diversity, mediates community stability across treatments. None of the treatments altered plant diversity, indicating that community stability was primarily driven by ANPP rather than diversity.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IPCC. AR6 Synthesis Report: Climate Change 2023. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 17 December 2024).
- Stott, P. How climate change affects extreme weather events. Research can increasingly determine the contribution of climate change to extreme events such as droughts. Science 2016, 352, 1517–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, L.; Li, Y.; Yang, P.; Liu, X.; Zhou, Z.; Zhong, H.; Ding, Y. Global extreme precipitation characteristics: The perspective of climate and large river basins. Clim. Dynam. 2024, 62, 1013–1030. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Zhang, Y.; Huang, C.; Pan, X.; Ma, M.; Song, M.; Zhao, H. More Extreme Precipitation in Chinese Deserts Prom 1960 to 2018. Earth Space Sci. 2019, 6, 1196–1204. [Google Scholar] [CrossRef]
- Donat, M.G.; Lowry, A.L.; Alexander, L.V.; O’Gorman, P.A.; Maher, N. More extreme precipitation in the world’s dry and wet regions. Nat. Clim. Change 2016, 6, 508–513. [Google Scholar] [CrossRef]
- Verrall, B.; Pickering, C.M. Alpine vegetation in the context of climate change: A global review of past research and future directions. Sci. Total Environ. 2020, 748, 141344. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, H.; Mi, Z.; Zhang, Z.; Wang, Y.; Xu, W.; Jiang, L.; He, J.-S. Climate warming reduces the temporal stability of plant community biomass production. Nat. Commun. 2017, 8, 15378. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Ho, C.-H.; Brown, M.E.; Kug, J.-S.; Piao, S. Browning in desert boundaries in Asia in recent decades. J. Geophys. Res. Atmos. 2011, 116, D02103. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, C.; Han, M.; Li, Y.; Yang, R. Temporal Patterns of Shrub Vegetation and Variation with Precipitation in Gurbantunggut Desert, Central Asia. Adv. Meteorol. 2015, 2015, 157245. [Google Scholar] [CrossRef]
- Li, F.; Zhao, W.; Liu, H. Productivity responses of desert vegetation to precipitation patterns across a rainfall gradient. J. Plant Res. 2015, 128, 283–294. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, H.; Li, Y.; Li, H.; Tang, Y. Analysis of the precipitation stability and variety trend in the desert region of northern China. Adv. Water Sci. 2008, 19, 792–799. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, T.; Walder, F.; Sun, Y.; Shi, Z.; Wagg, C.; Tian, C.; Feng, G. Can mycorrhizal fungi alleviate plant community instability caused by increased precipitation in arid ecosystems? Plant Soil 2022, 478, 559–577. [Google Scholar] [CrossRef]
- Lv, G.; He, M.; Wang, C.; Wang, Z. The stability of perennial grasses mediates the negative impacts of long-term warming and increasing precipitation on community stability in a desert steppe. Front. Plant Sci. 2023, 14, 1235510. [Google Scholar] [CrossRef] [PubMed]
- Makhalanyane, T.P.; Valverde, A.; Gunnigle, E.; Frossard, A.; Ramond, J.-B.; Cowan, D.A. Microbial ecology of hot desert edaphic systems. FEMS Microbiol. Rev. 2015, 39, 203–221. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-F.; He, Z.-B.; Zhao, W.-Z.; Liu, J.-L.; Zhou, H.; Li, J.; Meng, Y.-Y.; Wang, L.-S. Soil structure and nutrient supply drive changes in soil microbial communities during conversion of virgin desert soil to irrigated cropland. Eur. J. Soil Sci. 2020, 71, 768–781. [Google Scholar] [CrossRef]
- Maestre, F.T.; Biancari, L.; Chen, N.; Corrochano-Monsalve, M.; Jenerette, G.D.; Nelson, C.; Shilula, K.N.; Shpilkina, Y. Research needs on the biodiversity–ecosystem functioning relationship in drylands. NPJ Biodivers. 2024, 3, 12. [Google Scholar] [CrossRef]
