Major Bioactive Compounds in Seeds, Husks, and Leaves of Selected Genotypes of Coffea canephora cv. Conilon from Three Consecutive Crops
Abstract
1. Introduction
2. Results and Discussion
2.1. Water Content
2.2. Soluble Solids Contents of Green Seeds
2.3. Bioactive Compounds in Green Seeds, Husks, and Leaves
2.3.1. Chlorogenic Acids and Flavonoids
| (a) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 6.66 ± 0.03 a | 6.18 ± 0.08 b | 5.85 ± 0.03 c | 6.23 ± 0.40 | 6.48 | Z38 | 6.80 ± 0.05 b | 6.42 ± 0.05 c | 7.06 ± 0.10 a | 6.76 ± 0.32 | 4.78 |
| B01 | 7.02 ± 0.04 c | 8.77 ± 0.10 a | 7.87 ± 0.02 b | 7.89 ± 0.87 | 11.06 | Z18 | 6.90 ± 0.01 b | 7.41 ± 0.06 a | 6.91 ± 0.07 b | 7.07 ± 0.29 | 4.12 |
| Bicudo | 7.39 ± 0.04 a | 8.32 ± 0.08 b | 8.20 ± 0.01 b c | 7.97 ± 0.50 | 6.33 | Z17 | 8.60 ± 0.02 c | 9.16 ± 0.08 a | 8.83 ± 0.08 b | 8.86 ± 0.28 | 3.17 |
| Alecrim | 7.09 ± 0.04 c | 7.75 ± 0.05 b | 8.28 ± 0.04 a | 7.71 ± 0.60 | 7.75 | Z21 | 5.30 ± 0.02 c | 5.85 ± 0.02 b | 6.75 ± 0.02 a | 5.97 ± 0.74 | 12.32 |
| 700 | 6.33 ± 0.05 c | 6.81 ± 0.13 b | 7.85 ± 0.05 a | 7.00 ± 0.77 | 11.08 | Z36 | 7.07 ± 0.05 b | 7.94 ± 0.05 a | 7.85 ± 0.04 a | 7.62 ± 0.48 | 6.33 |
| CH1 | 5.22 ± 0.02 c | 6.06 ± 0.06 b | 7.94 ± 0.03 a | 6.41 ± 1.39 | 21.72 | Ouro negro | 7.60 ± 0.04 c | 8.54 ± 0.09 a | 8.09 ± 0.04 b | 8.08 ± 0.47 | 5.81 |
| Imbigudinho | 5.72 ± 0.01 c | 6.24 ± 0.03 b | 7.51 ± 0.08 a | 6.49 ± 0.92 | 14.15 | 18 | 5.51 ± 0.04 c | 6.45 ± 0.06 b | 7.70 ± 0.02 a | 6.55 ± 1.10 | 16.75 |
| AT | 5.01 ± 0.04 c | 6.03 ± 0.05 b | 8.59 ± 0.04 a | 6.55 ± 1.85 | 28.20 | Tardio C | 8.62 ± 0.02 b | 9.09 ± 0.01 a | 9.16 ± 0.08 a | 8.96 ± 0.29 | 3.27 |
| Graudão HP | 8.88 ± 0.05 c | 9.71 ± 0.06 a | 9.22 ± 0.08 b | 9.27 ± 0.42 | 4.50 | A1 | 7.56 ± 0.03 a | 6.46 ± 0.03 c | 7.22 ± 0.03 b | 7.08 ± 0.56 | 7.96 |
| Valcir P | 6.73 ± 0.04 b | 8.22 ± 0.11 a | 8.12 ± 0.07 a | 7.69 ± 0.83 | 10.82 | Cheique | 8.05 ± 0.03 c | 8.29 ± 0.02 b | 9.14 ± 0.02 a | 8.49 ± 0.57 | 6.72 |
| Beira Rio 8 | 6.70 ± 0.04 c | 8.82 ± 0.04 b | 8.95 ± 0.05 a | 8.16 ± 1.26 | 15.47 | P2 | 6.61 ± 0.07 b | 7.97 ± 0.01 a | 7.89 ± 0.03 a | 7.49 ± 0.76 | 10.15 |
| Tardio V | 8.15 ± 0.03 b | 9.50 ± 0.14 a | 8.29 ± 0.03 b | 8.65 ± 0.74 | 8.59 | Emcapa 02 | 8.72 ± 0.06 c | 9.39 ± 0.04 a | 9.11 ± 0.06 b | 9.07 ± 0.34 | 3.73 |
| AP | 6.43 ± 0.03 b | 7.53 ± 0.08 a | 7.40 ± 0.04 a | 7.12 ± 0.60 | 8.43 | Emcapa 153 | 6.26 ± 0.03 b | 5.95 ± 0.04 c | 7.10 ± 0.07 a | 6.44 ± 0.59 | 9.18 |
| L80 | 3.71 ± 0.04 c | 3.93 ± 0.03 b | 4.75 ± 0.05 a | 4.13 ± 0.05 | 13.29 | P1 | 7.11 ± 0.01 a | 5.99 ± 0.05 b | 7.03 ± 0.04 a | 6.71 ± 0.62 | 9.27 |
| Bamburral | 6.05 ± 0.04 c | 6.74 ± 0.04 b | 7.54 ± 0.01 a | 6.77 ± 0.75 | 11.00 | LB1 | 6.93 ± 0.02 b | 6.20 ± 0.02 c | 7.13 ± 0.08 a | 6.75 ± 0.49 | 7.22 |
| Pirata | 5.25 ± 0.04 c | 6.84 ± 0.09 b | 7.17 ± 0.02 a | 6.42 ± 1.03 | 15.98 | 122 | 8.49 ± 0.03 a | 8.55 ± 0.03 a | 8.37 ± 0.06 b | 8.47 ± 0.09 | 1.08 |
