Insights into the Genetics Underlying the Resistance to Root-Knot Nematode Reproduction in the Common Bean Ouro Negro
Abstract
1. Introduction
2. Results
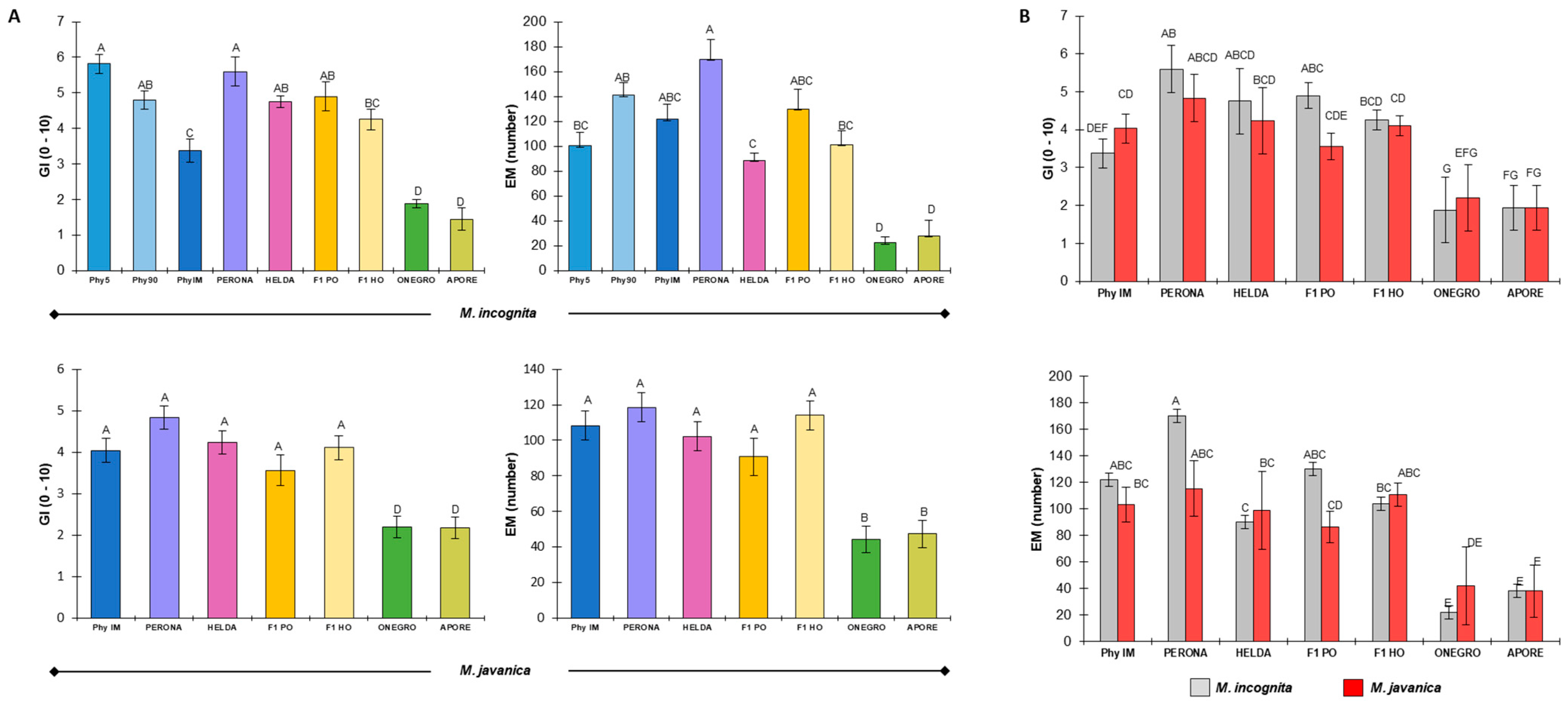
2.1. Response of Common Bean Genotypes to M. incognita and M. javanica Infection