- Madouh, T.A.A.; Quoreshi, A.M.M. The Function of Arbuscular Mycorrhizal Fungi Associated with Drought Stress Resistance in Native Plants of Arid Desert Ecosystems: A Review. Diversity 2023, 15, 391. [Google Scholar] [CrossRef]
- Shi, Z.; Mickan, B.; Feng, G.; Chen, Y. Arbuscular mycorrhizal fungi improved plant growth and nutrient acquisition of desert ephemeral Plantago minuta under variable soil water conditions. J. Arid Land 2015, 7, 414–420. [Google Scholar] [CrossRef]
- Shi, Z.Y.; Feng, G.; Christie, P.; Li, X.L. Arbuscular mycorrhizal status of spring ephemerals in the desert ecosystem of Junggar Basin, China. Mycorrhiza 2006, 16, 269–275. [Google Scholar] [CrossRef]
- Neuenkamp, L.; Moora, M.; Opik, M.; Davison, J.; Gerz, M.; Mannisto, M.; Jairus, T.; Vasar, M.; Zobel, M. The role of plant mycorrhizal type and status in modulating the relationship between plant and arbuscular mycorrhizal fungal communities. New Phytol. 2018, 220, 1236–1247. [Google Scholar] [CrossRef]
- He, S.; Guo, L.; Li, J.; Wang, Y.; Liu, Z.; Cheng, Y.; Hu, T.; Long, M. Advances in arbuscular mycorrhizal fungi and legumes symbiosis research. Acta Pratacult. Sin. 2017, 26, 187–194. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of Arbuscular Mycorrhizal Fungi and the Plant Growth-Promoting Rhizobacteria Improve Growth and Photosynthesis in Tobacco Under Drought Stress by Up-Regulating Antioxidant and Mineral Nutrition Metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Urcelay, C.; Díaz, S. The mycorrhizal dependence of subordinates determines the effect of arbuscular mycorrhizal fungi on plant diversity. Ecol. Lett. 2003, 6, 388–391. [Google Scholar] [CrossRef]
- Frosi, G.; Barros, V.A.; Oliveira, M.T.; Cavalcante, U.M.T.; Maia, L.C.; Santos, M.G. Increase in biomass of two woody species from a seasonal dry tropical forest in association with AMF with different phosphorus levels. Appl. Soil. Ecol. 2016, 102, 46–52. [Google Scholar] [CrossRef]
- Jia, Y.; Walder, F.; Wagg, C.; Feng, G. Mycorrhizal fungi maintain plant community stability by mitigating the negative effects of nitrogen deposition on subordinate species in Central Asia. J. Veg. Sci. 2021, 32, e12944. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liang, R.; Ji, B.; Wang, Z.; Wang, H.; Shen, Y. Negative effects of phosphorus addition outweigh effects of arbuscular mycorrhizal fungi and nitrogen addition on grassland temporal stability in the eastern Eurasian desert steppe. Ecol. Evol. 2023, 13, e10368. [Google Scholar] [CrossRef]
- Tilman, D. The ecological consequences of changes in biodiversity: A search for general principles. Ecology 1999, 80, 1455–1474. [Google Scholar] [CrossRef]
- Han, F.; Yu, C.; Fu, G. Non-growing/growing season non-uniform-warming increases precipitation use efficiency but reduces its temporal stability in an alpine meadow. Front. Plant Sci. 2023, 14, 1090204. [Google Scholar] [CrossRef]
- Godron, M.; Daget, P.; Poissonet, J.; Poissonet, P. Some aspects of heterogeneity in grasslands of Cantal (France). In Proceedings of the International Symposium on Statistical Ecology, New Haven, PA, USA, 3 August 1971. [Google Scholar]
- Zhou, S.; Peng, Y.; Ding, J.; Gao, P.; Li, G.; Wan, M.; Lu, F. Analysis on community stability and inter-specific correlations among dominant woody populations of the endangered plant Sinojackia rehderiana communities. Guihaia 2017, 37, 442–448. [Google Scholar] [CrossRef]
- Li, L.; Jiang, E.; Yin, H.; Wu, K.; Dong, G. Ultrashort-term responses of riparian vegetation restoration to adjacent cycles of ecological water conveyance scheduling in a hyperarid endorheic river basin. J. Environ. Manag. 2022, 320, 115803. [Google Scholar] [CrossRef]
- Chen, M.; Mo, F.; Zheng, L.; Bin, G.; Zou, Z.; Chen, P.; Xue, Y. Correlation and Community Stability Analysis of Herbaceous Plants in Dashiwei Tiankeng Group, China. Forests 2023, 14, 1244. [Google Scholar] [CrossRef]