| Peneirão | 7.36 ± 0.02 c | 8.26 ± 0.06 a | 7.73 ± 0.03 b | 7.78 ± 0.45 | 5.81 | Verdim D | 8.14 ± 0.01 c | 8.47 ± 0.03 b | 8.58 ± 0.05 a | 8.40 ± 0.23 | 2.70 |
| Z39 | 4.34 ± 0.05 c | 5.65 ± 0.03 b | 5.93 ± 0.07 a | 5.31 ± 0.85 | 15.96 | Emcapa 143 | 6.06 ± 0.05 c | 6.40 ± 0.06 b | 6.81 ± 0.04 a | 6.42 ± 0.38 | 5.84 |
| Z35 | 7.91 ± 0.05 b | 8.67 ± 0.02 a | 7.83 ± 0.04 b | 8.13 ± 0.46 | 5.69 | Ouro negro 1 | 6.85 ± 0.04 b | 7.21 ± 0.03 a | 6.90 ± 0.03 b | 6.99 ± 0.20 | 2.79 |
| Z40 | 5.99 ± 0.04 c | 7.42 ± 0.07 b | 7.77 ± 0.01 a | 7.06 ± 0.94 | 13.37 | Ouro negro 2 | 7.49 ± 0.07 b | 7.36 ± 0.05 c | 8.55 ± 0.02 a | 7.80 ± 0.65 | 8.39 |
| Z29 | 7.17 ± 0.05 a | 6.15 ± 0.14 b | 7.02 ± 0.02 c | 6.78 ± 0.05 | 8.14 | Clementino | 6.22 ± 0.08 c | 6.67 ± 0.05 b | 7.01 ± 0.05 a | 6.64 ± 0.40 | 5.95 |
| Note (a): * Average total CGA contents in the three consecutive crops (2018, 2019, and 2020). Total CGA: sum of CQA + FQA + diCQA. CQA: sum of 3-CQA (3-caffeoylquinic acid) + 4-CQA (4-caffeoylquinic acid) + 5-CQA (5-caffeoylquinic acid). FQA sum of 3-FQA (3-feruloylquinic acid) + 4-FQA (4-feruloylquinic acid) + 5-FQA (5-feruloylquinic acid). diCQA: sum of 3,4-diCQA (3,4-dicaffeoylquinic acid) + 3,5-diCQA (3,5-dicaffeoylquinic acid) + 4,5-diCQA (4,5-dicaffeoylquinic acid). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold indicate genotypes that stood out for consistently high contents of total CGA (>8%, CV < 9%), compared to average contents described in the literature [19] and with the remaining genotypes. | |||||||||||
| (b) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 0.72 ± 0.02 b | 0.95 ± 0.05 a | 1.01 ± 0.02 a | 0.89 ± 0.15 | 17.43 | Z38 | 0.48 ± 0.03 c | 0.55 ± 0.04 b | 0.67 ± 0.02 a | 0.57 ± 0.09 | 16.68 |
| B01 | 0.97 ± 0.05 a | 0.85 ± 0.03 b | 0.80 ± 0.05 b | 0.87 ± 0.09 | 10.08 | Z18 | 0.43 ± 0.01 b | 0.52 ± 0.05 a | 0.48 ± 0.03 b | 0.48 ± 0.04 | 9.30 |
| Bicudo | 0.79 ± 0.02 a | 0.77 ± 0.03 a | 0.68 ± 0.02 b | 0.75 ± 0.05 | 7.20 | Z17 | 0.65 ± 0.01 b | 0.92 ± 0.07 a | 0.91 ± 0.01 a | 0.83 ± 0.16 | 18.79 |
| Alecrim | 1.16 ± 0.03b | 1.37 ± 0.05 a | 1.38 ± 0.01 a | 1.30 ± 0.12 | 9.46 | Z21 | 0.65 ± 0.02 c | 0.75 ± 0.07 b | 0.82 ± 0.05 a | 0.74 ± 0.09 | 11.63 |
| 700 | 1.06 ± 0.02 b | 1.30 ± 0.03 a | 1.12 ± 0.05 b | 1.16 ± 0.03 | 10.82 | Z36 | 0.83 ± 0.04 c | 0.96 ± 0.04 b | 1.27 ± 0.05 a | 1.02 ± 0.22 | 21.83 |
| CH1 | 0.68 ± 0.03 b | 0.98 ± 0.04 a | 1.05 ± 0.02 a | 0.91 ± 0.20 | 21.72 | Ouro negro | 0.71 ± 0.02 c | 0.86 ± 0.04 a | 0.84 ± 0.03 b | 0.80 ± 0.08 | 9.73 |
| Imbigudinho | 0.58 ± 0.01 b | 0.71 ± 0.03 a | 0.68 ± 0.04 a | 0.66 ± 0.07 | 10.34 | 18 | 0.66 ± 0.00 c | 0.96 ± 0.02 b | 1.07 ± 0.01 a | 0.90 ± 0.22 | 24.10 |
| AT | 1.03 ± 0.03 c | 1.17 ± 0.04 b | 1.48 ± 0.02 a | 1.23 ± 0.23 | 18.78 | Tardio C | 1.51 ± 0.01 b | 1.65 ± 0.04 a | 1.45 ± 0.03 c | 1.54 ± 0.10 | 6.67 |
| Graudão HP | 0.53 ± 0.01 c | 0.81 ± 0.05 b | 1.27 ± 0.02 a | 0.87 ± 0.09 | 42.47 | A1 | 0.80 ± 0.02 b | 1.02 ± 0.06 a | 1.06 ± 0.05 a | 0.96 ± 0.14 | 14.46 |