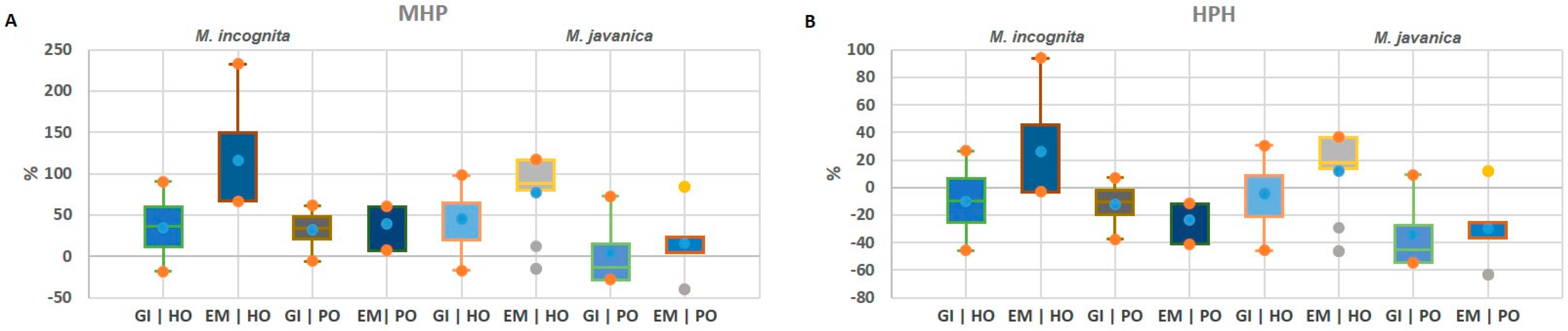
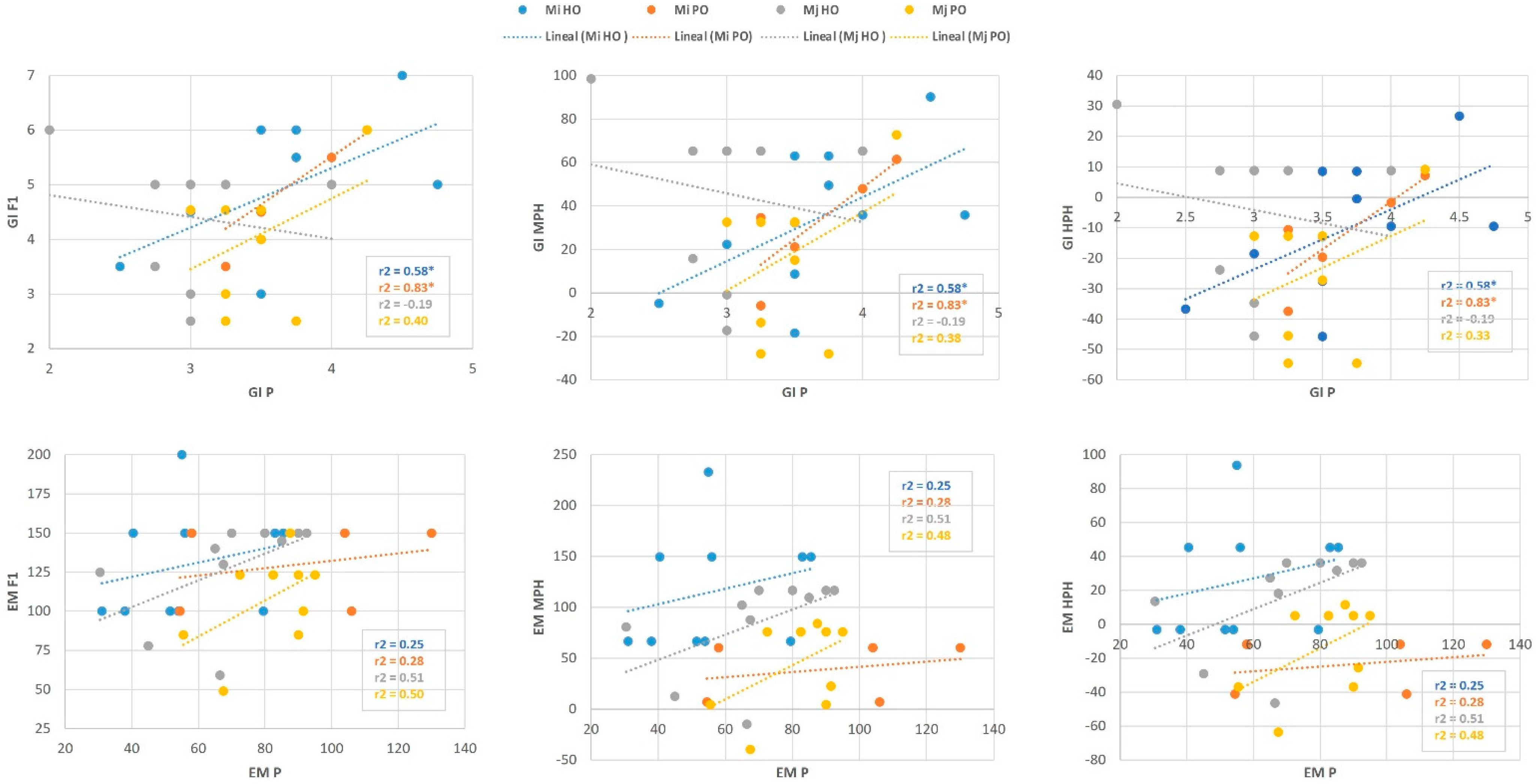
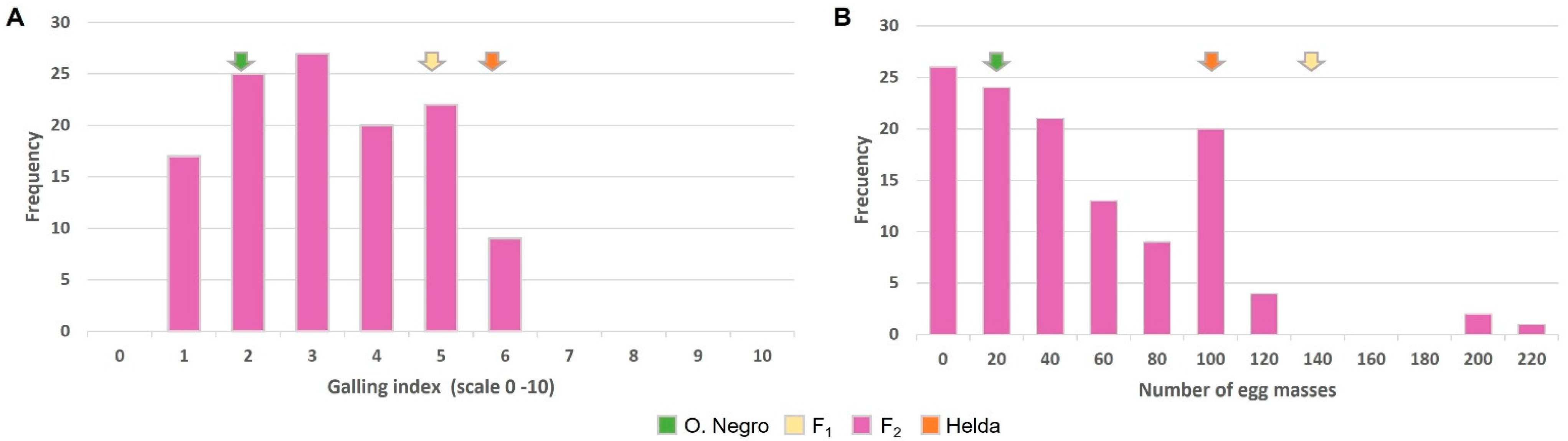
2.2. Heterosis Performance of F1 Hybrids
2.3. Inheritance Pattern of Resistance to Meloidogyne Derived from Ouro Negro
3. Discussion
