Predicting the Future Geographic Distribution of the Traditional Chinese Medicinal Plant Epimedium acuminatum Franch. in China Using Ensemble Models Based on Biomod2
Abstract
1. Introduction
2. Results
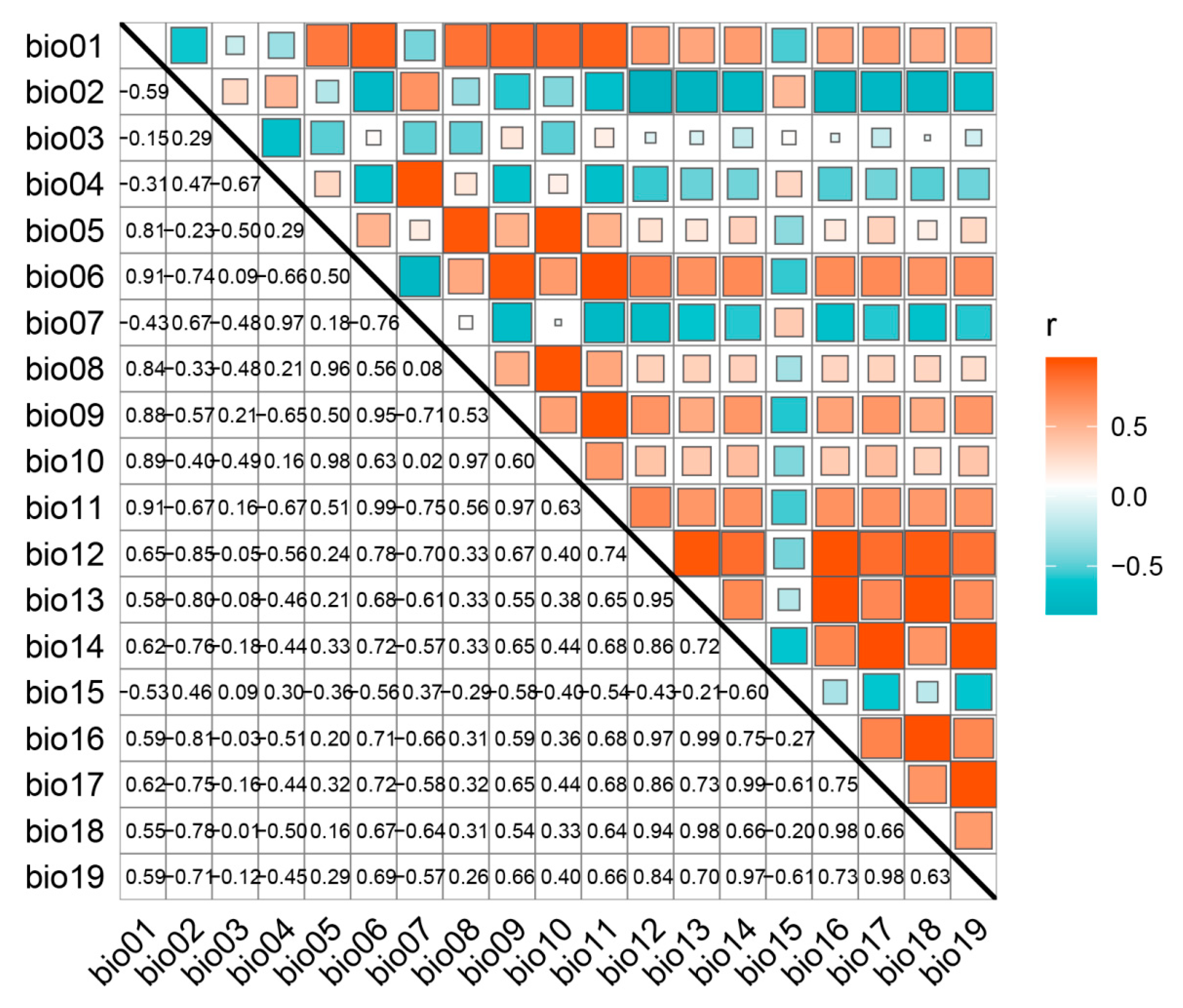
2.1. Evaluation of SDMs and Environmental Variables
2.2. Response Curve Analysis (RF)
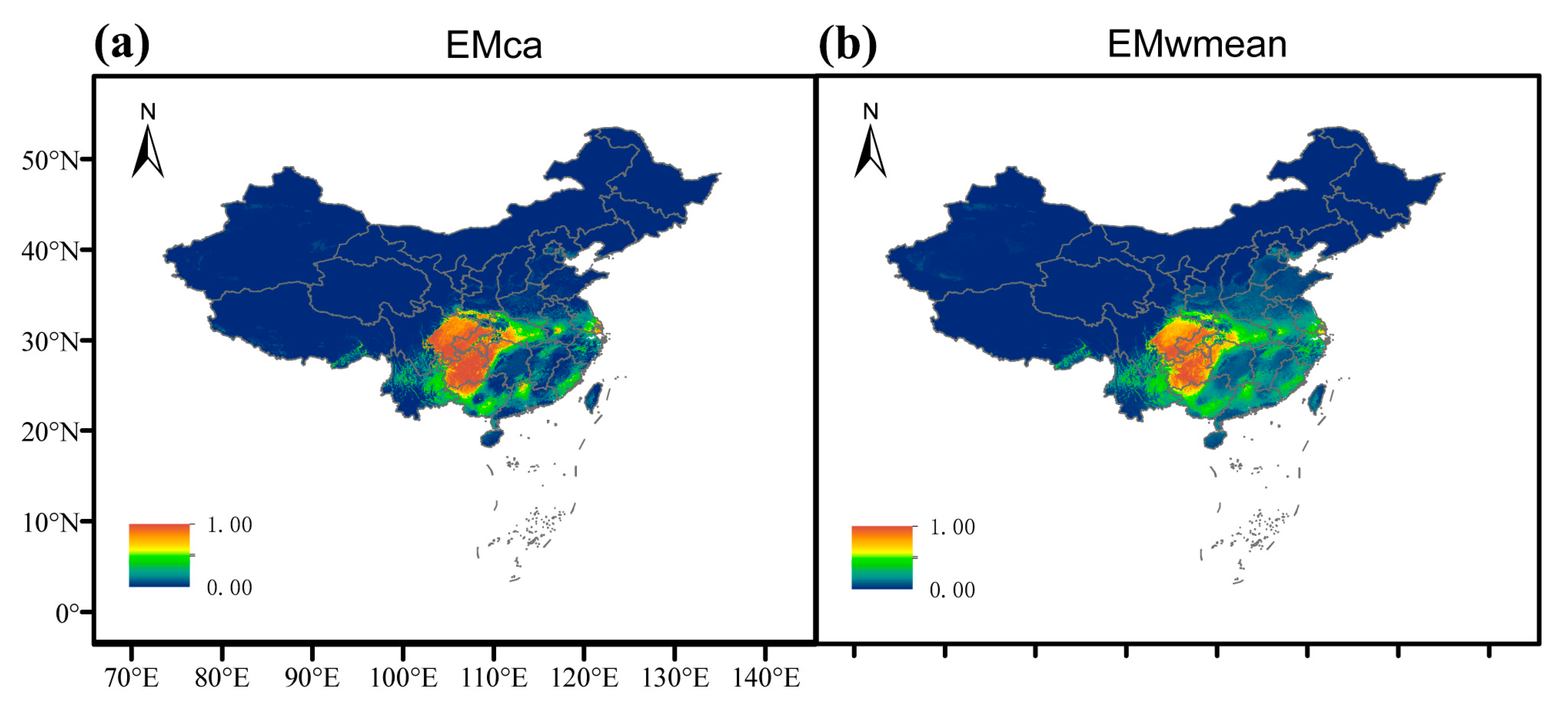
2.3. The Potential Suitable Habitat for E. acuminatum (Current)
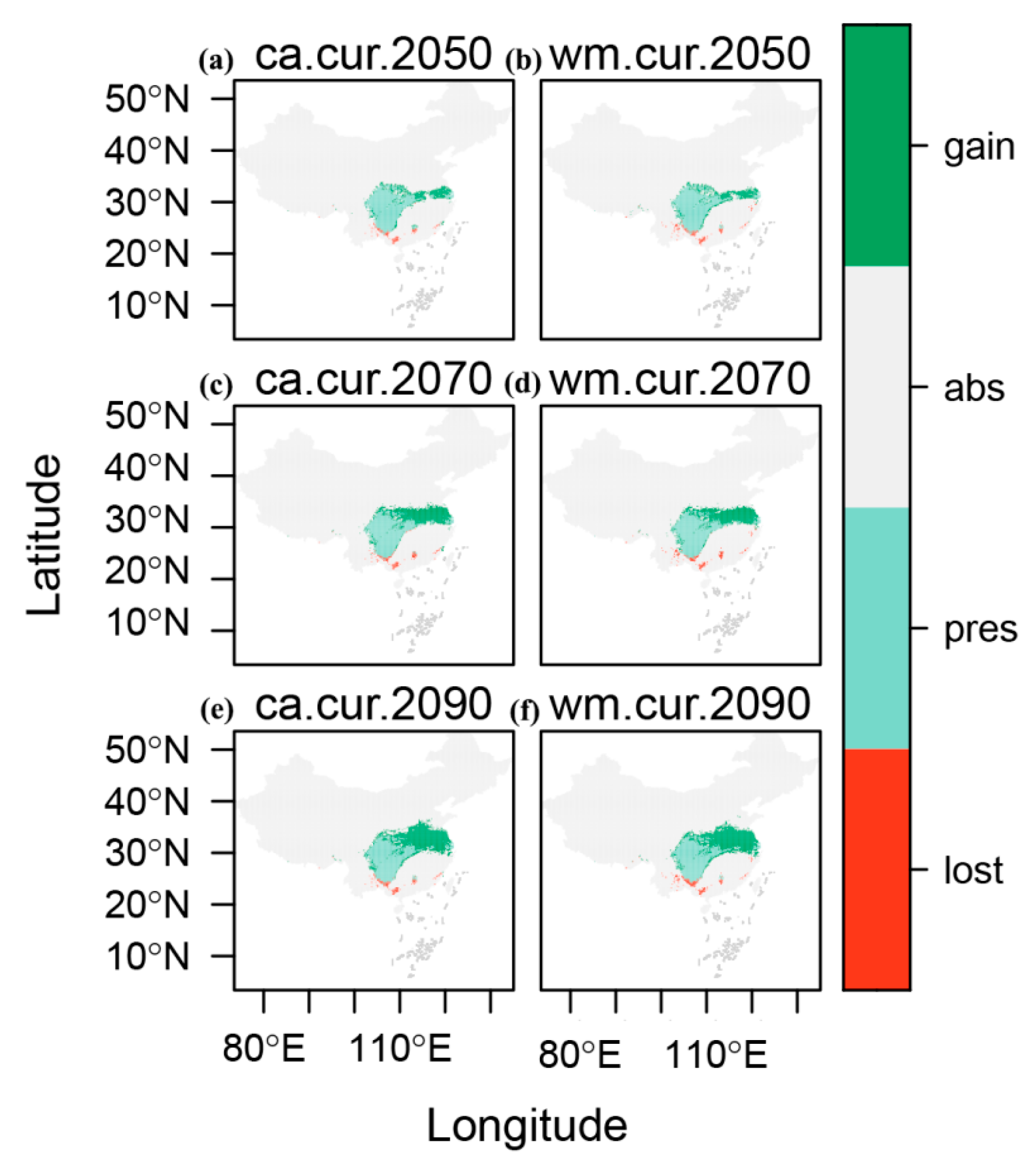
2.4. Dynamic Changes in Suitable Habitats for E. acuminatum Under Different Modeling Methods/Year Combinations
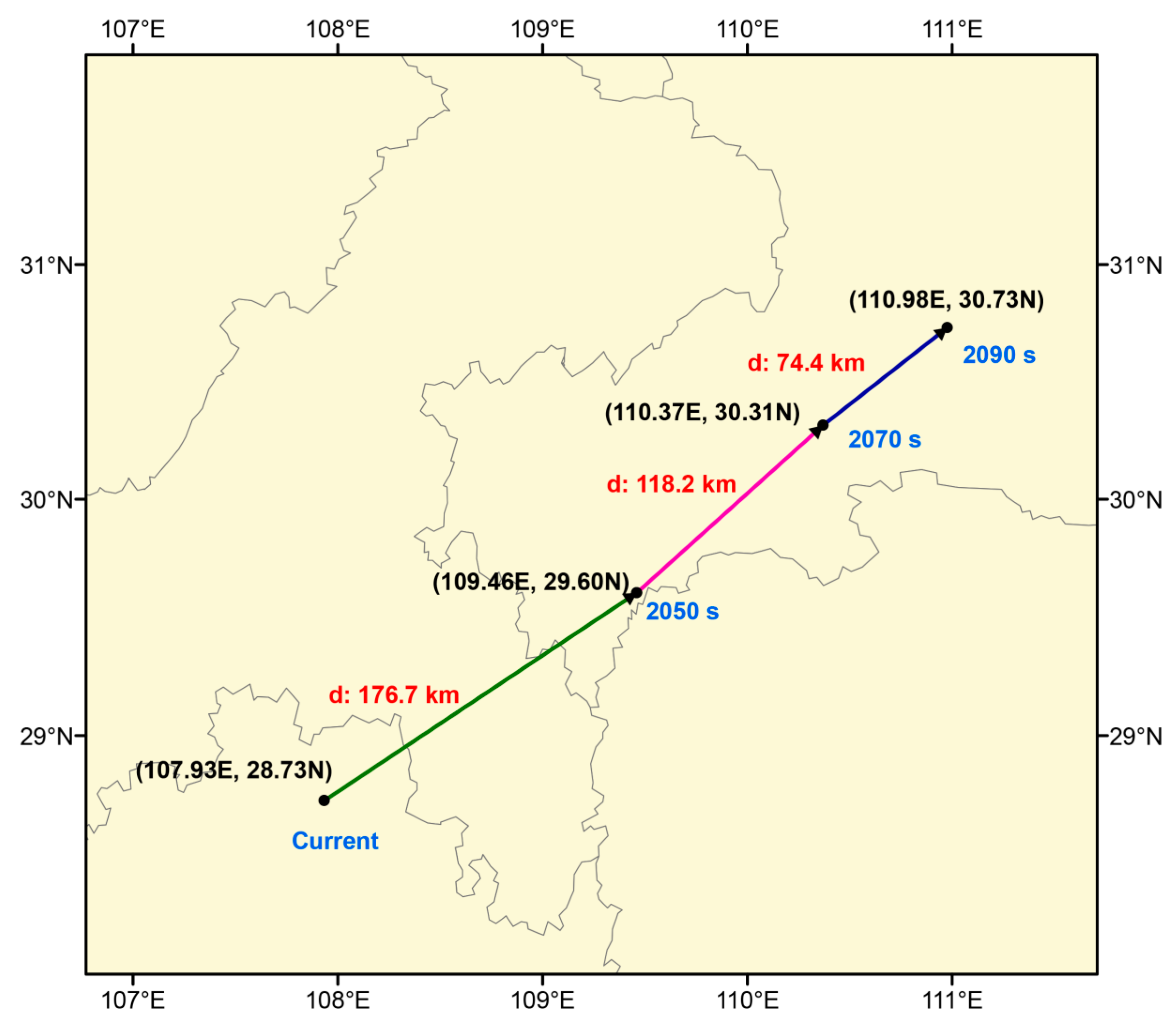
2.5. Migration of Centroid of Suitable Habitats for E. acuminatum
3. Discussion
4. Materials and Methods
4.1. Data Occurrence Points and Selection
4.2. Acquisition and Selection of Environmental Variables
4.3. Model Building
4.4. Data Processing and Habitat Suitability Map Visualization
4.5. Centroid Shift
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Xu, J.; Fang, H.; Li, Z.; Li, M. Advances and challenges in medicinal plant breeding. Plant Sci. 2020, 298, 110573. [Google Scholar] [CrossRef] [PubMed]
- Prinsloo, A.S.V.W. Medicinal plant harvesting, sustainability and cultivation in South Africa. Biol. Conserv. 2018, 227, 335–342. [Google Scholar]
- Tang, P.; Shen, T.; Wang, H.; Zhang, R.; Zhang, X.; Li, X.; Xiao, W. Challenges and opportunities for improving the druggability of natural product: Why need drug delivery system? Biomed. Pharmacother. 2023, 164, 114955. [Google Scholar] [CrossRef] [PubMed]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef]
- Zhuang, W.; Sun, N.; Gu, C.; Liu, S.; Zheng, Y.; Wang, H.; Tong, X.; Song, J. A literature review on Epimedium, a medicinal plant with promising slow aging properties. Heliyon 2023, 9, e21226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dang, H.; Li, S.; Li, J.; Wang, Y. Five new synonyms in Epimedium (Berberidaceae) from China. Phytokeys 2015, 49, 1–12. [Google Scholar] [CrossRef][Green Version]