| Valcir P | 0.93 ± 0.04 b | 1.00 ± 0.03 b | 1.32 ± 0.01 a | 1.08 ± 0.21 | 19.34 | Cheique | 1.43 ± 0.04 b | 1.59 ± 0.02 a | 1.27 ± 0.02 c | 1.43 ± 0.16 | 10.95 |
| Beira Rio 8 | 0.50 ± 0.01 b | 0.82 ± 0.06 a | 0.88 ± 0.03 a | 0.73 ± 0.20 | 27.80 | P2 | 1.54 ± 0.02 a | 1.39 ± 0.05 b | 1.24 ± 0.03 c | 1.39 ± 0.15 | 10.65 |
| Tardio V | 1.05 ± 0.02 c | 1.26 ± 0.01 a | 1.15 ± 0.04 b | 1.16 ± 0.10 | 9.04 | Emcapa 02 | 0.59 ± 0.03 b | 0.83 ± 0.03 a | 0.88 ± 0.01 a | 0.77 ± 0.15 | 19.99 |
| AP | 0.91 ± 0.01 c | 1.25 ± 0.00 a | 1.19 ± 0.03 b | 1.12 ± 0.18 | 15.99 | Emcapa 153 | 1.01 ± 0.02 c | 1.25 ± 0.05 b | 1.37 ± 0.03 a | 1.21 ± 0.18 | 14.84 |
| L80 | 0.69 ± 0.01 c | 0.82 ± 0.01 b | 0.95 ± 0.03 a | 0.82 ± 0.13 | 15.73 | P1 | 1.05 ± 0.01 c | 1.26 ± 0.02 b | 1.37 ± 0.01 a | 1.23 ± 0.16 | 13.36 |
| Bamburral | 0.98 ± 0.02 c | 1.13 ± 0.03 a | 1.05 ± 0.01 b | 1.06 ± 0.07 | 6.94 | LB1 | 0.70 ± 0.02 a | 0.59 ± 0.06 b | 0.59 ± 0.03 b | 0.63 ± 0.06 | 9.95 |
| Pirata | 0.62 ± 0.01 b | 0.55 ± 0.06 b | 0.71 ± 0.03 a | 0.63 ± 0.08 | 12.31 | 122 | 0.58 ± 0.02 c | 0.74 ± 0.02 b | 0.81 ± 0.02 a | 0.71 ± 0.12 | 17.02 |
| Peneirão | 0.60 ± 0.02 b | 0.62 ± 0.05 a b | 0.71 ± 0.04 a | 0.64 ± 0.06 | 9.14 | Verdim D | 0.64 ± 0.02 c | 0.93 ± 0.04 b | 1.03 ± 0.01 a | 0.87 ± 0.20 | 23.18 |
| Z39 | 0.57 ± 0.02 b | 0.48 ± 0.03 c | 0.69 ± 0.04 a | 0.58 ± 0.11 | 18.42 | Emcapa 143 | 0.79 ± 0.02 b | 1.07 ± 0.04 a | 1.03 ± 0.04 a | 0.96 ± 0.15 | 16.05 |
| Z35 | 0.46 ± 0.00 b | 0.47 ± 0.04 b | 0.55 ± 0.03 a | 0.50 ± 0.05 | 10.13 | Ouro negro 1 | 1.10 ± 0.01 b | 1.51 ± 0.05 a | 1.49 ± 0.03 a | 1.37 ± 0.23 | 16.86 |
| Z40 | 0.71 ± 0.02 a | 0.72 ± 0.05 a | 0.78 ± 0.04 a | 0.74 ± 0.04 | 5.29 | Ouro negro 2 | 0.99 ± 0.03 b | 1.22 ± 0.01 a | 1.24 ± 0.02 a | 1.15 ± 0.14 | 11.81 |
| Z29 | 0.66 ± 0.02 b | 0.80 ± 0.03 a | 0.67 ± 0.05 b | 0.71 ± 0.08 | 11.31 | Clementino | 0.94 ± 0.02 c | 1.23 ± 0.03 b | 1.32 ± 0.03 a | 1.16 ± 0.20 | 17.82 |
| Note (b): * Average total CGA contents of the three consecutive crops (2018, 2019, and 2020). Total CGA: sum of CQA + FQA + diCQA. CQA: sum of 3-CQA (3-caffeoylquinic acid) + 4-CQA (4-caffeoylquinic acid) + 5-CQA (5-caffeoylquinic acid). FQA: sum of 4-FQA (4-feruloylquinic acid) + 5-FQA (5-feruloylquinic acid). diCQA: sum of 3,4-diCQA (3,4-dicaffeoylquinic acid) + 3,5-diCQA (3,5-dicaffeoylquinic acid) + 4,5-diCQA (4,5-dicaffeoylquinic acid). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold were consistently outstanding compared to other genotypes. | |||||||||||
| (c) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 1.57 ± 0.01 a | 1.44 ± 0.03 b | 1.43 ± 0.03 b | 1.48 ± 0.08 | 5.18 | Z38 | 1.15 ± 0.02 c | 1.26 ± 0.01 b | 1.38 ± 0.02 a | 1.26 ± 0.11 | 9.09 |
| B01 | 0.97 ± 0.01 b | 0.91 ± 0.01 c | 1.20 ± 0.03 a | 1.03 ± 0.15 | 14.81 | Z18 | 1.57 ± 0.02 c | 1.73 ± 0.02 b | 1.86 ± 0.02 a | 1.72 ± 0.15 | 8.46 |
| Bicudo | 1.31 ± 0.01 b | 1.31 ± 0.03 b | 1.50 ± 0.02 a | 1.37 ± 0.11 | 7.74 | Z17 | 1.25 ± 0.03 b | 1.27 ± 0.07 b | 1.56 ± 0.03 a | 1.36 ± 0.17 | 12.74 |
