Phytochemical Investigation of Chamaemelum nobile L. and Evaluation of Acetylcholinesterase and Tyrosinase Inhibitory Activity
Abstract
1. Introduction
2. Results and Discussion
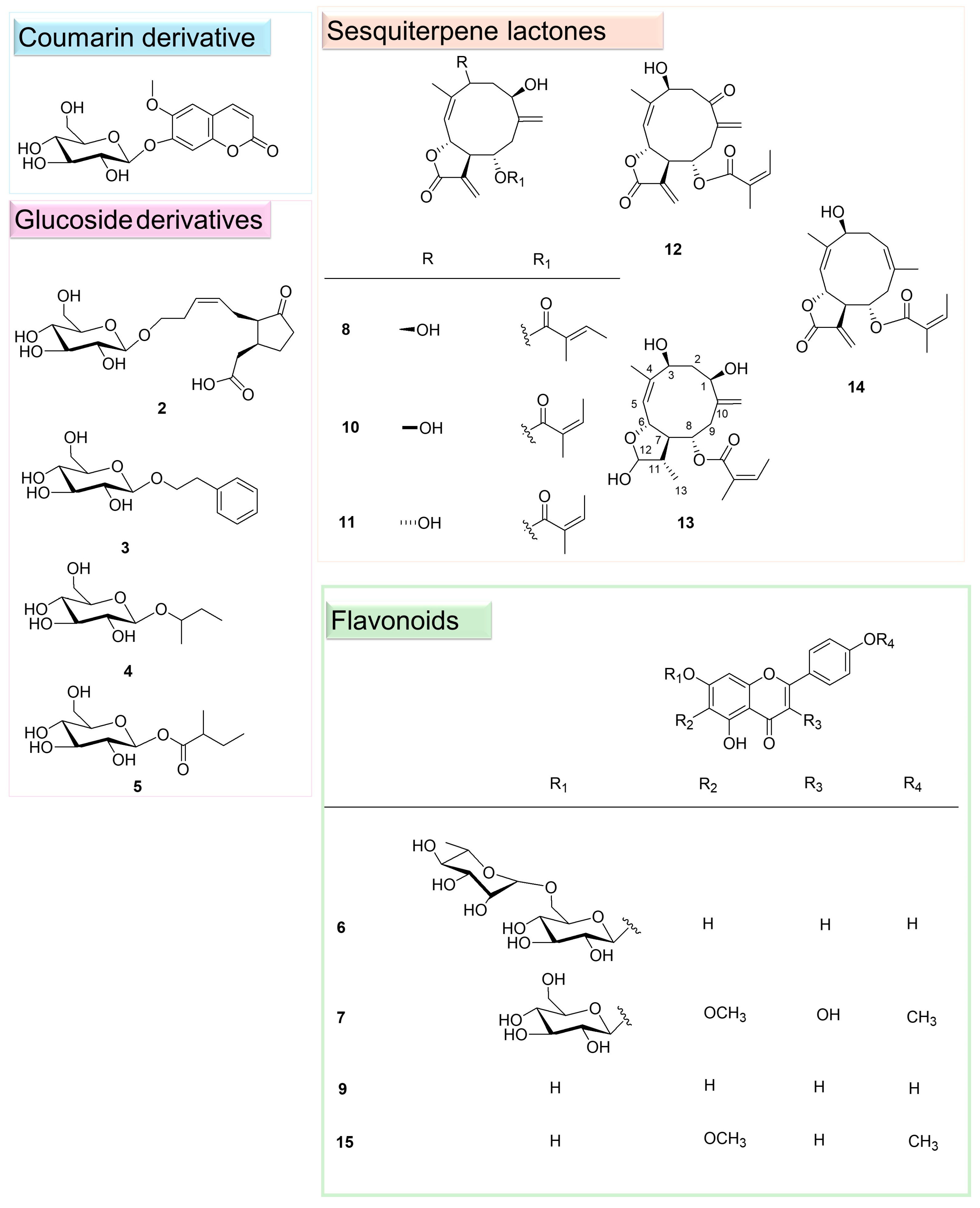
2.1. Isolation and Identification of Specialised Metabolites from C. nobile
2.2. Characterisation of 11,13-Dihydro-8-Tigloylhydroxyisonobilin (13)
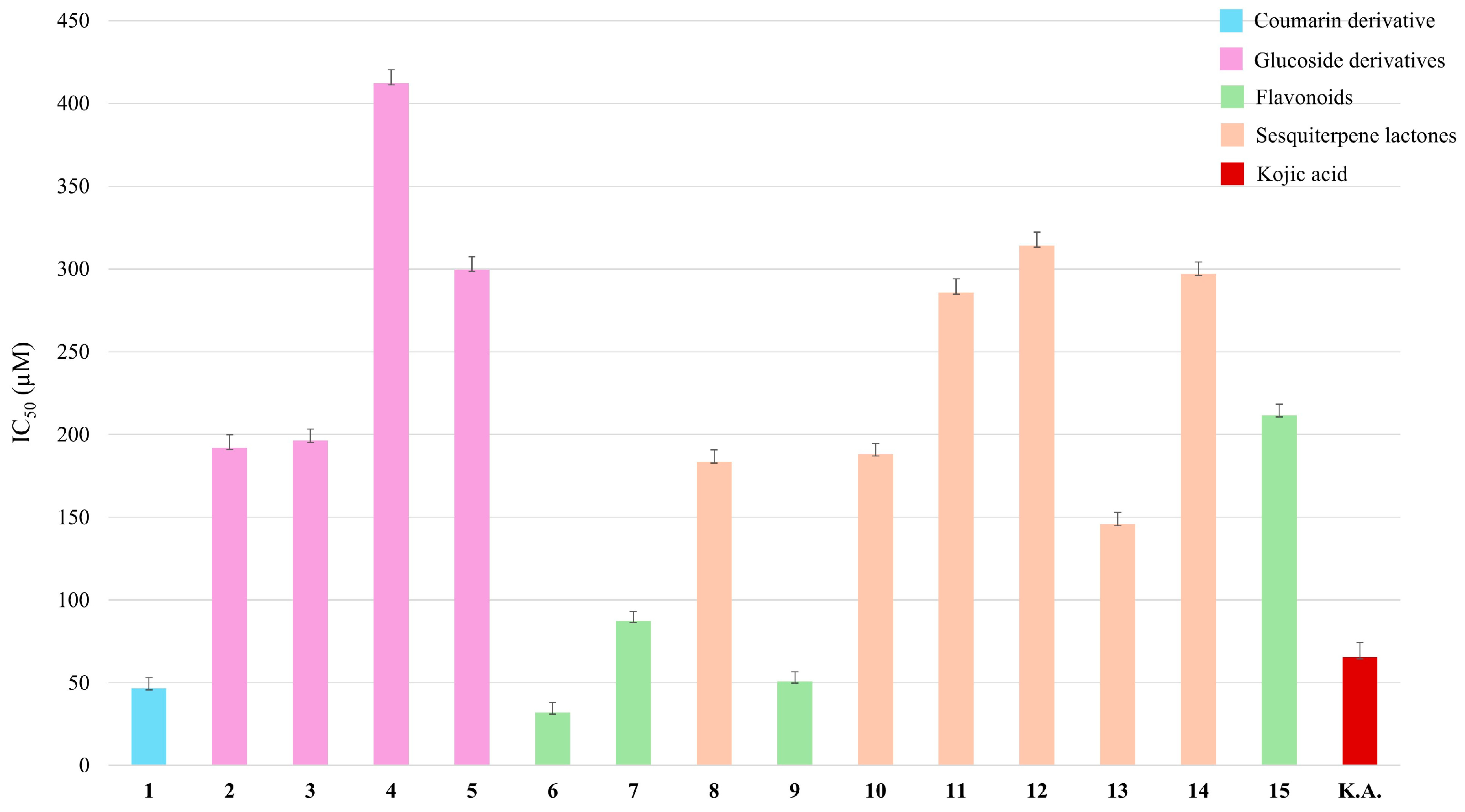
2.3. Tyrosinase Inhibition
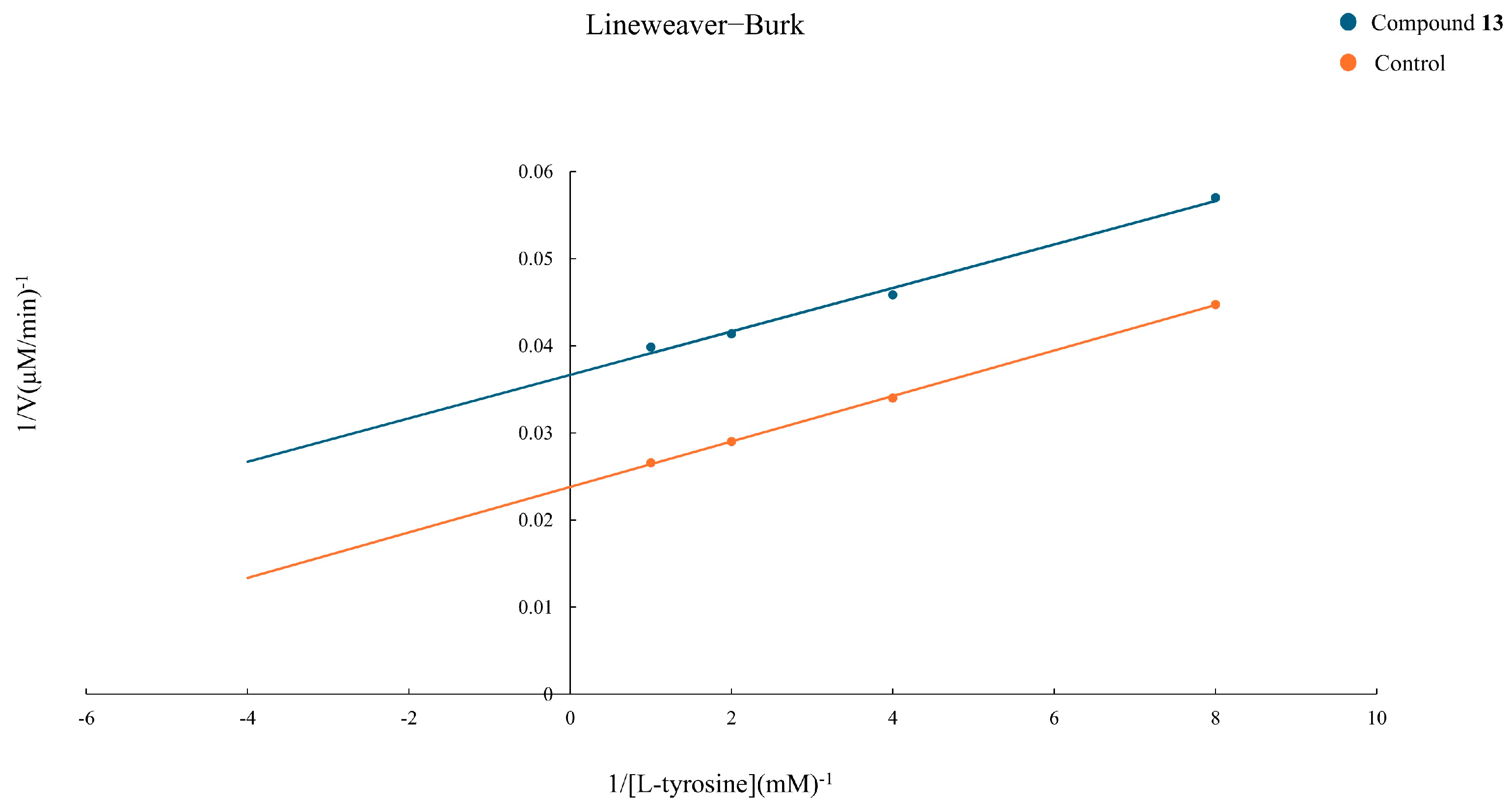
2.4. Evaluation of the Inhibition Kinetic of Compound 13
2.5. Acetylcholinesterase Inhibition Assay Results
3. Materials and Methods
3.1. Reagent and Solvents
3.2. Sample Preparation and Extraction
3.3. UPLC-HRMSMS Analysis
3.4. Isolation of Specialised Metabolites
3.5. 1D and 2D NMR Analysis
3.6. Tyrosinase Inhibition Assay
3.7. Tyrosinase Inhibition Kinetics Study
3.8. Acetylcholinesterase Inhibition Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sah, A.; Naseef, P.P.; Kuruniyan, M.S.; Jain, G.K.; Zakir, F.; Aggarwal, G. A Comprehensive Study of Therapeutic Applications of Chamomile. Pharmaceuticals 2022, 15, 1284. [Google Scholar] [CrossRef] [PubMed]
- Salamon, I. The Slovak gene pool of German chamomile (Matricaria recutita L.) and comparison in its parameters. Pharmaceuticals 2018, 31, 70–75. [Google Scholar]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with bright future. Mol. Med. Rep. 2010, 3, 895–901. [Google Scholar]