- Stearn, W.T. Genus Epimedium and Other Herbaceous Berberidaceae; Timber Press: Portland, OR, USA, 2002. [Google Scholar]
- Zhang, Y.; Chen, Y.; Li, Y.; Shi, X.; Guan, P. Observations on the formation of large spores and the development of female gametophytes in Epimedium vulgare. J. Mt. Agric. Biol. 2012, 31, 3. [Google Scholar] [CrossRef]
- Zhang, J. Graduation Dissertation for Master DegreeGuizhou Normal UniversityInducement of callus and establishing cellsuspension culture system of Epimediumacuminatum. Master’s Thesis, Guizhou Normal University, Guiyang, China, 2015. [Google Scholar]
- Sun, C.; Zhang, Y.; Lin, C.; Zou, J.; Zhong, Y. Study on the change of Glycoside Content of Epimedium acuminatim. Chin. J. Mod. Appl. Pharm. 2006, 23, 769–771. [Google Scholar] [CrossRef]
- He, S.; Fan, G. A study on the resources of medicinal plants of the genus Epimedium and commercial herbs in Guizhou Province. Chin. Pharm. J. 1992, 27, 7–9. [Google Scholar]
- Zhou, T.; Zhang, X.; Guo, L.; Lin, G.; Jiang, W.; Ai, Q.; Zhang, C. Analysis of changes in the content of Icariin and total flavonoids in different parts of Epimedium vulgare and in different habitats. Chin. J. Tradit. Chin. Med. 2012, 37, 1917–1921. [Google Scholar]
- Huang, X.; Wang, W.; Zhou, Y. Therapeutical Effect of Total Flavonoids of Epimedium on Experimental Myocardial Infarction in Dogs. Chin. J. Pharm. 2006, 41, 185. [Google Scholar]
- Chen, Y.; Shen, Z.Y.; Chen, W.H. Molecular Mechanism of Epimedium Flavonoids in Immune Homeostasis Remodeling in Aged Rats Revealed by Lymphocyte Gene Expression Profile. Chin. J. Integr. Med. 2004, 24, 59–62. [Google Scholar]