| Alecrim | 1.56 ± 0.01 b | 1.59 ± 0.07 b | 1.86 ± 0.02 a | 1.67 ± 0.16 | 9.71 | Z21 | 1.80 ± 0.02 a | 1.47 ± 0.06 b | 1.18 ± 0.07 c | 1.48 ± 0.31 | 20.97 |
| 700 | 1.70 ± 0.02 c | 1.81 ± 0.03 b | 1.94 ± 0.02 a | 1.82 ± 0.12 | 6.67 | Z36 | 1.24 ± 0.02 b | 1.27 ± 0.02 b | 1.34 ± 0.03 a | 1.28 ± 0.05 | 4.08 |
| CH1 | 1.01 ± 0.02 c | 1.16 ± 0.03 b | 1.40 ± 0.03 a | 1.19 ± 0.20 | 16.49 | Ouro negro | 1.24 ± 0.02 a | 1.09 ± 0.04 b | 1.27 ± 0.02 a | 1.20 ± 0.10 | 8.13 |
| Imbigudinho | 1.16 ± 0.00 b | 1.09 ± 0.05 c | 1.30 ± 0.02 a | 1.18 ± 0.11 | 9.01 | 18 | 0.97 ± 0.02 b | 0.84 ± 0.03 c | 1.20 ± 0.02 a | 1.00 ± 0.18 | 18.09 |
| AT | 1.15 ± 0.01 b | 1.27 ± 0.03 a | 1.16 ± 0.04 b | 1.19 ± 0.07 | 5.85 | Tardio C | 0.99 ± 0.03 c | 1.06 ± 0.00 b | 1.25 ± 0.02 a | 1.10 ± 0.13 | 12.22 |
| Graudão HP | 1.03 ± 0.02 c | 1.13 ± 0.02 b | 1.22 ± 0.02 a | 1.13 ± 0.10 | 8.50 | A1 | 1.90 ± 0.02 b | 1.91 ± 0.03 b | 2.00 ± 0.06 a | 1.94 ± 0.06 | 2.89 |
| Valcir P | 1.97 ± 0.02 c | 2.13 ± 0.03 b | 2.05 ± 0.02 b | 2.05 ± 0.08 | 3.73 | Cheique | 1.53 ± 0.01 b | 1.54 ± 0.02 b | 1.69 ± 0.02 a | 1.59 ± 0.09 | 5.70 |
| Beira Rio 8 | 1.54 ± 0.03 c | 1.68 ± 0.02 b | 1.78 ± 0.01 a | 1.67 ± 0.12 | 7.00 | P2 | 1.67 ± 0.02 a | 1.73 ± 0.03 a | 1.38 ± 0.03 b | 1.59 ± 0.19 | 11.90 |
| Tardio V | 0.87 ± 0.02 c | 1.04 ± 0.02 b | 1.26 ± 0.05 a | 1.06 ± 0.20 | 18.70 | Emcapa 02 | 1.83 ± 0.01 b | 2.22 ± 0.04 a | 1.80 ± 0.03 b | 1.95 ± 0.23 | 11.89 |
| AP | 1.73 ± 0.01 b | 2.01 ± 0.03 a | 1.50 ± 0.04 c | 1.75 ± 0.25 | 14.56 | Emcapa 153 | 1.25 ± 0.01 a | 1.31 ± 0.02 a | 1.28 ± 0.06 a | 1.28 ± 0.03 | 2.25 |
| L80 | 1.59 ± 0.02 b | 1.77 ± 0.02 a | 1.65 ± 0.04 b | 1.67 ± 0.09 | 5.62 | P1 | 0.92 ± 0.03 c | 1.09 ± 0.02 b | 1.15 ± 0.04 a | 1.05 ± 0.12 | 11.37 |
| Bamburral | 1.11 ± 0.01 c | 1.34 ± 0.02 b | 1.39 ± 0.03 b | 1.28 ± 0.15 | 11.82 | LB1 | 1.36 ± 0.01 a | 1.39 ± 0.02 a | 1.38 ± 0.04 a | 1.38 ± 0.02 | 1.23 |
| Pirata | 1.01 ± 0.01 c | 1.11 ± 0.01 b | 1.27 ± 0.04 a | 1.13 ± 0.13 | 11.58 | 122 | 1.93 ± 0.01 b | 2.09 ± 0.02 a | 2.07 ± 0.01 a | 2.03 ± 0.09 | 4.25 |
| Peneirão | 0.99 ± 0.01 c | 1.15 ± 0.03 b | 1.32 ± 0.04 a | 1.15 ± 0.17 | 14.42 | Verdim D | 1.38 ± 0.02 b | 1.45 ± 0.02 a | 1.44 ± 0.03 a | 1.42 ± 0.04 | 2.87 |
| Z39 | 1.22 ± 0.03 b | 1.37 ± 0.02 a | 1.40 ± 0.01 a | 1.33 ± 0.09 | 6.99 | Emcapa 143 | 0.80 ± 0.02 c | 0.87 ± 0.02 b | 1.56 ± 0.03 a | 1.08 ± 0.42 | 39.15 |
| Z35 | 0.95 ± 0.02 b | 0.97 ± 0.05 b | 1.27 ± 0.02 a | 1.06 ± 0.18 | 16.63 | Ouro negro 1 | 1.29 ± 0.02 c | 1.39 ± 0.01 b | 1.74 ± 0.01 a | 1.47 ± 0.24 | 16.17 |
| Z40 | 0.96 ± 0.01 b | 0.94 ± 0.05 b | 1.19 ± 0.02 a | 1.03 ± 0.14 | 13.64 | Ouro negro 2 | 1.03 ± 0.03 b | 0.97 ± 0.01 c | 1.35 ± 0.02 a | 1.12 ± 0.21 | 18.52 |
| Z29 | 1.11 ± 0.02 b | 1.05 ± 0.07 b | 1.29 ± 0.05 a | 1.15 ± 0.12 | 10.52 | Clementino | 1.77 ± 0.03 b | 1.84 ± 0.05 a | 1.74 ± 0.04 b | 1.78 ± 0.05 | 2.71 |