- Dai, Y.-L.; Li, Y.; Wang, Q.; Niu, F.-J.; Li, K.-W.; Wang, Y.-Y.; Wang, J.; Zhou, C.-Z.; Gao, L.-N. Chamomile: A Review of Its Traditional Uses, Chemical Constituents, Pharmacological Activities and Quality Control Studies. Molecules 2023, 28, 133. [Google Scholar] [CrossRef] [PubMed]
- De Mieri, M.; Monteleone, G.; Ismajili, I.; Kaiser, M.; Hamburger, M. Antiprotozoal Activity-Based Profiling of a Dichloromethane Extract from Anthemis nobilis Flowers. J. Nat. Prod. 2017, 80, 459–470. [Google Scholar] [CrossRef]
- Zaiter, L.; Bouheroum, M.; Benayache, S.; Benayache, F.; León, F.; Brouard, I.; Quintana, J.; Estévez, F.; Bermejo, J. Sesquiterpene lactones and other constituents from Matricaria chamomilla L. Biochem. Syst. Ecol. 2007, 35, 533–538. [Google Scholar] [CrossRef]
- Abdel-Mogib, M.; Jakupovic, J.; Dawidar, A.M.; Metwally, M.A.; Abou-Elzahab, M. Glaucolides from Achillea fragrantissima. Phytochemistry 1989, 28, 3528–3530. [Google Scholar] [CrossRef]
- Zarga, M.A.; Qauasmeh, R.; Sabri, S.; Munsoor, M.; Abdalla, S. Chemical Constituents of Artemisia arborescens and the Effect of the Aqueous Extract on Rat Isolated Smooth Muscle. Planta Med. 1995, 61, 242–245. [Google Scholar] [CrossRef]