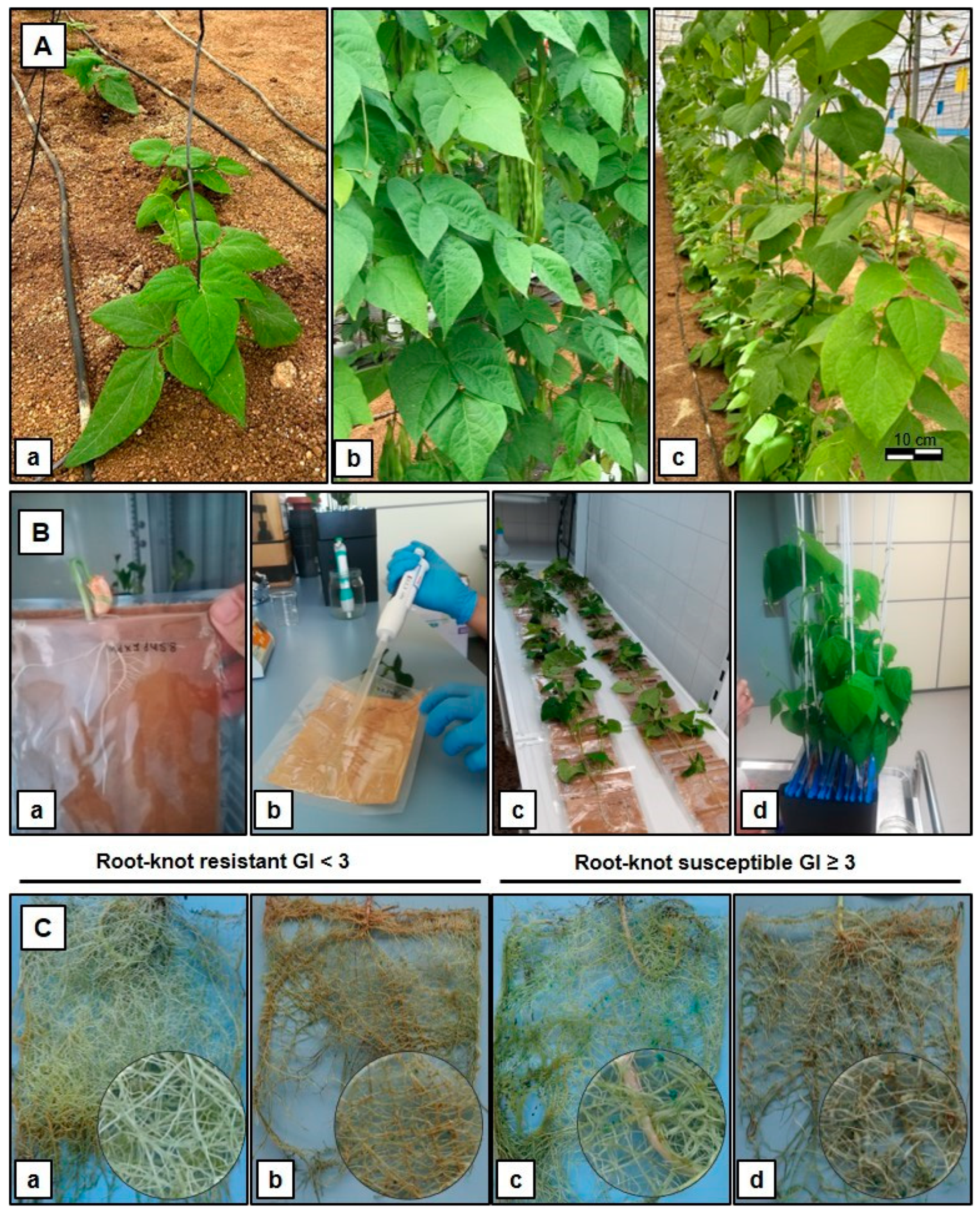
4. Materials and Methods
4.1. Plant Material
4.2. Nematode Isolates and Resistance Phenotyping Evaluation
4.3. Genetic Analyses of RKN Resistance: Heterosis, Inheritance and Allelism Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 16 January 2025).