| Note (c): * Average total CGA contents of the three consecutive crops (2018, 2019, and 2020). Total CGA: sum of CQA + FQA + diCQA. CQA: sum of 3-CQA (3-caffeoylquinic acid) + 4-CQA (4-caffeoylquinic acid) + 5-CQA (5-caffeoylquinic acid). FQA: sum of 4-FQA (4-feruloylquinic acid) + 5-FQA (5-feruloylquinic acid). diCQA: sum of 3,4-diCQA (3,4-dicaffeoylquinic acid) + 3,5-diCQA (3,5-dicaffeoylquinic acid) + 4,5-diCQA (4,5-dicaffeoylquinic acid). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold were consistently outstanding compared to other genotypes. | |||||||||||
2.3.2. Caffeine
| (a) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 2.08 ± 0.02 a | 1.95 ± 0.02 b | 1.94 ± 0.02 b | 1.99 ± 0.08 | 4.07 | Z38 | 1.81 ± 0.01 a | 1.77 ± 0.02 b | 1.56 ± 0.02 c | 1.71 ± 0.13 | 7.71 |
| B01 | 2.32 ± 0.02 a | 2.13 ± 0.02 b | 2.15 ± 0.01 b | 2.20 ± 0.10 | 4.58 | Z18 | 1.88 ± 0.01 a | 1.60 ± 0.02 b | 1.56 ± 0.01 c | 1.68 ± 0.18 | 10.47 |
| Bicudo | 1.87 ± 0.02 a | 1.79 ± 0.01 b | 1.84 ± 0.04 a | 1.83 ± 0.04 | 1.99 | Z17 | 1.80 ± 0.03 a | 1.84 ± 0.01 a | 1.70 ± 0.02 b | 1.78 ± 0.07 | 4.09 |
| Alecrim | 2.23 ± 0.02 b | 2.21 ± 0.01 b | 2.33 ± 0.04 a | 2.26 ± 0.07 | 2.99 | Z21 | 1.86 ± 0.02 c | 1.93 ± 0.02 b | 2.00 ± 0.02 a | 1.93 ± 0.07 | 3.44 |
| 700 | 1.74 ± 0.05 b | 1.90 ± 0.01 a | 1.79 ± 0.04 b | 1.81 ± 0.08 | 4.53 | Z36 | 1.82 ± 0.02 a | 1.74 ± 0.02 b | 1.62 ± 0.02 c | 1.73 ± 0.10 | 5.84 |
| CH1 | 1.70 ± 0.02 b | 1.74 ± 0.01 a | 1.54 ± 0.02 c | 1.66 ± 0.10 | 6.29 | Ouro negro | 1.63 ± 0.03 b | 1.54 ± 0.02 c | 1.82 ± 0.03 a | 1.66 ± 0.15 | 8.78 |
| Imbigudinho | 2.15 ± 0.03 b | 2.23 ± 0.01 a | 1.93 ± 0.03 c | 2.10 ± 0.16 | 7.43 | 18 | 1.66 ± 0.01 b | 1.43 ± 0.02 c | 1.76 ± 0.03 a | 1.61 ± 0.17 | 10.48 |
| AT | 1.50 ± 0.03 c | 1.64 ± 0.01 b | 1.72 ± 0.03 a | 1.62 ± 0.11 | 6.91 | Tardio C | 2.63 ± 0.03 a | 2.55 ± 0.03 b | 2.21 ± 0.04 c | 2.46 ± 0.23 | 9.22 |
| Graudão HP | 2.06 ± 0.04 a | 2.12 ± 0.02 a | 1.94 ± 0.04 b | 2.04 ± 0.09 | 4.38 | A1 | 1.87 ± 0.02 a | 1.77 ± 0.02 c | 1.82 ± 0.03 b | 1.82 ± 0.05 | 2.87 |
| Valcir P | 1.73 ± 0.02 c | 1.81 ± 0.01 b | 1.92 ± 0.02 a | 1.82 ± 0.10 | 5.37 | Cheique | 2.22 ± 0.03 a | 2.16 ± 0.02 b | 2.09 ± 0.02 c | 2.15 ± 0.07 | 3.02 |
| Beira Rio 8 | 2.53 ± 0.04 a | 2.45 ± 0.02 b | 2.55 ± 0.06 a | 2.51 ± 0.05 | 2.16 | P2 | 2.29 ± 0.01 a | 2.17 ± 0.01 c | 2.24 ± 0.03 b | 2.23 ± 0.06 | 2.62 |
| Tardio V | 2.25 ± 0.02 a | 2.29 ± 0.03 a | 1.90 ± 0.02 b | 2.15 ± 0.21 | 9.92 | Emcapa 02 | 1.72 ± 0.03 b | 1.53 ± 0.02 c | 1.85 ± 0.03 a | 1.70 ± 0.16 | 9.58 |
| AP | 2.03 ± 0.01 b | 2.13 ± 0.02 a | 1.95 ± 0.05 c | 2.04 ± 0.09 | 4.42 | Emcapa 153 | 1.55 ± 0.03 b | 1.41 ± 0.02 c | 1.71 ± 0.02 a | 1.56 ± 0.15 | 9.63 |
| L80 | 1.21 ± 0.02 c | 1.42 ± 0.02 a | 1.35 ± 0.03 b | 1.33 ± 0.11 | 8.02 | P1 | 1.88 ± 0.01 a | 1.83 ± 0.02 b | 1.80 ± 0.02 b | 1.83 ± 0.06 | 3.11 |
| Bamburral | 1.96 ± 0.01 c | 2.16 ± 0.01 b | 2.23 ± 0.04 a | 2.12 ± 0.14 | 6.73 | LB1 | 1.82 ± 0.03 a | 1.53 ± 0.02 c | 1.66 ± 0.05 b | 1.67 ± 0.15 | 8.79 |