- Gonzalez-Sepulveda, M.; Compte, J.; Cuadros, T.; Nicolau, A.; Guillard-Sirieix, C.; Peñuelas, N.; Lorente-Picon, M.; Parent, A.; Romero-Giménez, J.; Cladera-Sastre, J.M.; et al. In vivo reduction of age-dependent neuromelanin accumulation mitigates features of Parkinson’s disease. Brain 2023, 146, 1040–1052. [Google Scholar] [CrossRef]
- Polcaro, L.M.; Cerulli, A.; Montella, F.; Ciaglia, E.; Masullo, M.; Piacente, S. Chemical Profile and Antioxidant and Tyrosinase Inhibitory Activity of Chamaemelum nobile L. Green Extracts. Cosmetics 2024, 11, 94. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Mittal, P.; Gautam, R.K.; Kamal, M.A.; Perveen, A.; Garg, V.; Alexiou, A.; Saboor, M.; Haque, S.; Farhana, A.; et al. Natural products in the management of neurodegenerative diseases. Nutr. Metab. 2024, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.C.D.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.D.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase Inhibitory Potential of Various Sesquiterpene Analogues for Alzheimer’s Disease Therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.E.F.; Ibrahim, A.Y.; Mohamed, T.A.; Shahat, A.A.; El Halawany, A.M.; Abdel-Azim, N.S.; Alsaid, M.S.; Paré, P.W. Sesquiterpene Lactones from Cynara cornigera: Acetyl Cholinesterase Inhibition and In Silico Ligand Docking. Planta Medica 2015, 82, 138–146. [Google Scholar] [PubMed]