- Archidona-Yuste, A.; Cantalapiedra-Navarrete, C.; Liébanas, G.; Rapoport, H.F.; Castillo, P.; Palomares-Rius, J.E. Diversity of Root-Knot Nematodes of the Genus Meloidogyne Göeldi, 1892 (Nematoda: Meloidogynidae) Associated with Olive Plants and Environmental Cues Regarding Their Distribution in Southern Spain. PLoS ONE 2018, 13, e0198236. [Google Scholar] [CrossRef]
- Koenning, S.R.; Overstreet, C.; Noling, J.W.; Donald, P.A.; Becker, J.O.; Fortnum, B.A. Survey of Crop Losses in Response to Phytoparasitic Nematodes in the United States for 1994. J. Nematol. 1999, 31, 587–618. [Google Scholar]
- Di Vito, M.; Parisi, B.; Catalanol, F. Effect of Population Densities of Meloidogyne incognita on Common Bean. Nematol. Medit. 2004, 32, 81–85. [Google Scholar]
- Fullana, A.M.; Expósito, A.; Escudero, N.; Cunquero, M.; Loza-Alvarez, P.; Giné, A.; Sorribas, F.J. Crop Rotation with Meloidogyne-Resistant Germplasm Is Useful to Manage and Revert the (a)Virulent Populations of Mi1.2 Gene and Reduce Yield Losses. Front. Plant Sci. 2023, 14, 1133095. [Google Scholar] [CrossRef] [PubMed]
- Ornat, C.; Sorribas, F.J. Integrated Management of Root-Knot Nematodes in Mediterranean Horticultural Crops. Integr. Manag. Biocontrol Veg. Grain Crops Nematodes 2008, 2, 295–319. [Google Scholar] [CrossRef]
- Talavera, M.; Miranda, L.; Gómez-Mora, J.A.; Vela, M.D.; Verdejo-Lucas, S. Nematode Management in the Strawberry Fields of Southern Spain. Agronomy 2019, 9, 252. [Google Scholar] [CrossRef]
- Talavera, M.; Sayadi, S.; Chirosa-Ríos, M.; Salmerón, T.; Flor-Peregrín, E.; Verdejo-Lucas, S. Perception of the Impact of Root-Knot Nematode-Induced Diseases in Horticultural Protected Crops of South-Eastern Spain. Nematology 2012, 14, 517–527. [Google Scholar] [CrossRef]
- Zaumeyer, W.J.; Thomas, H.R. A Monographic Study of Bean Diseases and Methods for Their Control. In Technical Bulletins; US Department of Agriculture: Washington, DC, USA, 1957; p. 686. [Google Scholar] [CrossRef]
- Hartmann, R.W. Inheritance of Resistance to Root-Knot Nematodes (Meloidogyne incognita) in Beans (Phaseolus vulgaris L.). J. Am. Soc. Hortic. Sci. 1971, 96, 344–347. [Google Scholar] [CrossRef]
- Crozzoli, R.; Greco, N.; Audrey Suárez, C.; Rivas, D. Pathogenicity of the Root-Knot Nematode, Meloidogyne incognita, to Cultivars of Phaseolus vulgaris and Vigna unguiculata. Nematropica 1997, 27, 61–67. [Google Scholar]
- Chen, P.; Roberts, P.A. Virulence in Meloidogyne hapla Differentiated by Resistance in Common Bean (Phaseolus vulgaris). Nematology 2003, 5, 39–47. [Google Scholar] [CrossRef]
- Chen, P.; Roberts, P.A. Genetic Analysis of (a)Virulence in Meloidogyne hapla to Resistance in Bean (Phaseolus vulgaris). Nematology 2003, 5, 687–697. [Google Scholar] [CrossRef]
- Ferreira, S.; Gomes, L.A.A.; Maluf, W.R.; Campos, V.P.; de Carvalho Filho, J.L.S.; Santos, D.C. Resistance of Dry Bean and Snap Bean Cultivars to Root-Knot Nematodes. HortScience 2010, 45, 320–322. [Google Scholar] [CrossRef]
- Valentini, G.; Gonçalves-Vidigal, M.C.; Hurtado-Gonzales, O.P.; de Lima Castro, S.A.; Cregan, P.B.; Song, Q.; Pastor-Corrales, M.A. High-Resolution Mapping Reveals Linkage between Genes in Common Bean Cultivar Ouro Negro Conferring Resistance to the Rust, Anthracnose, and Angular Leaf Spot Diseases. Theor. Appl. Genet. 2017, 130, 1705–1722. [Google Scholar] [CrossRef]
- dos Santos, L.N.S.; Alves, F.R.; Belan, L.L.; Cabral, P.D.S.; de Matta, F.P.; de Jesus Junior, W.C.; de Moraes, W.B. Damage Quantification and Reaction of Bean Genotypes (Phaseolus vulgaris L.) to Meloidogyne incognita Race 3 and M. javanica. Summa Phytopathol. 2012, 38, 24–29. [Google Scholar] [CrossRef]