- Chen, Z.; Xiong, P.; Zhou, J.; Lai, S.; Jian, C.; Xu, W.; Xu, B. Effects of plant diversity on semiarid grassland stability depends on functional group composition and dynamics under N and P addition. Sci. Total Environ. 2021, 799, 149482. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Liu, X.; Zhou, S. Asynchrony among species and functional groups and temporal stability under perturbations: Patterns and consequences. J. Ecol. 2020, 108, 2038–2046. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Qiao, F.; Song, X.; Wang, C.; Hu, Y.; Li, X.; Yin, G.; Penuelas, J. Species Diversity and Stability of Dominant Species Dominate the Stability of Community Biomass in an Alpine Meadow Under Variable Precipitation. Ecosystems 2023, 26, 1441–1455. [Google Scholar] [CrossRef]
- Gao, W.; Li, L.; Munson, S.M.; Cui, X.; Wang, Y.; Hao, Y. Grasslands Maintain Stability in Productivity Through Compensatory Effects and Dominant Species Stability Under Extreme Precipitation Patterns. Ecosystems 2022, 25, 1150–1165. [Google Scholar] [CrossRef]
- Mariotte, P. Do subordinate species punch above their weight? Evidence from above- and below-ground. New Phytol. 2014, 203, 16–21. [Google Scholar] [CrossRef]
- Mariotte, P.; Robroek, B.J.M.; Jassey, V.E.J.; Buttler, A. Subordinate plants mitigate drought effects on soil ecosystem processes by stimulating fungi. Funct. Ecol. 2015, 29, 1578–1586. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Gao, Y.; Liu, H.; Gao, Y.-B.; van der Heijden, M.G.A.; Ren, A.-Z. Plant endophytes and arbuscular mycorrhizal fungi alter plant competition. Funct. Ecol. 2018, 32, 1168–1179. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Zhao, R.; Wang, J.; Hao, L.; Wang, M. Forb stability, dwarf shrub stability and species asynchrony regulate ecosystem stability along an experimental precipitation gradient in a semi-arid desert grassland. Plant Biol. 2024, 26, 378–389. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, L.; Jiang, C.; Zhang, X.; Ouyang, Z. Accounting of value of ecosystem services in the desert: An example of the Kubuqi Desert ecosystem. Front. Earth Sci. 2024, 11, 1247367. [Google Scholar] [CrossRef]
- Zhang, L.H.; Jiang, X.Y.; Wang, M.M.; Wang, J.F. Plant Growth and Functional Group Regulated Ecological Response to Precipitation Alteration on Desert Grassland. Appl. Ecol. Environ. Res. 2024, 22, 949–969. [Google Scholar] [CrossRef]
- Hadley, N.F.; Szarek, S.R. Productivity of Desert Ecosystems. BioScience 1981, 31, 747–753. [Google Scholar] [CrossRef]
- Burrell, A.L.; Evans, J.P.; De Kauwe, M.G. Anthropogenic climate change has driven over 5 million km2 of drylands towards desertification. Nat. Commun. 2020, 11, 3853. [Google Scholar] [CrossRef]
- Chen, F.; Huang, W.; Jin, L.; Chen, J.; Wang, J. Spatiotemporal precipitation variations in the arid Central Asia in the context of global warming. Sci. China Earth Sci. 2011, 54, 1812–1821. [Google Scholar] [CrossRef]
- Yao, J.; Liu, Z.; Yang, Q.; Liu, Y.; Li, C.; Hu, W. Temperature variability and its possible causes in the typical basins of the arid Central Asia in recent 130 years. Acta Geogr. Sin. 2014, 69, 291–302. [Google Scholar] [CrossRef]
- Sun, D.; Yang, J. Precipitation characteristics at the hinterland of Gurbantunggut Desert and the surrounding areas. Arid Land Geogr. 2010, 33, 769–774. [Google Scholar] [CrossRef]
- Zeng, G.Y.; Ye, M.; Li, M.M.; Chen, W.L.; He, Q.Z.; Pan, X.T.; Zhang, X.; Che, J.; Qian, J.R.; Lv, Y.X. The Relationships between Plant Community Stability and Diversity across Different Grassland Types and Their Association with Environmental Factors in the Habahe Forest Area, Xinjiang. Diversity 2024, 16, 499. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.L.; Chen, Y.K.; Nizamani, M.M.; Zhou, Q.; Su, X.T. Analyses of community stability and inter-specific associations between a plant species with extremely small populations (Hopea hainanensis) and its associated species. Front. Ecol. Evol. 2022, 10, 922829. [Google Scholar] [CrossRef]