- Hajimehdipoor, H.; Mosaddegh, M.; Naghibi, F.; Haeri, A.; Hamzeloo-Moghadam, M. Natural sesquiterpen lactones as acetylcholinesterase inhibitors. An. Acad. Bras. Cienc. 2014, 86, 801–806. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, M.; Farooq, T.; Hussain, N.; Hussain, A.; Gulzar, T.; Hussain, I.; Akash, M.S.H.; Rehmani, F.S. Acetyl and butyryl cholinesterase inhibitory sesquiterpene lactones from Amberboa ramosa. Chem. Cent. J. 2013, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Elsebai, M.F.; Ghabbour, H.A.; Marzouk, A.M.; Salmas, R.E.; Orhan, I.E.; Senol, F.S. Amberboin and lipidiol: X-ray crystalographic data, absolute configuration and inhibition of cholinesterase. Phytochem. Lett. 2018, 27, 44–48. [Google Scholar] [CrossRef]
- Polcaro, L.M.; Masullo, M.; Piacente, S. Effects of green extraction methods on the chemical profile of Schisandra chinensis fruit extracts and on their tyrosinase inhibitory activity. LWT 2024, 208, 116697. [Google Scholar] [CrossRef]
- Polcaro, L.M.; Cerulli, A.; Masullo, M.; Piacente, S. Metabolomics of Withania somnifera L. extracts by an integrated LC-MS and NMR approach and evaluation of their tyrosinase inhibitory activity. J. Pharm. Biomed. Anal. 2025, 253, 116520. [Google Scholar]
- Balkrishna, A.; Pokhrel, S.; Tomer, M.; Verma, S.; Kumar, A.; Nain, P.; Gupta, A.; Varshney, A. Anti-Acetylcholinesterase Activities of Mono-Herbal Extracts and Exhibited Synergistic Effects of the Phytoconstituents: A Biochemical and Computational Study. Molecules 2019, 24, 4175. [Google Scholar] [CrossRef] [PubMed]