- Giné, A.; Sanz-Prieto, A.; Gomes, L.A.A.; Expósito, A.; Escudero, N.; Sorribas, F.J. Host Suitability of Lettuce and Bean Germplasm for Meloidogyne incognita and M. javanica Isolates from Spain. Plants 2023, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.A. Conceptual and Practical Aspects of Variability in Root-Knot Nematodes Related to Host Plant Resistance. Annu. Rev. Phytopathol. 1995, 33, 199–221. [Google Scholar] [CrossRef]
- Jiang, L.; Ling, J.; Zhao, J.; Yang, Y.; Yang, Y.; Li, Y.; Jiao, Y.; Mao, Z.; Wang, Y.; Xie, B. Chromosome-Scale Genome Assembly-Assisted Identification of Mi-9 Gene in Solanum arcanum Accession LA2157, Conferring Heat-Stable Resistance to Meloidogyne incognita. Plant Biotechnol. J. 2023, 21, 1496–1509. [Google Scholar] [CrossRef] [PubMed]
- Tigano, M.; De Siqueira, K.; Castagnone-Sereno, P.; Mulet, K.; Queiroz, P.; Dos Santos, M.; Teixeira, C.; Almeida, M.; Silva, J.; Carneiro, R. Genetic diversity of the root-knot nematode Meloidogyne enterolobii and development of a SCAR marker for this guava-damaging species. Plant Patol. 2010, 59, 1054–1061. [Google Scholar] [CrossRef]
- Williamson, V.M.; Roberts, P.A. Mechanisms and Genetics of Resistance. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CABI Publishing: Wallingford, UK, 2009; pp. 301–325. ISBN 9781845934927. [Google Scholar]
- El-Sappah, A.H.; Islam, M.M.; El-Awady, H.H.; Yan, S.; Qi, S.; Liu, J.; Cheng, G.T.; Liang, Y. Tomato Natural Resistance Genes in Controlling the Root-Knot Nematode. Genes 2019, 10, 925. [Google Scholar] [CrossRef]
- Wu, W.-W.; Shen, H.-L.; Yang, W.-C. Sources for Heat-Stable Resistance to Southern Root-Knot Nematode (Meloidogyne incognita) in Solanum lycopersicum. Agric. Sci. China 2009, 8, 697–702. [Google Scholar] [CrossRef]
- Hendy, H.; Dalmasso, A.; Cardin, M.C. Differences in Resistant Capsicum annuum Attacked by Different Meloidogyne Species. Nematologica 1985, 31, 72–78. [Google Scholar] [CrossRef]
- Djian-Caporalino, C.; Fazari, A.; Arguel, M.J.; Vernie, T.; VandeCasteele, C.; Faure, I.; Brunoud, G.; Pijarowski, L.; Palloix, A.; Lefebvre, V.; et al. Root-Knot Nematode (Meloidogyne spp.) Me Resistance Genes in Pepper (Capsicum annuum L.) Are Clustered on the P9 Chromosome. Theor. Appl. Genet. 2007, 114, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Wu, C.L.; Holbrook, C.C.; Tillman, B.L.; Person, G.; Ozias-Akins, P. Marker-Assisted Selection to Pyramid Nematode Resistance and the High Oleic Trait in Peanut. Plant Genome 2011, 4, 110–117. [Google Scholar] [CrossRef]
- Garcia, G.M.; Stalker, H.T.; Shroeder, E.; Kochert, G. Identification of RAPD, SCAR, and RFLP Markers Tightly Linked to Nematode Resistance Genes Introgressed from Arachis cardenasii into Arachis hypogaea. Genome 1996, 39, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Cap, G.B.; Roberts, P.A.; Thomason, I.J. Inheritance of Heat-Stable Resistance to Meloidogyne incognita in Lycopersicon peruvianum and Its Relationship to the Mi Gene. Theor. Appl. Genet. 1993, 85, 777–783. [Google Scholar] [CrossRef]
- De Ilarduya, O.M.; Moore, A.E.; Kaloshian, I. The Tomato Rme1 Locus Is Required for Mi-1-Mediated Resistance to Root-Knot Nematodes and the Potato Aphid. Plant J. 2001, 27, 417–425. [Google Scholar] [CrossRef]