- De Boeck, H.J.; Bloor, J.M.G.; Kreyling, J.; Ransijn, J.C.G.; Nijs, I.; Jentsch, A.; Zeiter, M. Patterns and drivers of biodiversity-stability relationships under climate extremes. J. Ecol. 2018, 106, 890–902. [Google Scholar] [CrossRef]
- Cui, Y.; Hou, D.; Wang, Z.; Wang, J.; Qu, Z.; Wang, Y.; Han, G.; Li, Z.; Ren, H.; Wang, H. Increase in precipitation will facilitate the ecological stability of desert steppe in the future. Glob. Ecol. Conserv. 2024, 52, e02958. [Google Scholar] [CrossRef]
- Hallett, L.M.; Hsu, J.S.; Cleland, E.E.; Collins, S.L.; Dickson, T.L.; Farrer, E.C.; Gherardi, L.A.; Gross, K.L.; Hobbs, R.J.; Turnbull, L.; et al. Biotic mechanisms of community stability shift along a precipitation gradient. Ecology 2014, 95, 1693–1700. [Google Scholar] [CrossRef]
- Li, X.; Zuo, X.; Qiao, J.; Hu, Y.; Wang, S.; Yue, P.; Cheng, H.; Song, Z.; Chen, M.; Hautier, Y. Context-dependent impact of changes in precipitation on the stability of grassland biomass. Funct. Ecol. 2024, 38, 1185–1198. [Google Scholar] [CrossRef]
- Xu, Z.W.; Ren, H.Y.; Li, M.H.; van Ruijven, J.; Han, X.G.; Wan, S.Q.; Li, H.; Yu, Q.; Jiang, Y.; Jiang, L. Environmental changes drive the temporal stability of semi-arid natural grasslands through altering species asynchrony. J. Ecol. 2015, 103, 1308–1316. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.H.; Hautier, Y.; Qing, H.; Yang, J.; Bao, T.J.; Hajek, O.L.; Knapp, A.K. Effects of intra-annual precipitation patterns on grassland productivity moderated by the dominant species phenology. Front. Plant Sci. 2023, 14, 1142786. [Google Scholar] [CrossRef]
- Xu, Z.W.; Jiang, L.; Ren, H.Y.; Han, X.G. Opposing responses of temporal stability of aboveground and belowground net primary productivity to water and nitrogen enrichment in a temperate grassland. Global Change Biol. 2024, 30, e17071. [Google Scholar] [CrossRef]
- Collins, S.L.; Koerner, S.E.; Plaut, J.A.; Okie, J.G.; Brese, D.; Calabrese, L.B.; Carvajal, A.; Evansen, R.J.; Nonaka, E. Stability of tallgrass prairie during a 19-year increase in growing season precipitation. Funct. Ecol. 2012, 26, 1450–1459. [Google Scholar] [CrossRef]
- Hou, G.; Shi, P.L.; Zhou, T.C.; Sun, J.; Zong, N.; Song, M.H.; Zhang, X.Z. Dominant species play a leading role in shaping community stability in the northern Tibetan grasslands. J. Plant Ecol. 2023, 16, rtac110. [Google Scholar] [CrossRef]
- Grman, E.; Lau, J.A.; Schoolmaster, D.R.; Gross, K.L. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol. Lett. 2010, 13, 1400–1410. [Google Scholar] [CrossRef]
- Donohue, I.; Hillebrand, H.; Montoya, J.M.; Petchey, O.L.; Pimm, S.L.; Fowler, M.S.; Healy, K.; Jackson, A.L.; Lurgi, M.; McClean, D.; et al. Navigating the complexity of ecological stability. Ecol. Lett. 2016, 19, 1172–1185. [Google Scholar] [CrossRef]
- Avolio, M.L.; Forrestel, E.J.; Chang, C.C.; La Pierre, K.J.; Burghardt, K.T.; Smith, M.D. Demystifying dominant species. New Phytol. 2019, 223, 1106–1126. [Google Scholar] [CrossRef]
- Lisner, A.; Konecna, M.; Blazek, P.; Leps, J. Community biomass is driven by dominants and their characteristics—The insight from a field biodiversity experiment with realistic species loss scenario. J. Ecol. 2023, 111, 240–250. [Google Scholar] [CrossRef]
- Wang, B.; Huang, G.; Ma, J.; Li, Y. Responses of Nutrients Resorption of Five Desert Ephemeral Plants to Water and Nitrogen Additions. J. Desert Res. 2016, 36, 415–422. [Google Scholar]
- Mu, X.H.; Zheng, X.J.; Huang, G.; Tang, L.S.; Li, Y. Responses of Ephemeral Plants to Precipitation Changes and Their Effects on Community in Central Asia Cold Desert. Plants 2023, 12, 2841. [Google Scholar] [CrossRef]
- Tang, L. Effect of Precipitation Change on Germination Plasticity and Life History of Annual Ephemerals. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2016. (In Chinese). [Google Scholar]