- Zhong, Y.; Zhang, Y.; Lin, C.; Zou, J.; Sun, C. The Change of Glycoside Content of Epimedium acuminatium. Guizhou Agric. Sci. 2006, 34, 34. [Google Scholar]
- Sheng, M.; Yang, Q.; Cheng, Q. Studies on Extraction Technology of Epimedium acuminatum Flavones. Lishizhen Med. Mater. Medica Res. 2008, 19, 1899–1901. [Google Scholar]
- Zhou, T.; Zhang, X.; Guo, L.; Lin, G.; Jiang, W.; Ai, Q.; Zhang, C. Variation of icariin and total flavonoid of Epimedium acuminatum in different parts and habitats. Chin. J. Tradit. Chin. Med. 2012, 37, 1917–1921. [Google Scholar]
- Gafna, D.J.; Obando, J.A.; Reichelt, M.; Schmidtlein, S.; Dolos, K. Medicinal service supply by wild plants in Samburu, Kenya: Comparisons among medicinal plant assemblages. Glob. Ecol. Conserv. 2021, 30, e01749. [Google Scholar]
- Urban, M.C. Accelerating extinction risk from climate change. Science 2015, 348, 571–573. [Google Scholar] [CrossRef]
- Malcolm, J.R.; Liu, C.; Neilson, R.P.; Hansen, L.; Hannah, L. Global Warming and Extinctions of Endemic Species from Biodiversity Hotspots. Conserv. Biol. 2006, 20, 538–548. [Google Scholar]
- Mcclean, C.J.; Lovett, J.C.; Küper, W.; Hannah, L.; Sommer, J.H.; Barthlott, W.; Termansen, M.; Smith, G.F.; Taplin, T.J.R.D. African Plant Diversity and Climate Change. Ann. Mo. Bot. Gard. 2005, 92, 139–152. [Google Scholar]
- Mateo, R.G.; Felicísimo, Á.M.; Muñoz, J. Modelos de distribución de especies: Una revisión sintética. Rev. Chil. Hist. Nat. 2011, 84, 217–240. [Google Scholar]
- Candela, L.; Castelli, D.; Coro, G.; Pagano, P.; Sinibaldi, F. Species distribution modeling in the cloud. Concurr. Comput. Pract. Exp. 2016, 28, 1056–1079. [Google Scholar]
