Tocopherol and Tocotrienol Content in the Leaves of the Genus Hypericum: Impact of Species and Drying Technique
Abstract
1. Introduction
2. Results and Discussion
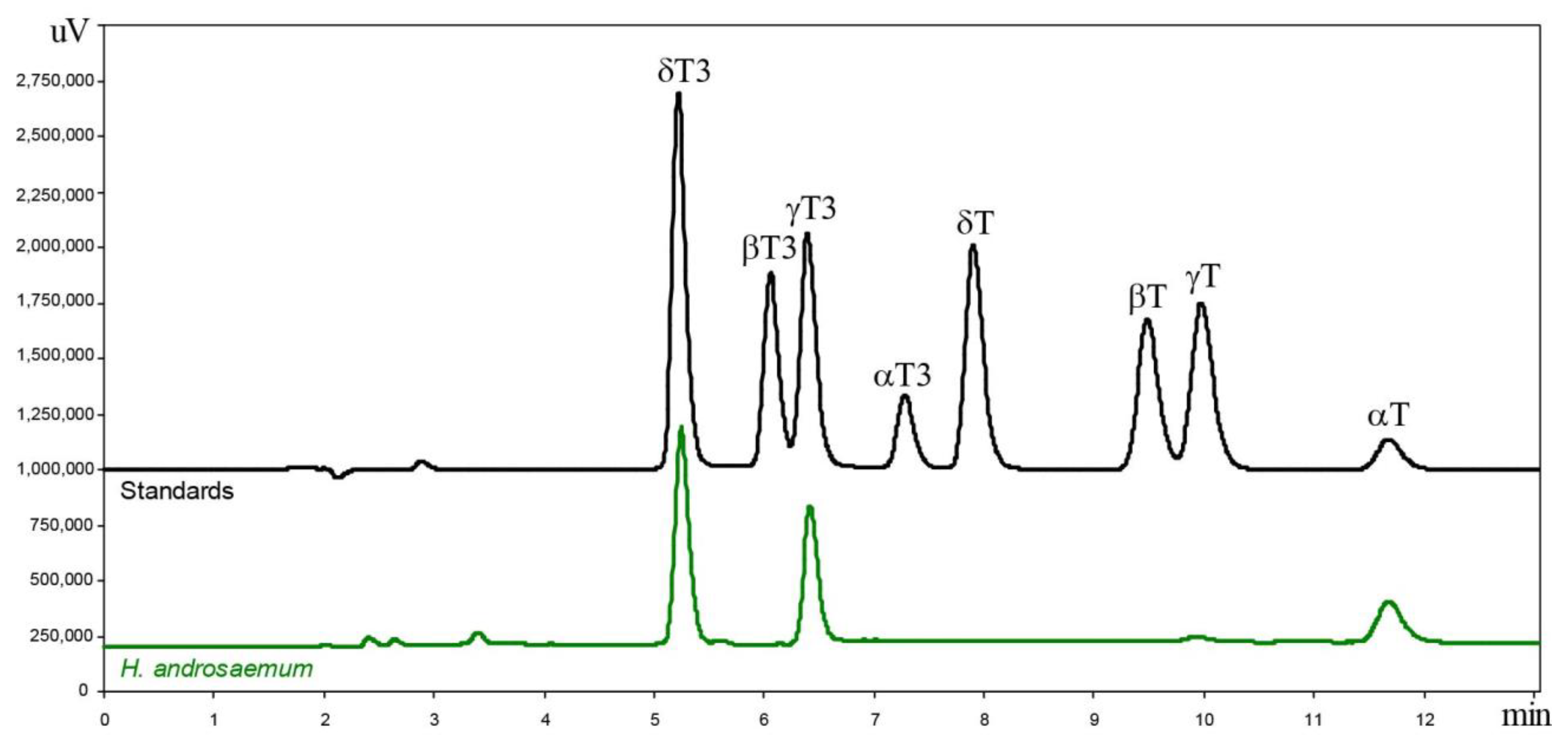
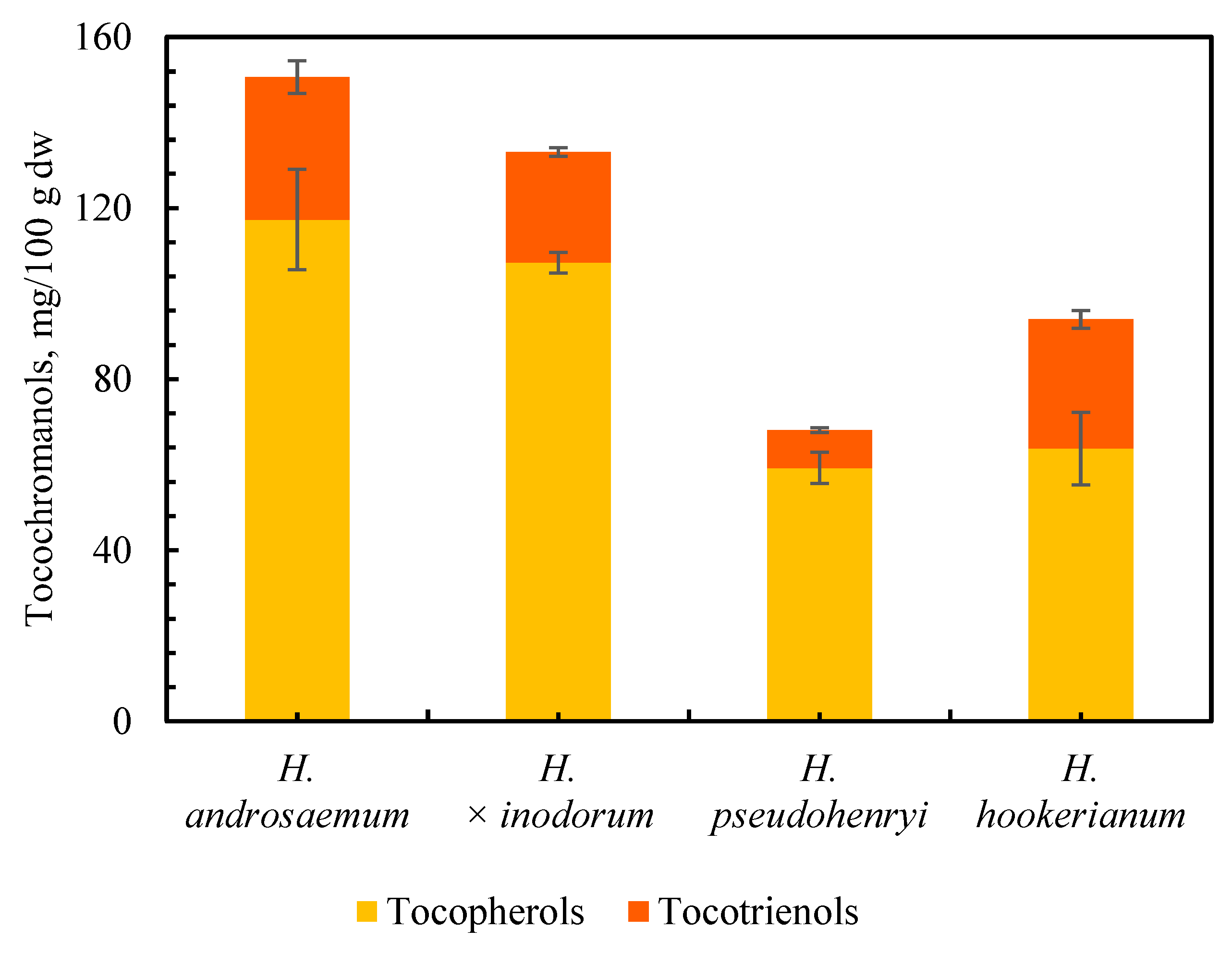
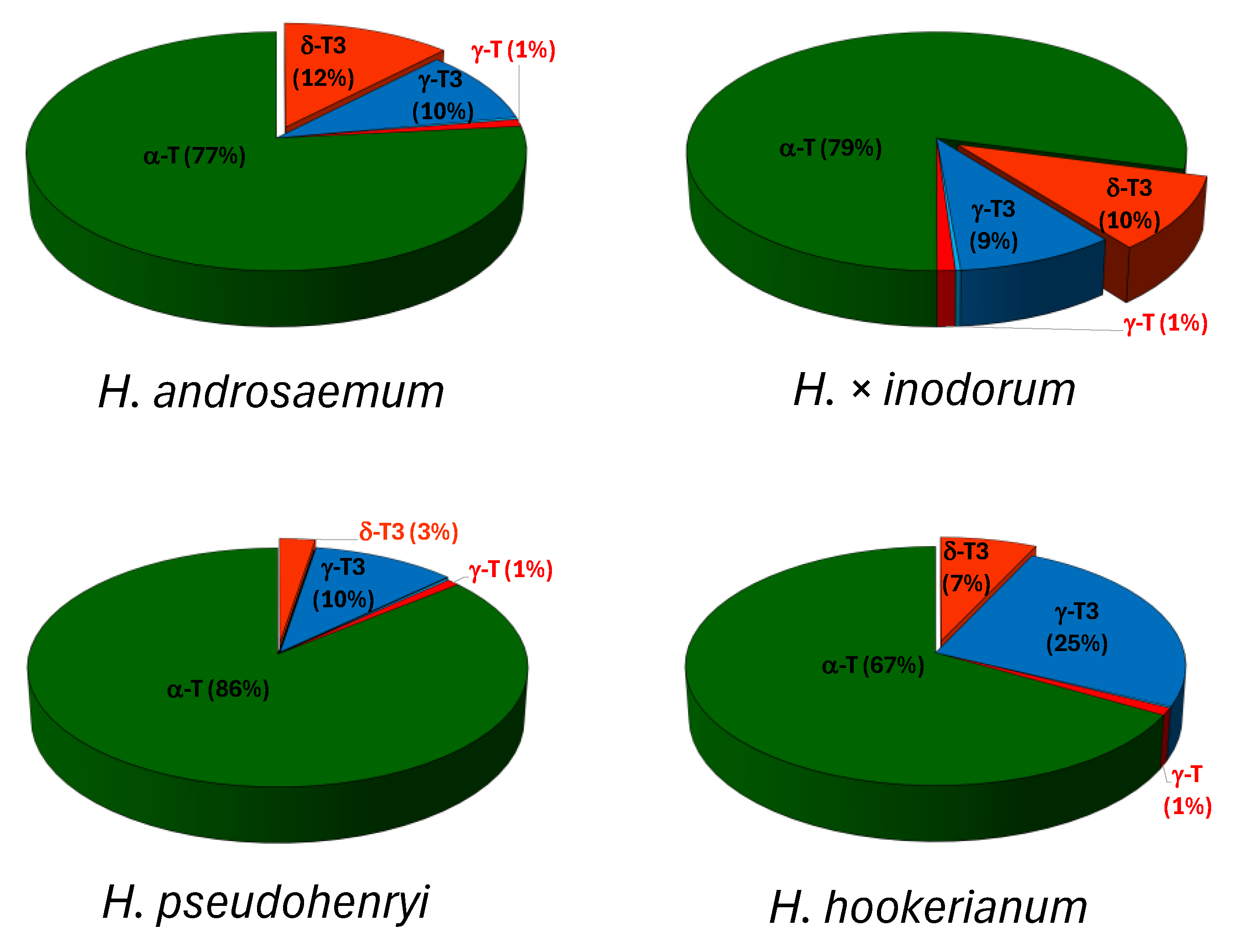
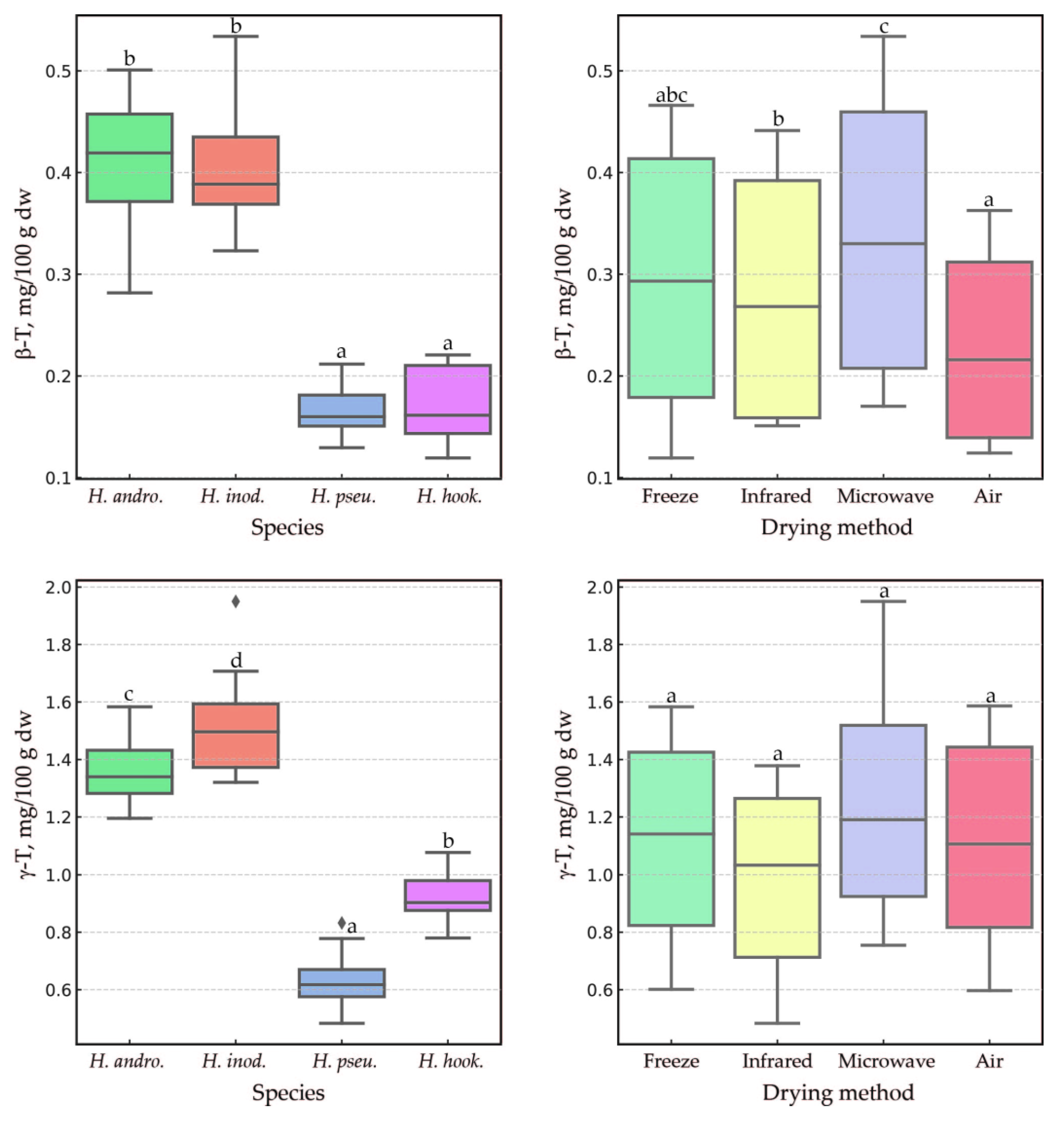
2.1. Tocochromanol Profile in the Freeze-Dried Leaves of the Genus Hypericum
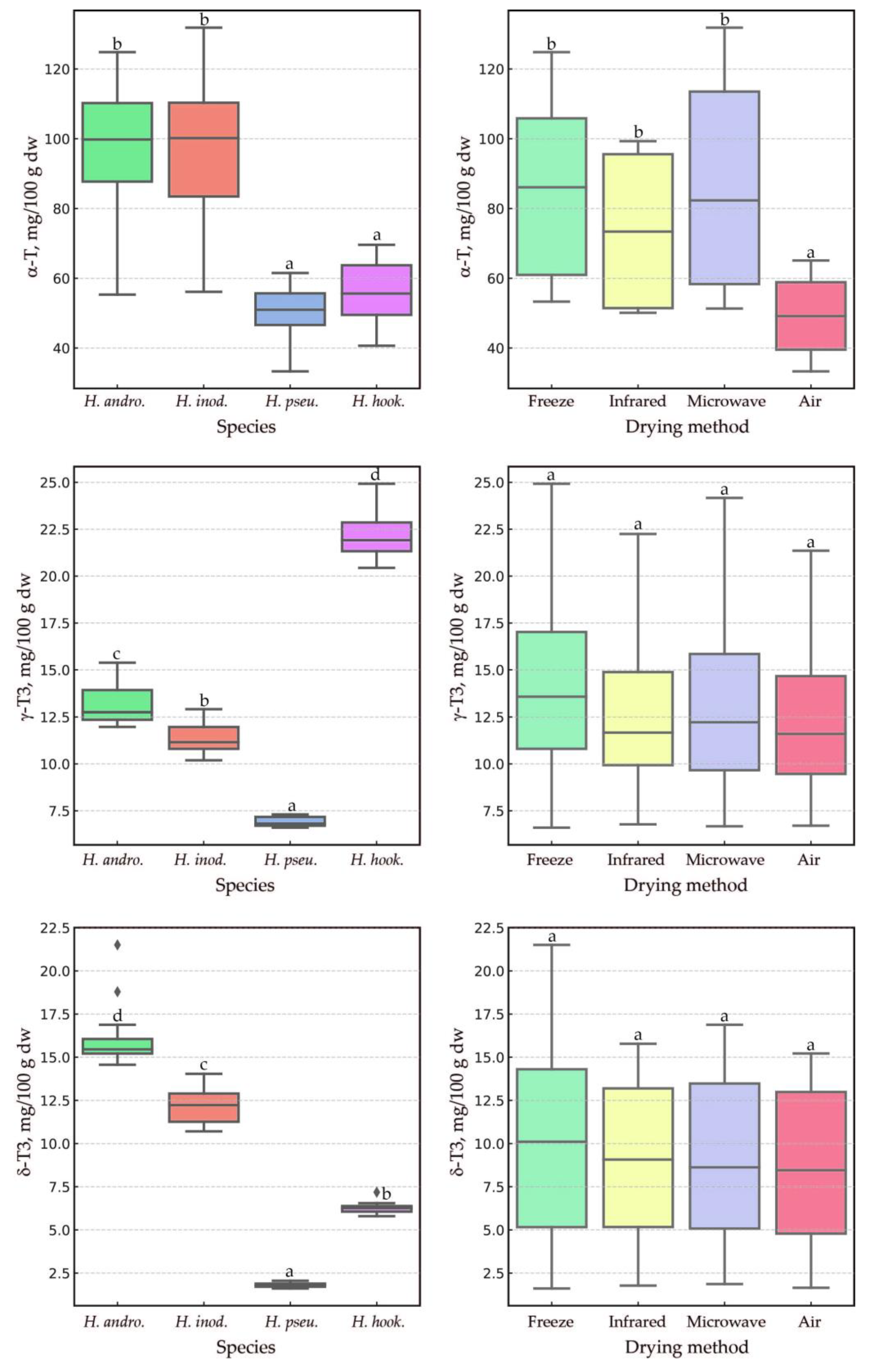
2.2. Impact of the Drying Method, Species, and Their Interaction on Tocochromanol Content in the Leaves of the Genus Hypericum
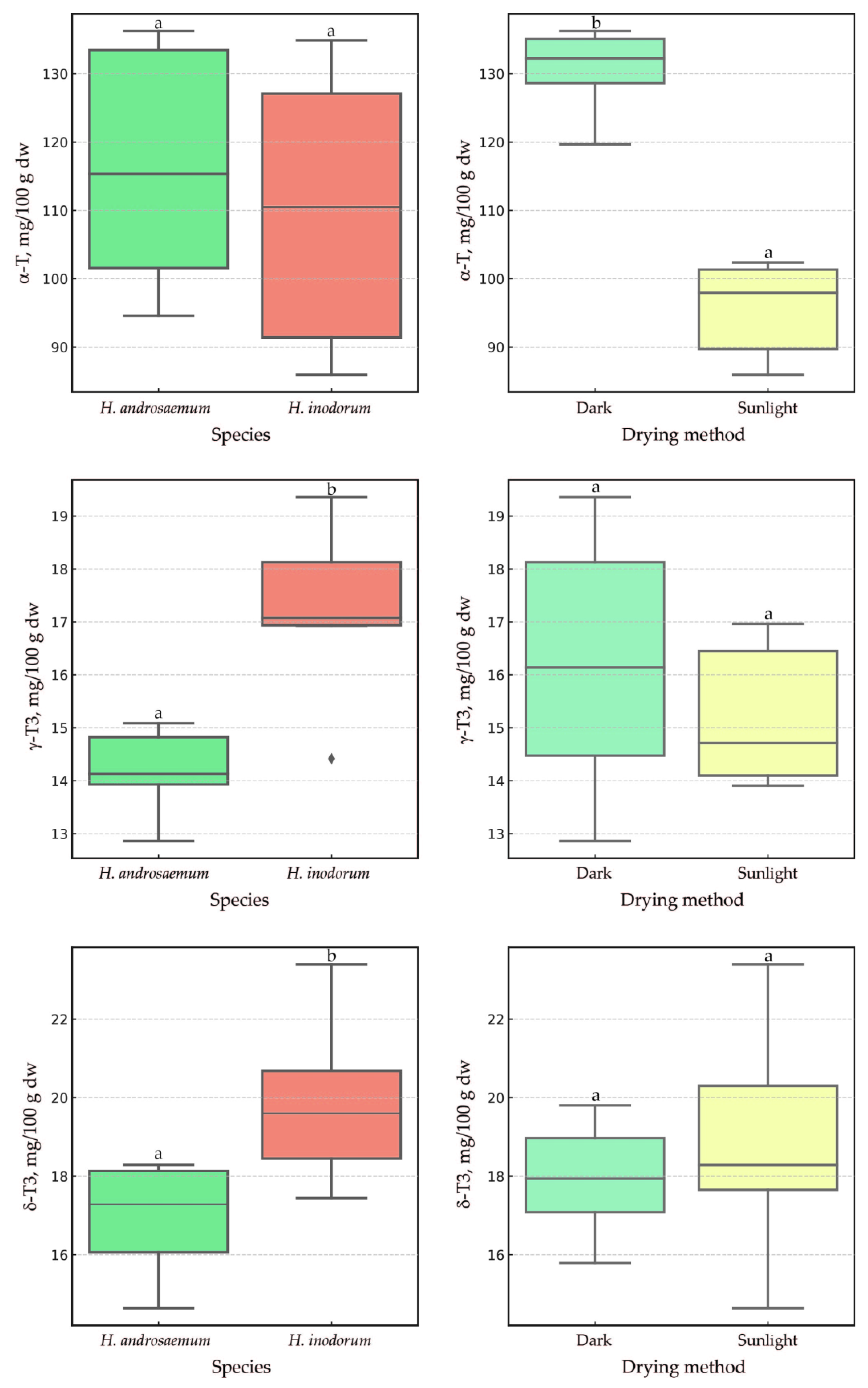
2.3. The Impact of Sunlight and a One-Month Delay in Leaf Harvesting of H. androsaemum and H. × inodorum
3. Materials and Methods
3.1. Reagents
3.2. Plant Material
3.3. Drying of Hypericum Leaves
3.3.1. Freeze Drying
3.3.2. Microwave–Vacuum Drying
3.3.3. Infrared Oven Drying
3.3.4. Air-Drying (Part Presence of Sunlight and Dark)
3.3.5. Powdering
3.4. Semi-Micro-Saponification Protocol
3.5. Tocopherol and Tocotrienol Determination via RP-HPLC-FLD
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| T | Tocopherol |
| T3 | Tocotrienol |
| RP-HPLC-FLD | Reverse-phase high-performance liquid chromatography with fluorescent light detector |
References
- The Angiosperm Phylogeny Group. An ordinal classification for the families of flowering plants. Ann. Mo. Bot. Gard. 1998, 85, 531–553. [Google Scholar]
- The Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [Google Scholar]