- Saterberg, T.; Jonsson, T.; Yearsley, J.; Berg, S.; Ebenman, B. A potential role for rare species in ecosystem dynamics. Sci. Rep. 2019, 9, 11107. [Google Scholar] [CrossRef]
- Nytko, A.G.; Senior, J.K.; Wooliver, R.C.; O’Reilly-Wapstra, J.; Schweitzer, J.A.; Bailey, J.K. An evolutionary case for plant rarity: Eucalyptus as a model system. Ecol. Evol. 2024, 14, e11440. [Google Scholar] [CrossRef]
- Bracken, M.E.S.; Low, N.H.N. Realistic losses of rare species disproportionately impact higher trophic levels. Ecol. Lett. 2012, 15, 461–467. [Google Scholar] [CrossRef]
- Holub, P.; Fabsicova, M.; Tuma, I.; Zahora, J.; Fiala, K. Effects of artificially varying amounts of rainfall on two semi-natural grassland types. J. Veg. Sci. 2013, 24, 518–529. [Google Scholar] [CrossRef]
- Li, X.e.; Zhu, X.; Wang, S.; Cui, S.; Luo, C.; Zhang, Z.; Zhang, L.; Jiang, L.; Lu, W. Responses of biotic interactions of dominant and subordinate species to decadal warming and simulated rotational grazing in Tibetan alpine meadow. Sci. China Life Sci. 2018, 61, 849–859. [Google Scholar] [CrossRef]
- Douda, J.; Doudova, J.; Hulik, J.; Havrdova, A.; Boublik, K. Reduced competition enhances community temporal stability under conditions of increasing environmental stress. Ecology 2018, 99, 2207–2216. [Google Scholar] [CrossRef]
- Valone, T.J.; Hoffman, C.D. Population stability is higher in more diverse annual plant communities. Ecol. Lett. 2003, 6, 90–95. [Google Scholar] [CrossRef]
- van Ruijven, J.; Berendse, F. Contrasting effects of diversity on the temporal stability of plant populations. Oikos 2007, 116, 1323–1330. [Google Scholar] [CrossRef]
- Token, S.; Jiang, L.; Zhang, L.; Ly, G. Effects of plant diversity on primary productivity and community stability along soil water and salinity gradients. Glob. Ecol. Conserv. 2022, 36, e02095. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, X.; Zhao, L.; Liang, C.; Miao, B.; Zhang, Q.; Zhang, J.; Schmid, B.; Ma, W. Biotic stability mechanisms in Inner Mongolian grassland. Proc. R. Soc. B 2020, 287, 20200675. [Google Scholar] [CrossRef]
- Kang, F.; Yang, B.; Wujisiguleng; Yang, X.; Wang, L.; Guo, J.; Sun, W.; Zhang, Q.; Zhang, T. Arbuscular mycorrhizal fungi alleviate the negative effect of nitrogen deposition on ecosystem functions in meadow grassland. Land Degrad. Dev. 2020, 31, 748–759. [Google Scholar] [CrossRef]
- Qin, M.; Li, L.; Miranda, J.-P.; Tang, Y.; Song, B.; Oosthuizen, M.K.; Wei, W. Experimental duration determines the effect of arbuscular mycorrhizal fungi on plant biomass in pot experiments: A meta-analysis. Front. Plant Sci. 2022, 13, 1024874. [Google Scholar] [CrossRef]
- Yang, X.; Mariotte, P.; Guo, J.; Hautier, Y.; Zhang, T. Suppression of arbuscular mycorrhizal fungi decreases the temporal stability of community productivity under elevated temperature and nitrogen addition in a temperate meadow. Sci. Total Environ. 2021, 762, 143137. [Google Scholar] [CrossRef]
- Sheng, Q.; Dong, L.; Liu, Z. Driving forces of herbaceous species diversity in natural broadleaf forests from in Maoershan from Northeast China. Front. Plant Sci. 2024, 15, 1449421. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Willing, C.E.; Wan, J.; Yeam, J.J.; Cessna, A.M.; Peay, K.G. Arbuscular mycorrhizal fungi equalize differences in plant fitness and facilitate plant species coexistence through niche differentiation. Nat. Ecol. Evol. 2024, 8, 2058–2071. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Liu, Z.; He, W.; Tang, J.; Hao, X.; Sun, Y.; Zeng, Y. Spatial and temporal distribution characteristics of ephemeral Corispermum lehmannianum in the Gurbantunggut Desert. Acta Ecol. Sin. 2016, 36, 4064–4073. [Google Scholar] [CrossRef]