- Sofaer, H.R.; Jarnevich, C.S.; Pearse, I.S.; Smyth, R.L.; Auer, S.; Cook, G.L.; Edwards, T.C.J.; Guala, G.F.; Howard, T.G.; Morisette, J.T.; et al. Development and Delivery of Species Distribution Models to Inform Decision-Making. Bioscience 2019, 69, 544–557. [Google Scholar] [CrossRef]
- Lifei, W.; Jackson, D.A. Effects of sample size, data quality, and species response in environmental space on modeling species distributions. Landscape Ecol. 2023, 38, 4009–4031. [Google Scholar]
- Lany, N.K.; Zarnetske, P.L.; Finley, A.O.; McCullough, D.G. Complementary strengths of spatially-explicit and multi-species distribution models. Ecography 2020, 43, 456–466. [Google Scholar] [CrossRef]
- Gregorietti, M.; Atzori, F.; Carosso, L.; Frau, F.; Pellegrino, G.; Sara, G.; Arcangeli, A. Cetacean presence and distribution in the central Mediterranean Sea and potential risks deriving from plastic pollution. Mar. Pollut. Bull. 2021, 173, 112943. [Google Scholar]
- Abou-Shaara, H.; Alashaal, S.A.; Hosni, E.M.; Nasser, M.G.; Alharbi, S.A. Modeling the Invasion of the Large Hive Beetle, Oplostomus fuligineus, into North Africa and South Europe under a Changing Climate. Insects 2021, 12, 275. [Google Scholar]
- Hebbar, K.B.; Abhin, S.P.; Sanjo, J.V.; Ramesh, S.V.; Bhat, R. Predicting current and future climate suitability for arecanut (Areca catechu L.) in India using ensemble model. Heliyon 2024, 10, e26382. [Google Scholar]
- Rew, J.; Cho, Y.; Hwang, E. A Robust Prediction Model for Species Distribution Using Bagging Ensembles with Deep Neural Networks. Remote Sens. 2021, 13, 1495. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Kindt, R. Ensemble species distribution modelling with transformed suitability values. Environ. Modell. Softw. 2018, 100, 136–145. [Google Scholar] [CrossRef]