- Isabel, U.-V.; Belén, A.D.L.R.M.; Elena, G.B. A new frontier in neuropharmacology: Recent progress in natural products research for blood–brain barrier crossing. Curr. Res. Biotechnol. 2024, 8, 100235. [Google Scholar] [CrossRef]
- Zhang, T.; Su, J.; Guo, B.; Wang, K.; Li, X.; Liang, G. Apigenin protects blood–brain barrier and ameliorates early brain injury by inhibiting TLR4-mediated inflammatory pathway in subarachnoid hemorrhage rats. Int. Immunopharmacol. 2015, 28, 79–87. [Google Scholar] [CrossRef]
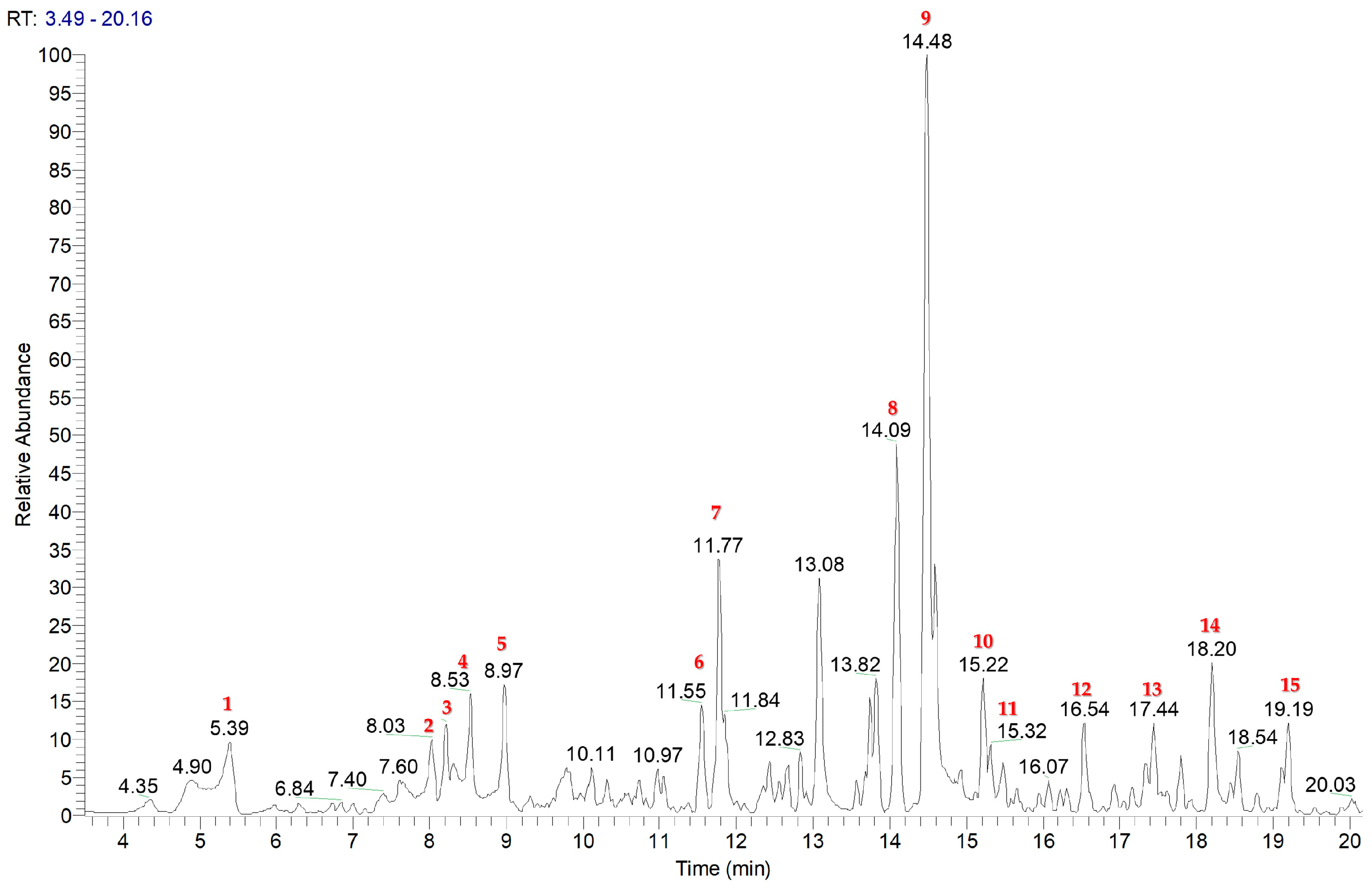

| Rt | [M + H]+ | [M + Na]+ | Mol Formula | Δppm | MS/MS | Name | |
|---|---|---|---|---|---|---|---|
| 1 | 6.97 | 377.0841 | C16H18O9 | −0.78 | 359.0734 (C16H16O8Na), 215.0527 (C7H12O6Na) | Scopolin | |
| 2 | 7.66 | 411.1619 | C18H28O9 | −1.64 | 249.1098 (C12H18O4Na), 203.0526 (C6H12O6Na) | Tuberonic acid glucoside | |
| 3 | 8.43 | 307.1150 | C14H20O6 | −0.93 | 232.0700 (C11H13O4Na) | Phenylethyl ꞵ-D-glucopyranoside | |
| 4 | 8.56 | 195.0875 | C7H14O6 | 2.38 | nf | 1-Methylpropyl ꞵ-D-glucopyranoside | |
| 5 | 8.97 | 299.1122 | C12H20O7 | −2.76 | nf | ꞵ-D-Glucopyranose, 1-[(2 Z)-2 methyl-2-butenoate] | |
| 6 | 10.33 | 601.1525 | C27H30O14 | −0.53 | 331.0998 (C12H20O9Na), 167.0704 (C9H11O3) | Apigenin 7-O-rutinoside | |