- Fan, L.L.; Li, Y.M.; Ma, J.; Mao, J.F.; Wang, L. Snow and rainfall independently affect the density, composition and productivity of ephemerals in a temperate desert. Sci. Total Environ. 2022, 807, 151033. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Jiang, L.; Yang, W.K.; Wang, L.; Wen, Z.B. Seed Germination Response and Tolerance to Different Abiotic Stresses of Four Salsola Species Growing in an Arid Environment. Front. Plant Sci. 2022, 13, 892667. [Google Scholar] [CrossRef]
- Zhang, S.; Zang, R.; Sheil, D. Rare and common species contribute disproportionately to the functional variation within tropical forests. J. Environ. Manag. 2022, 304, 114332. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.Y.; Zhang, L.Y.; Li, X.L.; Feng, G.; Tian, C.Y.; Christie, P. Diversity of arbuscular mycorrhizal fungi associated with desert ephemerals in plant communities of Junggar Basin, northwest China. Appl. Soil Ecol. 2007, 35, 10–20. [Google Scholar] [CrossRef]
- van der Heijden, M.G.A.; Klironomos, J.N.; Ursic, M.; Moutoglis, P.; Streitwolf-Engel, R.; Boller, T.; Wiemken, A.; Sanders, I.R. Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 1998, 396, 69–72. [Google Scholar] [CrossRef]
- Wagg, C.; Jansa, J.; Stadler, M.; Schmid, B.; van der Heijden, M.G.A. Mycorrhizal fungal identity and diversity relaxes plant–plant competition. Ecology 2011, 92, 1303–1313. [Google Scholar] [CrossRef]
- Klironomos, J.N. Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 2003, 84, 2292–2301. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, F.; Quan, Q.; Song, B.; Wang, J.; Zhou, Q.; Niu, S. Common Species Stability and Species Asynchrony Rather than Richness Determine Ecosystem Stability Under Nitrogen Enrichment. Ecosystems 2021, 24, 686–698. [Google Scholar] [CrossRef]
- Sun, S.; Liu, X.; He, Y.; Wei, S.; Zhang, L.; Lv, P.; Bao, C.; Wang, M.; Cheng, L. Responses of annual herb plant community characteristics to increased precipitation and reduced wind velocity in semiarid sandy grassland. Ecol. Evol. 2019, 9, 10654–10664. [Google Scholar] [CrossRef]
- Zhang, T.; Yu, G.; Chen, Z.; Hu, Z.; Jiao, C.; Yang, M.; Fu, Z.; Zhang, W.; Han, L.; Fan, M.; et al. Patterns and controls of vegetation productivity and precipitation-use efficiency across Eurasian grasslands. Sci. Total Environ. 2020, 741, 140204. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, Y.; Zhang, T.; Shi, Z.; Maimaitiaili, B.; Tian, C.; Feng, G. Elevated precipitation alters the community structure of spring ephemerals by changing dominant species density in Central Asia. Ecol. Evol. 2020, 10, 2196–2212. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Cheng, H.; Zhao, S.; Yue, P.; Liu, X.; Wang, S.; Liu, L.; Xu, C.; Luo, W.; Knops, J.M.H.; et al. Observational and experimental evidence for the effect of altered precipitation on desert and steppe communities. Glob. Ecol. Conserv. 2020, 21, e00864. [Google Scholar] [CrossRef]
- Xie, M.; Li, L.; Liu, B.; Liu, Y.; Wan, Q. Responses of terrestrial ecosystem productivity and community structure to intra-annual precipitation patterns: A meta-analysis. Front. Plant Sci. 2023, 13, 1088202. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, T.; Zhou, J.; Cai, W.; Gao, N.; Du, H.; Jiang, L.; Lai, L.; Zheng, Y. Community Characteristics and Leaf Stoichiometric Traits of Desert Ecosystems Regulated by Precipitation and Soil in an Arid Area of China. Int. J. Environ. Res. Public Health 2018, 15, 109. [Google Scholar] [CrossRef]
- Su, F.; Wei, Y.; Wang, F.; Guo, J.; Zhang, J.; Wang, Y.; Guo, H.; Hu, S. Sensitivity of plant species to warming and altered precipitation dominates the community productivity in a semiarid grassland on the Loess Plateau. Ecol. Evol. 2019, 9, 7628–7638. [Google Scholar] [CrossRef]
- Vincent, H.; Bornand, C.N.; Kempel, A.; Fischer, M. Rare species perform worse than widespread species under changed climate. Biol. Conserv. 2020, 246, 108586. [Google Scholar] [CrossRef]