- Breiner, F.T.; Nobis, M.P.; Bergamini, A.; Guisan, A. Optimizing ensembles of small models for predicting the distribution of species with few occurrences. Methods Ecol. Evol. 2018, 9, 802–808. [Google Scholar] [CrossRef]
- Powell-Romero, F.; Fountain-Jones, N.M.; Norberg, A.; Clark, N.J. Improving the predictability and interpretability of co-occurrence modelling through feature-based joint species distribution ensembles. Methods Ecol. Evol. 2023, 14, 146–161. [Google Scholar] [CrossRef]
- Woodman, S.M.; Forney, K.A.; Becker, E.A.; DeAngelis, M.L.; Hazen, E.L.; Palacios, D.M.; Redfern, J.V. esdm: A tool for creating and exploring ensembles of predictions from species distribution and abundance models. Methods Ecol. Evol. 2019, 10, 1923–1933. [Google Scholar] [CrossRef]
- Kaky, E.; Nolan, V.; Alatawi, A.; Gilbert, F. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: A case study with Egyptian medicinal plants. Ecol. Inform. 2020, 60, 101150. [Google Scholar] [CrossRef]
- Yunfeng, L.; Ping, H.; Yun, M.F. Quality change analysis of Epimedium brevicornu Maxim. in Taohe River basin in southern Gansu Province based on the MaxEnt model. J. Sichuan Univ. (Nat. Sci. Ed.) 2023, 60, 156–162. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W. Feasibility analysis of kiwifruit-epimedium three-dimensional cultivation. Northwest Hortic. 2024, 8, 50–51. [Google Scholar]
- Li, L.; Chen, J. Influence of climate change on wild plants and the conservation strategies. Biol. Divers. 2014, 22, 549–563. [Google Scholar]
- Fu, S.; Li, L.; Hu, H.; Yan, Y. Factors influencing the diversity of terrestrial plant communities in China. J. Appl. Ecol. 2025, 36, 113–120. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, H.; Yang, Z.; Luo, W.; Chen, K.; Zhou, W.; Qin, J. Feasibility Analysis of Cultivation Base of Cultivation under Forest of Epimedium koreanum. Ginseng Res. 2021, 33, 62–64. [Google Scholar] [CrossRef]