- Mišina, I.; Lazdiņa, D.; Górnaś, P. Tocochromanols in the leaves of plants in the Hypericum and Clusia genera. Molecules 2025, 30, 709. [Google Scholar] [CrossRef] [PubMed]
- Robson, N.K.B. Studies in the genus Hypericum L. (Clusiaceae). Section 9. Hypericum sensu lato (part 3): Subsection 1. Hypericum series 2. Senanensia, subsection 2. Erecta and section 9b. Graveolentia. Syst. Biodivers. 2006, 4, 19–98. [Google Scholar]
- Christenhusz, M.J.M.; Byng, J.W. The number of known plants species in the world and its annual increase. Phytotaxa 2016, 261, 201–217. [Google Scholar]
- Wu, T.-R.; Xu, J.; An, M.-T.; Yu, J.-H.; Liu, F.; Chen, Z.-R. Hypericum liboense (Hypericaceae), a new species from Guizhou, China. PhytoKeys 2024, 237, 37–49. [Google Scholar]
- Caldeira, G.I.; Gouveia, L.P.; Serrano, R.; Silva, O.D. Hypericum genus as a natural source for biologically active compounds. Plants 2022, 11, 2509. [Google Scholar] [CrossRef]
- Cirak, C.; Seyis, F.; Özcan, A.; Yurteri, E. Ontogenetic changes in phenolic contents and volatile composition of Hypericum androsaemum and Hypericum xylosteifolium. Biochem. Syst. Ecol. 2022, 102, 104429. [Google Scholar]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species–A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar]