| Pirata | 1.60 ± 0.00 a | 1.42 ± 0.01 b | 1.37 ± 0.03 c | 1.46 ± 0.12 | 8.47 | 122 | 2.00 ± 0.01 b | 2.11 ± 0.01 a | 1.83 ± 0.03 c | 1.98 ± 0.14 | 7.22 |
| Peneirão | 1.86 ± 0.01 a | 1.67 ± 0.02 c | 1.72 ± 0.03 b | 1.75 ± 0.10 | 5.81 | Verdim D | 1.97 ± 0.01 b | 2.12 ± 0.01 a | 2.07 ± 0.06 a | 2.05 ± 0.07 | 3.62 |
| Z39 | 1.63 ± 0.04 a | 1.33 ± 0.01 b | 1.35 ± 0.05 b | 1.44 ± 0.17 | 11.70 | Emcapa 143 | 1.91 ± 0.02 c | 2.14 ± 0.02 b | 2.33 ± 0.02 a | 2.13 ± 0.21 | 9.75 |
| Z35 | 1.60 ± 0.01 a b | 1.54 ± 0.02 b | 1.72 ± 0.07 a | 1.62 ± 0.09 | 5.62 | Ouro negro 1 | 1.65 ± 0.02 c | 1.77 ± 0.01 b | 1.87 ± 0.06 a | 1.77 ± 0.11 | 6.19 |
| Z40 | 1.95 ± 0.02 b | 2.17 ± 0.03 a | 2.06 ± 0.04 c | 2.06 ± 0.11 | 5.37 | Ouro negro 2 | 1.54 ± 0.01 b | 1.44 ± 0.02 c | 1.66 ± 0.02 a | 1.54 ± 0.11 | 7.12 |
| Z29 | 1.81 ± 0.02 b | 2.02 ± 0.02 a | 1.72 ± 0.03 c | 1.85 ± 0.16 | 8.42 | Clementino | 1.77 ± 0.01 b | 1.77 ± 0.01 b | 1.95 ± 0.03 a | 1.83 ± 0.10 | 5.53 |
| Note (a): * Average caffeine contents of the three consecutive crops (2018, 2019, and 2020). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold indicate genotypes that stood out for their consistently high contents of caffeine (>2%, CV < 10%) in all crops evaluated, compared to average contents described in the literature [36] and with the remaining genotypes. | |||||||||||
| (b) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 1.04 ± 0.03 a | 0.94 ± 0.02 b | 0.84 ± 0.02 c | 0.94 ± 0.10 | 10.48 | Z38 | 1.15 ± 0.01 a | 0.97 ± 0.02 b | 0.96 ± 0.01 b | 1.03 ± 0.11 | 10.60 |
| B01 | 0.85 ± 0.01 b | 0.95 ± 0.02 a | 0.86 ± 0.01 b | 0.88 ± 0.05 | 6.19 | Z18 | 1.02 ± 0.01 a | 0.95 ± 0.03 b | 0.81 ± 0.01 c | 0.93 ± 0.11 | 11.46 |
| Bicudo | 0.51 ± 0.02 b | 0.65 ± 0.04 a | 0.63 ± 0.01 a | 0.60 ± 0.07 | 12.29 | Z17 | 0.21 ± 0.02 c | 0.37 ± 0.02 a | 0.30 ± 0.02 b | 0.29 ± 0.08 | 27.00 |
| Alecrim | 1.41 ± 0.01 b | 1.46 ± 0.02 a | 1.34 ± 0.02 c | 1.41 ± 0.06 | 4.28 | Z21 | 0.22 ± 0.01 c | 0.36 ± 0.04 a | 0.35 ± 0.01 b | 0.31 ± 0.08 | 24.87 |
| 700 | 0.87 ± 0.01 c | 0.95 ± 0.03 b | 1.11 ± 0.01 a | 0.98 ± 0.12 | 12.55 | Z36 | 0.45 ± 0.00 a | 0.36 ± 0.01 b | 0.36 ± 0.02 b | 0.39 ± 0.05 | 13.66 |
| CH1 | 0.38 ± 0.04 c | 0.46 ± 0.03 a | 0.44 ± 0.03 b | 0.43 ± 0.04 | 9.14 | Ouro negro | 0.88 ± 0.00 c | 1.02 ± 0.01 a | 0.95 ± 0.05 b | 0.95 ± 0.07 | 7.52 |
| Imbigudinho | 1.22 ± 0.02 b | 1.14 ± 0.01 c | 1.32 ± 0.02 a | 1.23 ± 0.09 | 7.22 | 18 | 0.90 ± 0.01 b | 0.95 ± 0.02 a | 0.86 ± 0.03 c | 0.91 ± 0.05 | 4.98 |
| AT | 0.81 ± 0.02 b | 0.92 ± 0.02 a | 0.84 ± 0.02 b | 0.86 ± 0.06 | 6.85 | Tardio C | 1.70 ± 0.01 b | 1.84 ± 0.02 a | 1.53 ± 0.02 c | 1.69 ± 0.16 | 9.21 |
| Graudão HP | 0.92 ± 0.01 a | 0.73 ± 0.02 b | 0.75 ± 0.01 b | 0.80 ± 0.10 | 13.02 | A1 | 0.22 ± 0.01 a | 0.24 ± 0.01 a | 0.15 ± 0.03 b | 0.21 ± 0.05 | 22.45 |
| Valcir P | 0.22 ± 0.02 b | 0.28 ± 0.01 a | 0.30 ± 0.02 a | 0.27 ± 0.04 | 14.91 | Cheique | 0.44 ± 0.01 a | 0.47 ± 0.01 a | 0.25 ± 0.02 b | 0.39 ± 0.12 | 30.98 |