| 7 | 11.56 | 493.1334 | C23H24O12 | −1.37 | 331.080 (C17H15O7), 316.0577 (C16H12O7) | Camaraside | |
| 8 | 14.20 | 385.1616 | C20H26O6 | −0.59 | 303.1209 (C15H20O5Na), 285.1098 (C15H18O4Na) | 8-Tigloylhydroxyisonobilin | |
| 9 | 14.52 | 271.0595 | C15H10O5 | −2.51 | 119.0492 (C8H7O) | Apigenin | |
| 10 | 16.64 | 385.1616 | C20H26O6 | −0.72 | 303.1209 (C15H20O5Na), 285.1098 (C15H18O4Na) | Hydroxyisonobilin | |
| 11 | 16.66 | 385.1619 | C20H26O6 | −0.83 | 303.1209 (C15H20O5Na), 285.1098 (C15H18O4Na) | 3-Epi-hydroxyisonobilin | |
| 12 | 17.49 | 383.1459 | C20H24O6 | −0.44 | 283.0945 (C15H16O4Na), 239.1050 (C14H16O2Na) | Nobilinon A | |
| 13 | 17.85 | 387.1806 | C20H28O6 | −1.07 | 287.1254 (C15H20O4Na) | 11,13-Dihydro-8-tigloylhydroxyisonobilin | |
| 14 | 17.90 | 369.1671 | C20H26O5 | 0.84 | 269.1140 (C15H18O3Na), 251.1039 (C15H16O2Na) | Nobilin | |
| 15 | 19.19 | 315.0862 | C17H14O6 | −1.49 | 300.0627 (C16H12O6) | 5,7-Dihydroxy-6-methoxy-2-(4-methoxyphenyl)-4H-1-benzopyran-4-one |
| 13 | ||
| δH (J in Hz) | δc | |
| 1 | 3.93 (brdd, 9.5, 5.8) | 73.5 CH |
| 2 | 2.28 (m), 2.25 (m) | 38.0 CH2 |
| 3 | 4.47 (brt, 4.4) | 73.0 CH |
| 4 | - | 144.0 C |
| 5 | 5.30 (m) | 125.9 CH |
| 6 | 6.14 (dd, 9.4, 7.8) | 76.5 CH |
| 7 | 2.32 (m) | 55.1 CH |
| 8 | 5.31 (m) | 77.3 CH |
| 9 | 2.94 (dd, 14.8, 2.3) 2.35 (m) | 39.7 CH2 |
| 10 | - | 145.5 C |
| 11 | 2.60 (dq, 12.3, 7.2) | 40.5 CH |