- Lazdiņa, D.; Mišina, I.; Górnaś, P. Tocotrienols in eleven species of Hypericum genus leaves. Molecules 2025, 30, 662. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Perkons, I.; Segliņa, D.; Czlapka-Matyasik, M. Characterization of tocochromanols in wild Hypericum perforatum populations in Latvia. Horticulturae 2025, 11, 205. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Lazdiņa, D. Tocopherol and tocotrienol homologue recovery from Hypericum perforatum L. and extraction residues after hydroethanolic extraction. Ind. Crops Prod. 2025, 224, 120321. [Google Scholar] [CrossRef]
- Górnaś, P.; Siger, A. Rare prenyllipids in wild St. John’s wort during three harvest seasons. Molecules 2025, 30, 901. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-M.; Wang, F.-F.; Zhao, Y.-Y.; Lu, L.-F.; Jiang, X.-Y.; Huang, T.-Q.; Lin, Y.; Guo, L.; Weng, Z.-B.; Liu, E.-H. Hypericum perforatum L. attenuates depression by regulating Akkermansia muciniphila, tryptophan metabolism and NFκB-NLRP2-Caspase1-IL1β pathway. Phytomedicine 2024, 132, 155847. [Google Scholar] [CrossRef]
- Górnaś, P.; Mišina, I.; Lazdiņa, D. Annatto (Bixa orellana) seeds, palm (Elaeis guineensis) oils, and St. John’s wort (Hypericum perforatum) aerial parts as sources of rare prenyllipids. Eur. Food Res. Technol. 2025. [Google Scholar] [CrossRef]
- Inoue, T.; Tatemori, S.; Muranaka, N.; Hirahara, Y.; Homma, S.; Nakane, T.; Takano, A.; Nomi, Y.; Otsuka, Y. The Identification of Vitamin E Homologues in Medicinal Plant Samples Using ESI (+)-LC-MS3. J. Agric. Food Chem. 2012, 60, 9581–9588. [Google Scholar] [CrossRef]