- Xi, H.; Feng, Q.; Zhang, L.; Si, J.; Chang, Z.; Yu, T.; Guo, R. Effects of water and salinity on plant species composition and community succession in Ejina Desert Oasis, northwest China. Environ. Earth Sci. 2016, 75, 138. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Herren, C.M.; McMahon, K.D. Keystone taxa predict compositional change in microbial communities. Environ. Earth Sci. 2018, 20, 2207–2217. [Google Scholar] [CrossRef]
- Tang, B.; Man, J.; Romero, F.; Bergmann, J.; Lehmann, A.; Rillig, M.C. Mycorrhization enhances plant growth and stabilizes biomass allocation under drought. Glob. Change Biol. 2024, 30, e17438. [Google Scholar] [CrossRef]
- Kebede, T.G.G.; Birhane, E.; Ayimut, K.-M.; Egziabher, Y.G.G. Arbuscular mycorrhizal fungi improve biomass, photosynthesis, and water use efficiency of Opuntia ficus-indica (L.) Miller under different water levels. J. Arid Land 2023, 15, 975–988. [Google Scholar] [CrossRef]
- Augé, R.M. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 2001, 11, 3–42. [Google Scholar] [CrossRef]
- Kakouridis, A.; Hagen, J.A.; Kan, M.P.; Mambelli, S.; Feldman, L.J.; Herman, D.J.; Weber, P.K.; Pett-Ridge, J.; Firestone, M.K. Routes to roots: Direct evidence of water transport by arbuscular mycorrhizal fungi to host plants. New Phytol. 2022, 236, 210–221. [Google Scholar] [CrossRef]
- Tisserant, E.; Malbreil, M.; Kuo, A.; Kohler, A.; Symeonidi, A.; Balestrini, R.; Charron, P.; Duensing, N.; Frey, N.F.D.; Gianinazzi-Pearson, V.; et al. Genome of an arbuscular mycorrhizal fungus provides insight into the oldest plant symbiosis. Proc. Natl. Acad. Sci. USA 2013, 110, 20117–20122. [Google Scholar] [CrossRef]
- Li, B.; Feng, Q.; Li, Z.; Wang, F.; Luo, C.; Li, R.; Hu, H. Provenance of surface dune sands in the Gurbantunggut Desert, northwestern China: Qualitative and quantitative assessment using geochemical fingerprinting. Funct. Ecol. 2024, 452, 109115. [Google Scholar] [CrossRef]
- Heng, R. NPP Simulation Analysis of Vegetation in Gurbantunggut Desert Under CMIP6 Mode. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2023. (In Chinese). [Google Scholar]
- Du, H.Y.; Zhou, C.; Tang, H.Q.; Jin, X.L.; Chen, D.S.; Jiang, P.H.; Li, M.C. Simulation and estimation of future precipitation changes in arid regions: A case study of Xinjiang, Northwest China. Clim. Change 2021, 167, 43. [Google Scholar] [CrossRef]
- Cui, X.; Yue, P.; Gong, Y.; Li, K.; Tan, D.; Goulding, K.; Liu, X. Impacts of water and nitrogen addition on nitrogen recovery in Haloxylon ammodendron dominated desert ecosystems. Sci. Total Environ. 2017, 601, 1280–1288. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, L.; Wang, X.; Li, Y.; Zhang, G.; Li, P. Downscaling analysis of SMAP soil moisture products in Gurbantunggut Desert. Arid Zone Res. 2023, 40, 583–593. [Google Scholar] [CrossRef]
- Fan, L.L.; Li, Y.; Tang, L.S.; Ma, J. Combined effects of snow depth and nitrogen addition on ephemeral growth at the southern edge of the Gurbantunggut Desert, China. J. Arid Land 2013, 5, 500–510. [Google Scholar] [CrossRef]
- Zhang, T.; Tian, C.Y.; Sun, Y.; Bai, D.S.; Feng, G. Dynamics of arbuscular mycorrhizal fungi associated with desert ephemeral plants in Gurbantunggut Desert. J. Arid. Land 2012, 4, 43–51. [Google Scholar] [CrossRef]
- Takayabu, I.; Kato, H.; Nishizawa, K.; Takayabu, Y.N.; Sato, Y.; Sasaki, H.; Kurihara, K.; Kitoh, A. Future projections in precipitation over Asia simulated by two RCMs nested into MRI-CGCM2.2. J. Meteorol. Soc. Jpn. 2007, 85, 511–519. [Google Scholar] [CrossRef]
- Zhao, Z.; Ding, Y.; Xu, Y.; Zhang, J. Detection and Prediction of Climate Change for the 20th and 21st Century Due to Human Activity in Northwest China. Clim. Environ. Res. 2003, 8, 26–34. [Google Scholar] [CrossRef]