- Ho, J.-Y.; Weide, R.; Ma, H.M.; van Wordragen, M.F.; Lambert, K.N.; Koornneef, M.; Zabel, P.; Williamson, V.M. The Root-knot Nematode Resistance Gene (Mi) in Tomato: Construction of a Molecular Linkage Map and Identification of Dominant CDNA Markers in Resistant Genotypes. Plant J. 1992, 2, 971–982. [Google Scholar] [CrossRef]
- Roberts, P.A.; Matthews, W.C.; Ehlers, J.D.; Helms, D. Genetic Determinants of Differential Resistance to Root-knot Nematode Reproduction and Galling in Lima Bean. Crop Sci. 2008, 48, 553–561. [Google Scholar] [CrossRef]
- Omwega, C.O.; Roberts, P.A. Inheritance of Resistance to Meloidogyne spp. in Common Bean and the Genetic Basis of Its Sensitivity to Temperature. Theor. Appl. Genet. 1992, 83, 720–726. [Google Scholar] [CrossRef]
- Freitas, L.C.; Ramalho, M.; Silva, F.B. Controle Genético Da Resistência Aos Nematoides de Galhas Em Feijoeiro. In Proceedings of the Congresso Nacional de Pesquisa de Feijão, Viçosa, MG, Brasil, 8–12 September 2002; pp. 361–362. [Google Scholar]
- Di Vito, M.; Parisi, B.; Carboni, A.; Ranalli, P.; Catalano, F. Genetics and Introgression of Resistance to Root-Knot Nematodes (Meloidogyne spp.) in Common Bean (Phaseolus vulgaris L.). Nematol. Mediterr. 2007, 35, 193–198. [Google Scholar]
- Ferreira, S.; Gomes, L.A.A.; Maluf, W.R.; Furtini, I.V.; Campos, V.P. Genetic Control of Resistance to Meloidogyne incognita Race 1 in the Brazilian Common Bean (Phaseolus vulgaris L.) Cv. Aporé. Euphytica 2012, 186, 867–873. [Google Scholar] [CrossRef]
- Faleiro, F.G.; Nietsche, S.; Ragagnin, V.A.; Borém, A.; Moreira, M.A.; De Barros, E.G. Resistance of Common Bean to Rust and Angular Leaf Spot Under Greenhouse Conditions. Fitopatol. Bras. 2001, 26, 86–89. [Google Scholar] [CrossRef]
- Gonçalves-Vidigal, M.C.; Kelly, J.D.; Gonçalves-Vidigal, M.C. Inheritance of Anthracnose Resistance in the Common Bean Cultivar Widusa. Euphytica 2006, 151, 411–419. [Google Scholar] [CrossRef]
- Lilia Alzate-Marin, A.; Regina Costa, M.; Marcio Arruda, K.; Gonçalves de Barros, E.; Alves Moreira, M. Characterization of the Anthracnose Resistance Gene Present in Ouro Negro (Honduras 35) Common Bean Cultivar. Euphytica 2003, 133, 165–169. [Google Scholar]
- Omwega, C.O.; Thomason, I.J.; Roberts, P.A. A Single Dominant Gene in Common Bean Conferring Resistance to Three Root-Knot Nematode Species. Phytopathology 1990, 80, 745. [Google Scholar] [CrossRef]
- Barrons, K.C. Root-Knot Resistance in Beans. J. Hered. 1940, 31, 35–38. [Google Scholar]
- Blazey, D.A.; Smith, P.G.; Gentile, A.G.; Miyagawa, S.T. Nematode Resistance in the Common Bean. J. Hered. 1964, 55, 20–22. [Google Scholar]
- Ndeve, A.D.; Santos, J.R.P.; Matthews, W.C.; Huynh, B.L.; Guo, Y.N.; Lo, S.; Muñoz-Amatriaín, M.; Roberts, P.A. A Novel Root-Knot Nematode Resistance QTL on Chromosome Vu01 in Cowpea. G3 Genes Genome Genet. 2019, 9, 1199–1209. [Google Scholar] [CrossRef]
- Kagoda, F.; Derera, J.; Tongoona, P.; Coyne, D.L.; Talwana, H.L. Grain Yield and Heterosis of Maize Hybrids under Nematode Infested and Nematicide Treated Conditions. J. Nematol. 2011, 43, 209–2019. [Google Scholar]
- González, A.M.; Godoy, L.; Santalla, M. Dissection of Resistance Genes to Pseudomonas syringae Pv. phaseolicola in UI3 Common Bean Cultivar. Int. J. Mol. Sci. 2017, 18, 2503. [Google Scholar] [CrossRef]
- Burton, J.W.; Brownie, C. Heterosis and Inbreeding Depression in Two Soybean Single Crosses. Crop Sci. 2006, 46, 2643–2648. [Google Scholar] [CrossRef]