- Zhao, J.; Jiang, C.; Ding, Y.; Li, G.; Li, Q. Analyzing potential distribution and disturbance intensity of plateau pika in the source region of the Yellow River via BIOMOD2 integrated model. J. Ecol. 2024, 43, 1192–1201. [Google Scholar] [CrossRef]
- Yang, X.; Kushwaha, S.; Saran, S.; Xu, J.; Roy, P. Maxent modeling for predicting the potential distribution of medicinal plant, Justicia adhatoda L. in Lesser Himalayan foothills. Ecol. Eng. 2013, 51, 83–87. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, Kappa and the True Skill Statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fawcett, T. Introduction to ROC analysis. Pattern Recogn. Lett. 2006, 27, 861–874. [Google Scholar] [CrossRef]

| Model | Data | KAPPA | TSS | AUC | |
|---|---|---|---|---|---|
| RF | PA1 | 0.965 | 0.989 | 1.000 | |
| PA2 | 0.965 | 0.985 | 0.999 | ||
| average value | 0.965 | 0.987 | 1.000 | ||
| GAM | PA1 | 0.917 | 0.982 | 0.994 | |
| PA2 | 0.948 | 0.989 | 0.997 | ||
| average value | 0.948 | 0.989 | 0.997 | ||
| GBM | PA1 | 0.813 | 0.948 | 0.989 | |
| PA2 | 0.813 | 0.946 | 0.988 | ||
| average value | 0.813 | 0.947 | 0.989 | ||
| ANN | PA1 | 0.727 | 0.855 | 0.967 | |
| PA2 | 0.810 | 0.916 | 0.974 | ||
| average value | 0.769 | 0.886 | 0.971 | ||
| FDA | PA1 | 0.761 | 0.847 | 0.960 | |
| PA2 | 0.750 | 0.848 | 0.958 | ||
| average value | 0.756 | 0.848 | 0.959 | ||
| MARS | PA1 | 0.721 | 0.866 | 0.974 | |
| PA2 | 0.744 | 0.879 | 0.971 | ||
| average value | 0.733 | 0.873 | 0.973 | ||
| GLM | PA1 | 0.737 | 0.863 | 0.975 | |
| PA2 | 0.735 | 0.872 | 0.972 | ||
| average value | 0.736 | 0.868 | 0.974 | ||
| CTA | PA1 | 0.686 | 0.858 | 0.941 | |
| PA2 | 0.694 | 0.868 | 0.943 | ||
| average value | 0.690 | 0.863 | 0.942 | ||
| SRE | PA1 | 0.686 | 0.705 | 0.853 | |
| PA2 | 0.665 | 0.699 | 0.850 | ||