- Babu, A.K.; Kumaresan, G.; Raj, V.A.A.; Velraj, R. Review of leaf drying: Mechanism and influencing parameters, drying methods, nutrient preservation, and mathematical models. Renew. Sustain. Energy Rev. 2018, 90, 536–556. [Google Scholar] [CrossRef]
- Górnaś, P.; Šnē, E.; Siger, A.; Segliņa, D. Sea buckthorn (Hippophae rhamnoides L.) leaves as valuable source of lipophilic antioxidants: The effect of harvest time, sex, drying and extraction methods. Ind. Crops Prod. 2014, 60, 1–7. [Google Scholar] [CrossRef]
- Silva, E.M.; Da Silva, J.S.; Pena, R.S.; Rogez, H. A combined approach to optimize the drying process of flavonoid-rich leaves (Inga edulis) using experimental design and mathematical modelling. Food Bioprod. Process. 2011, 89, 39–46. [Google Scholar] [CrossRef]
- Pyrka, I.; Mantzouridou, F.T.; Nenadis, N. Optimization of olive leaves’ thin layer, intermittent near-infrared-drying. Innov. Food Sci. Emerg. Technol. 2023, 84, 103264. [Google Scholar]
- Górnaś, P.; Baškirovs, G.; Siger, A. Free and esterified tocopherols, tocotrienols and other extractable and non-extractable tocochromanol-related molecules: Compendium of knowledge, future perspectives and recommendations for chromatographic techniques, tools, and approaches used for tocochromanol determination. Molecules 2022, 27, 6560. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Mišina, I.; Waśkiewicz, A.; Perkons, I.; Pugajeva, I.; Segliņa, D. Simultaneous extraction of tocochromanols and flavan-3-ols from the grape seeds: Analytical and industrial aspects. Food Chem. 2025, 462, 140913. [Google Scholar] [PubMed]