- Paril, J.; Reif, J.; Fournier-Level, A.; Pourkheirandish, M. Heterosis in Crop Improvement. Plant J. 2024, 117, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Hochholdinger, F.; Yu, P. Molecular Concepts to Explain Heterosis in Crops. Trends Plant Sci. 2025, 30, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Fassuliotis, G.; Deakin, J.; Hoffman, J. Root-Knot Nematode Resistance in Snap Beans: Breeding and Nature of Resistance. J. Am. Soc. Hortic. Sci. 1970, 95, 640–645. [Google Scholar] [CrossRef]
- Mullin, B.A.; Abawi, G.S.; Pastor-Corrales, M.A. Modification of Resistance Expression of Phaseolus vulgaris to Meloidogyne incognita by Elevated Soil Temperatures. J. Nematol. 1991, 23, 182–187. [Google Scholar]
- Harris, D.K.; Roger Boerma, H.R.; Hussey, R.S.; Finnerty, S.L. Additional Sources of Soybean Germplasm Resistant to Two Species of Root-knot Nematode. Crop Sci. 2003, 43, 1848–1851. [Google Scholar] [CrossRef]
- Liu, S.; Kandoth, P.K.; Lakhssassi, N.; Kang, J.; Colantonio, V.; Heinz, R.; Yeckel, G.; Zhou, Z.; Bekal, S.; Dapprich, J.; et al. The soybean GmSNAP18 gene underlies two types of resistance to soybean cyst nematode. Nat. Commun. 2017, 8, 14822. [Google Scholar] [CrossRef]
- Singh, J.; van der Knaap, E. Unintended consequences of plant domestication. Plant Cell Physiol. 2022, 63, 1573–1583. [Google Scholar] [CrossRef]
- Córdova-Campos, O.; Adame-Álvarez, R.M.; Acosta-Gallegos, J.A.; Heil, M. Domestication affected the basal and induced disease resistance in common bean (Phaseolus vulgaris). Eur. J. Plant Pathol. 2012, 134, 367–379. [Google Scholar] [CrossRef]
- Trucchi, E.; Benazzo, A.; Lari, M.; Iob, A.; Vai, S.; Nanni, L.; Bellucci, E.; Bitocchi, E.; Raffini, F.; Xu, C.; et al. Ancient Genomes Reveal Early Andean Farmers Selected Common Beans While Preserving Diversity. Nat. Plants 2021, 7, 123–128. [Google Scholar] [CrossRef]
- de Melo, O.D.; Maluf, W.R.; de Gonçalves, R.J.S.; Neto, Á.C.G.; Gomes, L.A.A.; de Carvalho, R.C. Triagem de Genótipos de Hortaliças Para Resistência a Meloidogyne enterolobii. Pesqui. Agropecuária Bras. 2011, 46, 829–835. [Google Scholar] [CrossRef]
- de Oliveira, C.L.; Oliveira, N.S.; de Oliveira, M.S.; Campos, V.P.; Maluf, W.R.; Augusto Gomes, L.A. Reaction of Common Bean to Meloidogyne incognita Race 1 and Meloidogyne javanica. Rev. Ceres 2018, 65, 321–328. [Google Scholar] [CrossRef]
- Hartman, K.M.; Sasser, S.J.N. Identification of Meloidogyne Species on the Basis of Differential Host Test and Perineal-Pattern Morphology. In An Advalued Treatise on Meloidogyne; Barker, K.R., Carter, C.C., Sasser, J.N., Eds.; North Carolina State University Graphic: Raleigh, NC, USA, 1985; Volume 2, pp. 66–77. [Google Scholar]
- Whitehead, A.G.; Hemming, J.R. A Comparison of Some Quantitative Methods of Extracting Small Vermiform Nematodes from Soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Atamian, H.S.; Roberts, P.A.; Kaloshian, I. High and Low Throughput Screens with Root-Knot Nematodes Meloidogyne spp. J. Vis. Exp. 2012, 61, e3629. [Google Scholar] [CrossRef]
- Omwega, C.O. A Nondestructive Technique for Screening Bean Germ Plasm for Resistance to Meloidogyne incognita. Plant Dis. 1988, 72, 970. [Google Scholar] [CrossRef]
- Bridge, J.; Page, S.L.J. Estimation of Root-Knot Nematode Infestation Levels on Roots Using a Rating Chart. Int. J. Pest. Manag. 1980, 26, 296–298. [Google Scholar] [CrossRef]
- Fery, R.L.; Dukes, P.D.; Thies, J.A. Characterization of New Sources of Resistance in Cowpea to the Southern Root-Knot Nematode. HortScience 1994, 29, 678–679. [Google Scholar] [CrossRef]
- Addinsoft, A. XLSTAT Statistical and Data Analysis Solution 2024. Available online: https://www.xlstat.com/en/ (accessed on 1 November 2024).