| average value | 0.676 | 0.702 | 0.852 | ||
| MaxEnt | PA1 | NA | NA | NA | |
| PA2 | NA | NA | NA | ||
| average value | |||||
| Variable | Percent Contribution (%) |
|---|---|
| Minimum Temperature of the Coldest Month (Bio 6) | 64.80 |
| Mean Temperature of the Coldest Quarter (Bio 11) | 36.80 |
| Temperature Seasonality (standard deviation × 100) (Bio 4) | 33.38 |
| Annual Mean Temperature (Bio 1) | 28.69 |
| Precipitation of the Driest Quarter (Bio 17) | 27.45 |
| Mean Temperature of the Driest Quarter (Bio 9) | 21.55 |
| Maximum Temperature of the Warmest Month (Bio 5) | 13.16 |
| Isothermally (Bio 2/BIO 7) (×100) (Bio 3) | 11.03 |
| Elevation (Elev) | 10.88 |
| Aspect | 3.14 |
| Slope (°) | 3.05 |
| Period | Model | Loss (km2) | Stable (km2) | Gain (km2) | PercLoss (%) | PercGain (%) |
|---|---|---|---|---|---|---|
| 2050s | EMca | 2215 | 505,940 | 12,843 | 6.32 | 36.62 |
| EMwm | 2846 | 506,489 | 12,487 | 8.16 | 35.80 | |
| 2070s | EMca | 2367 | 494,820 | 23,963 | 6.75 | 68.32 |
| EMwm | 2862 | 495,803 | 23,173 | 8.21 | 66.44 | |
| 2090s | EMca | 2458 | 485,436 | 33,347 | 7.01 | 95.08 |
| EMwm | 3418 | 486,595 | 32,381 | 9.80 | 92.84 |
| Abbreviation | Description |
|---|---|
| BIO 1 | Annual mean temperature (°C) |
| BIO 2 | Mean diurnal range (mean of monthly (max temp-min temp)) (°C) |
| BIO 3 | Isothermally (Bio 2/BIO 7) × 100 |
| BIO 4 | Temperature seasonality (standard deviation × 100) (C of V) |
| BIO 5 | Maximum temperature of the warmest month (°C) |
| BIO 6 | Minimum temperature of the coldest month (°C) |
| BIO 7 | Temperature annual range (Bio 5-BIO 6) (°C) |
| BIO 8 | Mean temperature of the wettest quarter (°C) |
| BIO 9 | Mean temperature of the driest quarter (°C) |
| BIO 10 | Mean temperature of the warmest quarter (°C) |