- Górnaś, P.; Siger, A.; Czubinski, J.; Dwiecki, K.; Segliņa, D.; Nogala-Kalucka, M. An alternative RP-HPLC method for the separation and determination of tocopherol and tocotrienol homologues as butter authenticity markers: A comparative study between two European countries. Eur. J. Lipid Sci. Technol. 2014, 116, 895–903. [Google Scholar]
- Slavin, M.; Yu, L.L. A single extraction and HPLC procedure for simultaneous analysis of phytosterols, tocopherols and lutein in soybeans. Food Chem. 2012, 135, 2789–2795. [Google Scholar]
- Górnaś, P.; Segliņa, D.; Lācis, G.; Pugajeva, I. Dessert and crab apple seeds as a promising and rich source of all four homologues of tocopherol (α, β, γ and δ). LWT-Food Sci. Technol. 2014, 59, 211–214. [Google Scholar]
- Buddrick, O.; Jones, O.A.; Morrison, P.D.; Small, D.M. Heptane as a less toxic option than hexane for the separation of vitamin E from food products using normal phase HPLC. RSC Adv. 2013, 3, 24063–24068. [Google Scholar]
- Górnaś, P.; Lazdiņa, D.; Mišina, I.; Sipeniece, E.; Segliņa, D. Cranberry (Vaccinium macrocarpon Aiton) seeds: An exceptional source of tocotrienols. Sci. Hortic. 2024, 331, 113107. [Google Scholar]