- Stuber, C.W.; Edwards, M.D.; Wendel, J.F. Molecular Marker-Facilitated Investigations of Quantitative Trait Loci in Maize. II. Factors Influencing Yield and Its Component Traits. Crop Sci. 1987, 27, 639–648. [Google Scholar] [CrossRef]
- Holland, J.B.; Nyquist, W.E.; Cervantes-Martínez, C.T. Estimating and Interpreting Heritability for Plant Breeding: An Update. Plant Breed. Rev. 2003, 22, 9–112. [Google Scholar] [CrossRef]
- Wright, J.R.C. I. GERMANY. History 1968, 53, 385–388. [Google Scholar] [CrossRef]
- Lande, R. Models of Speciation by Sexual Selection on Polygenic Traits. Proc. Natl. Acad. Sci. USA 1981, 78, 3721–3725. [Google Scholar] [CrossRef] [PubMed]
| Genotype | GI 1 | EM | ||||||
|---|---|---|---|---|---|---|---|---|
| M. incognita | M. javanica | M. incognita | M. javanica | |||||
| Mean | S.E | Mean | S.E | Mean | S.E | Mean | S.E | |
| Ouro Negro | 1.88 | 0.08 | 1.82 | 0.25 | 22.15 | 2.02 | 31.86 | 3.84 |
| Aporé | 1.44 | 0.19 | 0.83 | 0.22 | 28.00 | 4.44 | 7.89 | 2.03 |
| PhyIM | 3.30 | 0.26 | 4.64 | 0.36 | 128.57 | 10.10 | 132.86 | 11.28 |
| Phy90 | 4.91 | 0.24 | Ne | Ne | 147.18 | 23.25 | Ne | Ne |
| Phy5 | 6.05 | 0.12 | Ne | Ne | 103.10 | 10.05 | Ne | Ne |
| F1 HO | 4.95 | 0.39 | 4.40 | 0.35 | 130.00 | 11.06 | 127.70 | 10.35 |
| F1 PO | 4.90 | 0.43 | 3.60 | 0.66 | 130.00 | 12.25 | 93.80 | 16.37 |
| Helda | 5.10 | 0.24 | 4.60 | 0.34 | 99.48 | 8.74 | 110.00 | 12.47 |
| Perona | 5.60 | 0.46 | 5.50 | 0.13 | 170.00 | 30.00 | 134.50 | 5.98 |
| Genotype | No. Plants a,b | |||
|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 3 | Exp. 4 | |
| M. incognita | M. javanica | M. incognita | M. incognita | |
| Helda parent | 5 | 10 | 20 | |
| Perona parent | 5 | 10 | ||
| F1 Helda × Ouro Negro line | 7 | 10 | 6 | |
| F1 Perona × Ouro Negro line | 5 | 4 | ||
| Ouro Negro parent | 18 | 8 | 27 | |
| Aporé resistant control | 8 | 9 | ||
| Phy5 susceptible control | 11 | |||
| Phy90 susceptible control | 12 | |||
| PhyIM susceptible control | 8 | 15 | ||
| F2 Helda × Ouro Negro lines | 121 | |||
| F3 Helda × Ouro Negro lines | 57 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesqueira, A.M.; González, A.M.; Barragán-Lozano, T.; Arnedo, M.S.; Lozano, R.; Santalla, M. Insights into the Genetics Underlying the Resistance to Root-Knot Nematode Reproduction in the Common Bean Ouro Negro. Plants 2025, 14, 1073. https://doi.org/10.3390/plants14071073
Pesqueira AM, González AM, Barragán-Lozano T, Arnedo MS, Lozano R, Santalla M. Insights into the Genetics Underlying the Resistance to Root-Knot Nematode Reproduction in the Common Bean Ouro Negro. Plants. 2025; 14(7):1073. https://doi.org/10.3390/plants14071073
Chicago/Turabian StylePesqueira, Ana M., Ana M. González, Teresa Barragán-Lozano, María S. Arnedo, Rafael Lozano, and Marta Santalla. 2025. "Insights into the Genetics Underlying the Resistance to Root-Knot Nematode Reproduction in the Common Bean Ouro Negro" Plants 14, no. 7: 1073. https://doi.org/10.3390/plants14071073
APA StylePesqueira, A. M., González, A. M., Barragán-Lozano, T., Arnedo, M. S., Lozano, R., & Santalla, M. (2025). Insights into the Genetics Underlying the Resistance to Root-Knot Nematode Reproduction in the Common Bean Ouro Negro. Plants, 14(7), 1073. https://doi.org/10.3390/plants14071073