| 12 | - | 180.0 C |
| 13 | 1.30 (d, 6.8) | 16.7 CH3 |
| 14 | 5.55 (d, 0.5) 5.49 (d, 0.5) | 117.8 CH2 |
| 15 | 1.83 (s) | 23.5 CH3 |
| 16 | - | 167.3 C |
| 17 | - | 128.0 C |
| 18 | 6.22 (brdd, 7.3, 1.3) | 139.1 CH |
| 19 | 1.94 (brd, 1.3) | 20.3 CH3 |
| 20 | 2.03 (d, 7.3) | 15.7 CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polcaro, L.M.; Cerulli, A.; Masullo, M.; Piacente, S. Phytochemical Investigation of Chamaemelum nobile L. and Evaluation of Acetylcholinesterase and Tyrosinase Inhibitory Activity. Plants 2025, 14, 595. https://doi.org/10.3390/plants14040595
Polcaro LM, Cerulli A, Masullo M, Piacente S. Phytochemical Investigation of Chamaemelum nobile L. and Evaluation of Acetylcholinesterase and Tyrosinase Inhibitory Activity. Plants. 2025; 14(4):595. https://doi.org/10.3390/plants14040595
Chicago/Turabian StylePolcaro, Luciana Maria, Antonietta Cerulli, Milena Masullo, and Sonia Piacente. 2025. "Phytochemical Investigation of Chamaemelum nobile L. and Evaluation of Acetylcholinesterase and Tyrosinase Inhibitory Activity" Plants 14, no. 4: 595. https://doi.org/10.3390/plants14040595
APA StylePolcaro, L. M., Cerulli, A., Masullo, M., & Piacente, S. (2025). Phytochemical Investigation of Chamaemelum nobile L. and Evaluation of Acetylcholinesterase and Tyrosinase Inhibitory Activity. Plants, 14(4), 595. https://doi.org/10.3390/plants14040595