- Hosni, K.; Msaâda, K.; Taârit, M.B.; Marzouk, B. Fatty acid composition and tocopherol content in four Tunisian Hypericum species: Hypericum perforatum, Hypericum tomentosum, Hypericum perfoliatum and Hypericum ericoides Ssp. Roberti. Arab. J. Chem. 2017, 10, S2736–S2741. [Google Scholar]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar]
- Szymańska, R.; Kruk, J. Tocopherol content and isomers’ composition in selected plant species. Plant Physiol. Biochem. 2008, 46, 29–33. [Google Scholar]
- Singh, R. Chemotaxonomy: A tool for plant classification. J. Med. Plants Stud. 2016, 4, 90–93. [Google Scholar]
- Bagci, E. Fatty acids and tocochromanol patterns of some Turkish Apiaceae (Umbelliferae) plants; a chemotaxonomic approach. Acta Bot. Gall. 2007, 154, 143–151. [Google Scholar]
- Siger, A.; Górnaś, P. Free tocopherols and tocotrienols in 82 plant species’ oil: Chemotaxonomic relation as demonstrated by PCA and HCA. Food Res. Int. 2023, 164, 112386. [Google Scholar]
- Morales, M.; Garcia, Q.S.; Siqueira-Silva, A.I.; Silva, M.C.; Munné-Bosch, S. Tocotrienols in Vellozia gigantea leaves: Occurrence and modulation by seasonal and plant size effects. Planta 2014, 240, 437–446. [Google Scholar]
- Hormaetxe, K.; Esteban, R.; Becerril, J.M.; García-Plazaola, J.I. Dynamics of the α-tocopherol pool as affected by external (environmental) and internal (leaf age) factors in Buxus sempervirens leaves. Physiol. Plant. 2005, 125, 333–344. [Google Scholar]
- Miķelsone, I.; Sipeniece, E.; Mišina, I.; Bondarenko, E.; Górnaś, P. Cultivated St. John’s wort flower heads accumulate tocotrienols over tocopherols, regardless of the year of the plant. Plants 2025, 14, 852. [Google Scholar] [CrossRef]