| BIO 11 | Mean temperature of the coldest quarter (°C) |
| BIO 12 | Annual precipitation (mm) |
| BIO 13 | Precipitation of the wettest month (mm) |
| BIO 14 | Precipitation of the driest month (mm) |
| BIO 15 | Precipitation seasonality (C of V) |
| BIO 16 | Precipitation of the wettest quarter (mm) |
| BIO 17 | Precipitation of the driest quarter (mm) |
| BIO 18 | Precipitation of the warmest quarter (mm) |
| BIO 19 | Precipitation of the coldest quarter (mm) |
| Elevation (Elev) | Elevation of the terrain |
| Slope | Slope or obliquity of the terrain |
| Aspect | The direction or orientation of the earth’s surface |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhuo, Z.; Liu, B.; Peng, Y.; Xu, D. Predicting the Future Geographic Distribution of the Traditional Chinese Medicinal Plant Epimedium acuminatum Franch. in China Using Ensemble Models Based on Biomod2. Plants 2025, 14, 1065. https://doi.org/10.3390/plants14071065
Wang Z, Zhuo Z, Liu B, Peng Y, Xu D. Predicting the Future Geographic Distribution of the Traditional Chinese Medicinal Plant Epimedium acuminatum Franch. in China Using Ensemble Models Based on Biomod2. Plants. 2025; 14(7):1065. https://doi.org/10.3390/plants14071065
Chicago/Turabian StyleWang, Zhiling, Zhihang Zhuo, Biyu Liu, Yaqin Peng, and Danping Xu. 2025. "Predicting the Future Geographic Distribution of the Traditional Chinese Medicinal Plant Epimedium acuminatum Franch. in China Using Ensemble Models Based on Biomod2" Plants 14, no. 7: 1065. https://doi.org/10.3390/plants14071065
APA StyleWang, Z., Zhuo, Z., Liu, B., Peng, Y., & Xu, D. (2025). Predicting the Future Geographic Distribution of the Traditional Chinese Medicinal Plant Epimedium acuminatum Franch. in China Using Ensemble Models Based on Biomod2. Plants, 14(7), 1065. https://doi.org/10.3390/plants14071065