| Beira Rio 8 | 0.87 ± 0.02 a | 0.88 ± 0.02 a | 0.76 ± 0.02 b | 0.83 ± 0.07 | 7.79 | P2 | 1.23 ± 0.01 a | 1.16 ± 0.01 b | 1.16 ± 0.02 b | 1.18 ± 0.04 | 3.61 |
| Tardio V | 1.10 ± 0.02 b | 1.04 ± 0.01 c | 1.15 ± 0.03 a | 1.09 ± 0.05 | 4.88 | Emcapa 02 | 0.70 ± 0.01 b | 0.85 ± 0.01 a | 0.83 ± 0.02 a | 0.79 ± 0.08 | 10.66 |
| AP | 0.89 ± 0.01 b | 0.95 ± 0.02 a | 0.77 ± 0.01 c | 0.87 ± 0.09 | 10.54 | Emcapa 153 | 1.07 ± 0.01 b | 1.16 ± 0.02 a | 0.84 ± 0.02 c | 1.03 ± 0.17 | 16.29 |
| L80 | 0.55 ± 0.02 b | 0.65 ± 0.02 a | 0.55 ± 0.02 b | 0.59 ± 0.06 | 9.85 | P1 | 0.63 ± 0.00 b | 0.74 ± 0.02 a | 0.72 ± 0.02 a | 0.70 ± 0.06 | 8.01 |
| Bamburral | 1.35 ± 0.01 b | 1.24 ± 0.03 c | 1.44 ± 0.02 a | 1.34 ± 0.10 | 7.21 | LB1 | 1.00 ± 0.01 a | 1.02 ± 0.02 a | 0.64 ± 0.02 b | 0.89 ± 0.21 | 24.09 |
| Pirata | 0.31 ± 0.03 b | 0.42 ± 0.02 a | 0.33 ± 0.03 b | 0.35 ± 0.06 | 16.23 | 122 | 1.03 ± 0.01 b | 1.10 ± 0.02 a | 0.78 ± 0.02 c | 0.97 ± 0.17 | 17.20 |
| Peneirão | 1.11 ± 0.01 b | 1.02 ± 0.02 c | 1.25 ± 0.04 a | 1.13 ± 0.11 | 10.13 | Verdim D | 0.87 ± 0.02 a | 0.99 ± 0.01 b | 1.04 ± 0.03 b | 0.96 ± 0.08 | 8.78 |
| Z39 | 0.40 ± 0.02 a | 0.41 ± 0.03 a | 0.45 ± 0.04 a | 0.42 ± 0.03 | 6.46 | Emcapa 143 | 0.62 ± 0.02 b | 0.86 ± 0.01 a | 0.62 ± 0.03 b | 0.70 ± 0.14 | 19.80 |
| Z35 | 0.13 ± 0.00 c | 0.20 ± 0.01 a | 0.17 ± 0.01 b | 0.17 ± 0.03 | 20.08 | Ouro negro 1 | 1.08 ± 0.01 a | 1.11 ± 0.01 a | 0.82 ± 0.02 b | 1.00 ± 0.16 | 15.69 |
| Z40 | 0.22 ± 0.01 b | 0.24 ± 0.03 b | 0.35 ± 0.01 a | 0.27 ± 0.07 | 25.54 | Ouro negro 2 | 1.12 ± 0.01 a | 1.10 ± 0.01 b | 0.83 ± 0.01 c | 1.01 ± 0.16 | 15.78 |
| Z29 | 1.13 ± 0.01 a | 1.03 ± 0.01 b | 0.82 ± 0.02 c | 0.99 ± 0.16 | 16.08 | Clementino | 0.41 ± 0.01 c | 0.57 ± 0.02 a | 0.47 ± 0.02 b | 0.48 ± 0.08 | 17.14 |
| Note (b): * Average caffeine contents of the three consecutive crops (2018, 2019, and 2020). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold were consistently outstanding compared to other genotypes. | |||||||||||
| (c) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 1.50 ± 0.01 a | 1.12 ± 0.02 b | 1.07 ± 0.02 c | 1.23 ± 0.24 | 19.34 | Z38 | 1.17 ± 0.00 a | 1.18 ± 0.02 a | 0.97 ± 0.02 b | 1.11 ± 0.12 | 10.50 |
| B01 | 1.41 ± 0.02 a | 1.15 ± 0.02 b | 0.96 ± 0.03 c | 1.17 ± 0.23 | 19.22 | Z18 | 1.59 ± 0.01 a | 1.61 ± 0.01 a | 1.26 ± 0.03 b | 1.49 ± 0.20 | 13.29 |
| Bicudo | 0.87 ± 0.01 b | 0.93 ± 0.01 a | 0.95 ± 0.03 a | 0.92 ± 0.04 | 4.40 | Z17 | 0.41 ± 0.01 c | 0.63 ± 0.02 a | 0.55 ± 0.03 b | 0.53 ± 0.11 | 21.28 |
| Alecrim | 1.44 ± 0.01 a | 1.25 ± 0.01 b | 1.44 ± 0.02 a | 1.38 ± 0.11 | 8.13 | Z21 | 1.07 ± 0.02 b | 1.13 ± 0.03 a | 1.11 ± 0.02 a | 1.11 ± 0.03 | 2.58 |
| 700 | 1.01 ± 0.01 a | 1.04 ± 0.02 a | 0.96 ± 0.02 b | 1.01 ± 0.04 | 3.99 | Z36 | 1.20 ± 0.01 b | 1.22 ± 0.01 b | 1.26 ± 0.02 a | 1.23 ± 0.03 | 2.78 |
| CH1 | 0.85 ± 0.01 c | 0.96 ± 0.02 b | 1.06 ± 0.01 a | 0.96 ± 0.11 | 11.15 | Ouro negro | 1.37 ± 0.01 a | 1.20 ± 0.02 b | 1.02 ± 0.03 c | 1.20 ± 0.17 | 14.61 |