- Franzen, J.; Bausch, J.; Glatzle, D.; Wagner, E. Distribution of vitamin E in spruce seedling and mature tree organs, and within the genus. Phytochemistry 1991, 30, 147–151. [Google Scholar]
- Matringe, M.; Ksas, B.; Rey, P.; Havaux, M. Tocotrienols, the unsaturated forms of vitamin E, can function as antioxidants and lipid protectors in tobacco leaves. Plant Physiol. 2008, 147, 764–778. [Google Scholar]
- Niu, Y.; Zhang, Q.; Wang, J.; Li, Y.; Wang, X.; Bao, Y. Vitamin E synthesis and response in plants. Front. Plant Sci. 2022, 13, 994058. [Google Scholar]
- Korus, A. Effect of pre-treatment and drying methods on the content of minerals, B-group vitamins and tocopherols in kale (Brassica oleracea L. var. acephala) leaves. J. Food Sci. Technol. 2022, 59, 279–287. [Google Scholar]
- Spicher, L.; Almeida, J.; Gutbrod, K.; Pipitone, R.; Dörmann, P.; Glauser, G.; Rossi, M.; Kessler, F. Essential role for phytol kinase and tocopherol in tolerance to combined light and temperature stress in tomato. J. Exp. Bot. 2017, 68, 5845–5856. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [PubMed]
- Szymańska, R.; Kruk, J. γ-Tocopherol dominates in young leaves of runner bean (Phaseolus coccineus) under a variety of growing conditions: The possible functions of γ-tocopherol. Phytochemistry 2008, 69, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Kofler, M.; Sommer, P.F.; Bolliger, H.R.; Schmidli, B.; Vecchi, M. Physicochemical properties and assay of the tocopherols. In Vitamins & Hormones; Elsevier: Amsterdam, The Netherlands, 1962; Volume 20, pp. 407–440. [Google Scholar]