- O’Connor, P.J.; Smith, S.E.; Smith, F.A. Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol. 2002, 154, 209–218. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, N.; Bai, D.; Chen, Y.; Feng, G. Arbuscular Mycorrhizal Fungi Promote the Growth of Ceratocarpus arenarius (Chenopodiaceae) with No Enhancement of Phosphorus Nutrition. PLoS ONE 2012, 7, e41151. [Google Scholar] [CrossRef]
- Yang, G.; Liu, N.; Lu, W.; Wang, S.; Kan, H.; Zhang, Y.; Xu, L.; Chen, Y. The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J. Ecol. 2014, 102, 1072–1082. [Google Scholar] [CrossRef]
- Hartnett, D.C.; Wilson, G.W.T. Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 1999, 80, 1187–1195. [Google Scholar] [CrossRef]
- Shi, Y.; Sun, Z.; Yang, Q. Characteristics of Area Precipitation in Xinjiang Region with Its Variations. J. Appl. Meteorol. Sci. 2008, 19, 326–332. [Google Scholar] [CrossRef]
- Rawls, W.J.; Brakensiek, D.L.; Saxtonn, K.E. Estimation of Soil Water Properties. Trans. ASAE 1982, 25, 1316–1320. [Google Scholar] [CrossRef]
- Mariotte, P.; Buttler, A.; Kohler, F.; Gilgen, A.K.; Spiegelberger, T. How do subordinate and dominant species in semi-natural mountain grasslands relate to productivity and land-use change? Basic Appl. Ecol. 2013, 14, 217–224. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Smith, B.; Wilson, J. A Consumer’s Guide to Evenness Indices. Oikos 1996, 76, 70–82. [Google Scholar] [CrossRef]
- Zheng, Y. Comparison of methods for studying stability of forest community. Sci. Silvae Sin. 2000, 36, 28–32. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, J.; Zhao, W.; Dong, L.; Zheng, J. Effect of fences on functional groups and stability of the alpine meadow plant community in the Qinghai-Tibet Plateau. Pratacul. Sci. 2017, 34, 565–574. [Google Scholar] [CrossRef]
- Lehman, C.L.; Tilman, D. Biodiversity, Stability, and Productivity in Competitive Communities. Am. Nat. 2000, 156, 534–552. [Google Scholar] [CrossRef]
- Downing, A.L.; Jackson, C.; Plunkett, C.; Lockhart, J.A.; Schlater, S.M.; Leibold, M.A. Temporal stability vs. community matrix measures of stability and the role of weak interactions. Ecol. Lett. 2020, 23, 1468–1478. [Google Scholar] [CrossRef]
- Grace, J.B. Structural Equation Modeling and Natural Systems; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
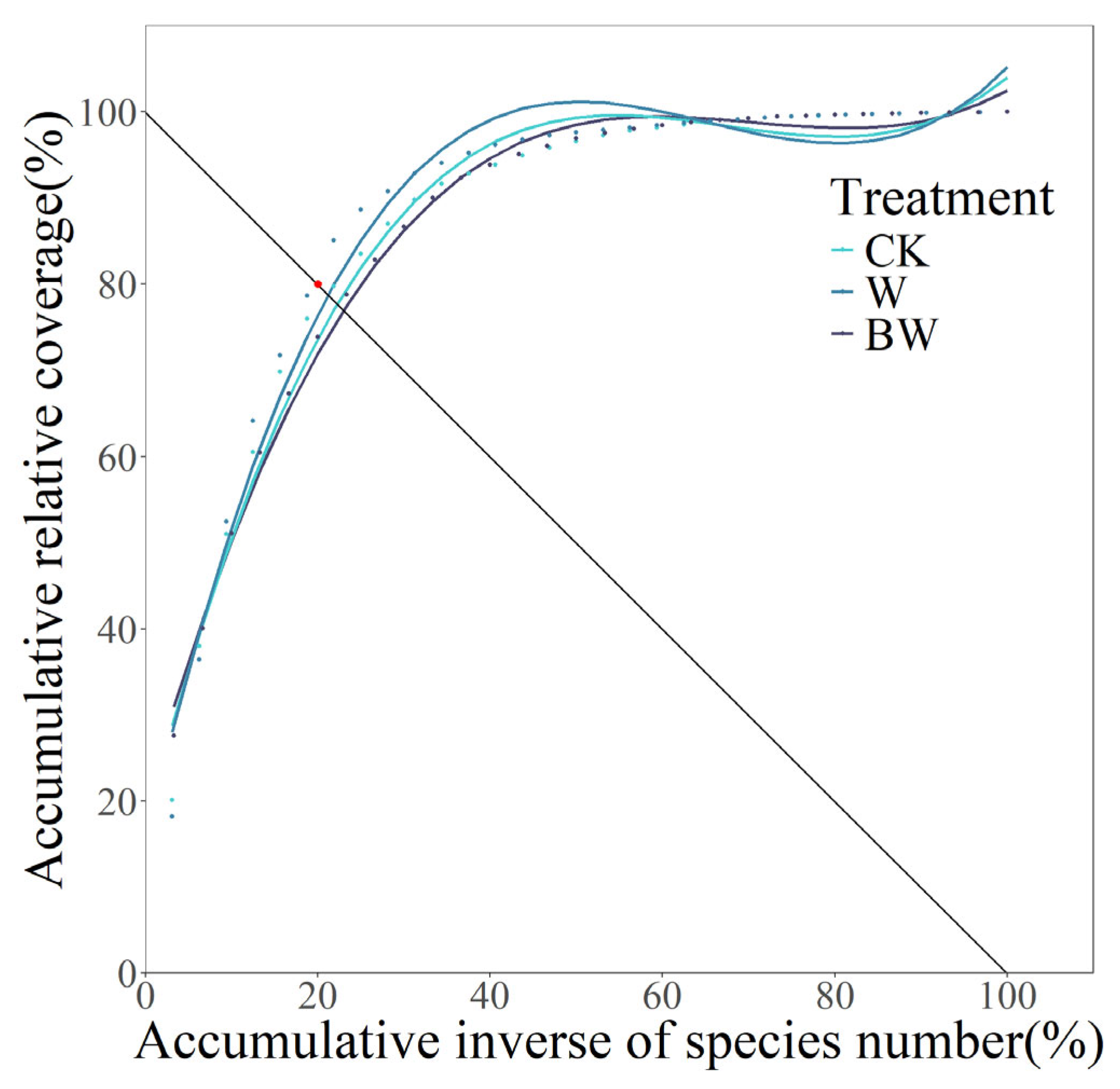

| Treatment | Fitted Curve | Correlation Coefficient (R2) | p Value | Intersection Coordinate | Euclidean Distance |
|---|---|---|---|---|---|
| CK | y = 0.0003x3 − 0.0602x2 + 3.9091x + 17.1632 | 0.9794 | p < 0.01 | (22.29, 77.71) | 3.2385 |
| W | y = 0.0004x3 − 0.0698x2 + 4.3200x + 15.1589 | 0.9682 | p < 0.01 | (21.06, 78.94) | 1.4991 |
| BW | y = 0.0002x3 − 0.0517x2 + 3.5506x + 19.6804 | 0.9948 | p < 0.01 | (23.23, 76.77) | 4.5679 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Z.; Dong, Q.; Yang, R.; Qin, W.; Peng, Y.; Jia, Y. From Ordinary to Extraordinary: The Crucial Role of Common Species in Desert Plant Community Stability with Arbuscular Mycorrhizal (AM) Fungi Under Increased Precipitation. Plants 2025, 14, 1099. https://doi.org/10.3390/plants14071099
Ji Z, Dong Q, Yang R, Qin W, Peng Y, Jia Y. From Ordinary to Extraordinary: The Crucial Role of Common Species in Desert Plant Community Stability with Arbuscular Mycorrhizal (AM) Fungi Under Increased Precipitation. Plants. 2025; 14(7):1099. https://doi.org/10.3390/plants14071099
Chicago/Turabian StyleJi, Zhanquan, Qianqian Dong, Rong Yang, Wenhao Qin, Yi Peng, and Yangyang Jia. 2025. "From Ordinary to Extraordinary: The Crucial Role of Common Species in Desert Plant Community Stability with Arbuscular Mycorrhizal (AM) Fungi Under Increased Precipitation" Plants 14, no. 7: 1099. https://doi.org/10.3390/plants14071099
APA StyleJi, Z., Dong, Q., Yang, R., Qin, W., Peng, Y., & Jia, Y. (2025). From Ordinary to Extraordinary: The Crucial Role of Common Species in Desert Plant Community Stability with Arbuscular Mycorrhizal (AM) Fungi Under Increased Precipitation. Plants, 14(7), 1099. https://doi.org/10.3390/plants14071099