- Mišina, I.; Perkons, I.; Siger, A.; Soliven, A.; Górnaś, P. Residues of St. John’s wort (Hypericum perforatum) tea infusions/water extracts as a valuable source of tocotrienols: An extraction study. Appl. Sci. 2025, 15, 2047. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miķelsone, I.; Sipeniece, E.; Segliņa, D.; Górnaś, P. Tocopherol and Tocotrienol Content in the Leaves of the Genus Hypericum: Impact of Species and Drying Technique. Plants 2025, 14, 1079. https://doi.org/10.3390/plants14071079
Miķelsone I, Sipeniece E, Segliņa D, Górnaś P. Tocopherol and Tocotrienol Content in the Leaves of the Genus Hypericum: Impact of Species and Drying Technique. Plants. 2025; 14(7):1079. https://doi.org/10.3390/plants14071079
Chicago/Turabian StyleMiķelsone, Ieva, Elise Sipeniece, Dalija Segliņa, and Paweł Górnaś. 2025. "Tocopherol and Tocotrienol Content in the Leaves of the Genus Hypericum: Impact of Species and Drying Technique" Plants 14, no. 7: 1079. https://doi.org/10.3390/plants14071079
APA StyleMiķelsone, I., Sipeniece, E., Segliņa, D., & Górnaś, P. (2025). Tocopherol and Tocotrienol Content in the Leaves of the Genus Hypericum: Impact of Species and Drying Technique. Plants, 14(7), 1079. https://doi.org/10.3390/plants14071079