| Imbigudinho | 1.64 ± 0.03 b | 1.78 ± 0.02 a | 1.26 ± 0.02 c | 1.56 ± 0.27 | 17.23 | 18 | 1.44 ± 0.01 a | 1.06 ± 0.01 b | 1.04 ± 0.02 b | 1.18 ± 0.22 | 19.02 |
| AT | 0.94 ± 0.01 b | 1.00 ± 0.05 a b | 1.07 ± 0.03 a | 1.01 ± 0.06 | 6.24 | Tardio C | 1.42 ± 0.01 a | 1.21 ± 0.03 b | 1.16 ± 0.03 b | 1.27 ± 0.14 | 10.88 |
| Graudão HP | 1.42 ± 0.02 a | 1.34 ± 0.02 b | 1.15 ± 0.01 c | 1.30 ± 0.14 | 10.53 | A1 | 1.03 ± 0.01 c | 1.14 ± 0.02 b | 1.28 ± 0.03 a | 1.15 ± 0.13 | 10.97 |
| Valcir P | 0.65 ± 0.01 b | 0.76 ± 0.02 a | 0.56 ± 0.00 c | 0.66 ± 0.10 | 15.46 | Cheique | 1.07 ± 0.01 b | 1.14 ± 0.02 a | 1.10 ± 0.04 b | 1.10 ± 0.03 | 3.00 |
| Beira Rio 8 | 1.01 ± 0.01 a | 1.03 ± 0.01 a | 0.94 ± 0.02 b | 0.99 ± 0.05 | 4.73 | P2 | 1.40 ± 0.01 c | 1.51 ± 0.02 b | 1.85 ± 0.03 a | 1.59 ± 0.23 | 14.73 |
| Tardio V | 0.33 ± 0.01 c | 0.44 ± 0.02 b | 0.54 ± 0.02 a | 0.44 ± 0.11 | 24.65 | Emcapa 02 | 1.76 ± 0.01 b | 1.82 ± 0.01 a | 1.02 ± 0.04 c | 1.53 ± 0.45 | 29.27 |
| AP | 2.01 ± 0.02 a | 1.78 ± 0.02 b | 1.81 ± 0.03 c | 1.87 ± 0.13 | 6.78 | Emcapa 153 | 1.10 ± 0.01 c | 1.50 ± 0.02 b | 1.66 ± 0.04 a | 1.42 ± 0.29 | 20.20 |
| L80 | 0.86 ± 0.02 b | 0.93 ± 0.03 a | 0.74 ± 0.01 c | 0.84 ± 0.09 | 11.23 | P1 | 0.81 ± 0.01 c | 0.94 ± 0.02 b | 0.99 ± 0.03 a | 0.91 ± 0.09 | 10.11 |
| Bamburral | 1.47 ± 0.02 b | 1.56 ± 0.03 a | 1.36 ± 0.02 c | 1.46 ± 0.10 | 7.07 | LB1 | 0.96 ± 0.01 b | 1.04 ± 0.02 a | 0.86 ± 0.04 c | 0.95 ± 0.09 | 9.65 |
| Pirata | 0.59 ± 0.02 c | 0.77 ± 0.02 b | 0.86 ± 0.03 a | 0.74 ± 0.14 | 18.28 | 122 | 1.12 ± 0.02 b | 1.13 ± 0.03 b | 1.34 ± 0.02 a | 1.20 ± 0.13 | 10.52 |
| Peneirão | 0.82 ± 0.02 b | 0.92 ± 0.02 a | 0.77 ± 0.02 c | 0.83 ± 0.07 | 8.68 | Verdim D | 1.29 ± 0.03 a | 1.10 ± 0.01 b | 0.83 ± 0.02 c | 1.07 ± 0.23 | 21.38 |
| Z39 | 0.79 ± 0.01 b | 1.02 ± 0.02 a | 1.05 ± 0.03 a | 0.95 ± 0.14 | 14.96 | Emcapa 143 | 1.11 ± 0.02 b | 1.16 ± 0.02 a | 0.97 ± 0.01 c | 1.08 ± 0.10 | 9.24 |
| Z35 | 0.35 ± 0.02 c | 0.67 ± 0.02 a | 0.44 ± 0.04 b | 0.49 ± 0.16 | 33.55 | Ouro negro 1 | 1.00 ± 0.01 a | 1.06 ± 0.03 a | 0.86 ± 0.03 b | 0.97 ± 0.10 | 10.57 |
| Z40 | 0.79 ± 0.01 a b | 0.90 ± 0.07 a | 0.76 ± 0.03 b | 0.82 ± 0.07 | 9.10 | Ouro negro 2 | 1.66 ± 0.03 b | 1.72 ± 0.02 a | 1.07 ± 0.03 c | 1.48 ± 0.36 | 24.36 |
| Z29 | 1.50 ± 0.01 b | 1.69 ± 0.01 a | 1.24 ± 0.03 c | 1.47 ± 0.23 | 15.33 | Clementino | 0.57 ± 0.02 c | 0.77 ± 0.02 b | 0.87 ± 0.02 a | 0.74 ± 0.15 | 20.64 |
| Note (c): * Average caffeine contents of the three consecutive crops (2018, 2019, and 2020). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold were consistently outstanding compared to other genotypes. | |||||||||||
2.3.3. Trigonelline
| (a) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 0.93 ± 0.03 a | 0.94 ± 0.01 a | 0.89 ± 0.02 b | 0.92 ± 0.03 | 2.89 | Z38 | 1.05 ± 0.02 a | 1.03 ± 0.03 b | 1.06 ± 0.01 a | 1.05 ± 0.02 | 1.63 |
| B01 | 0.95 ± 0.02 c | 1.04 ± 0.02 b | 1.08 ± 0.02 a | 1.02 ± 0.07 | 6.56 | Z18 | 1.10 ± 0.02 a | 0.95 ± 0.02 b c | 0.99 ± 0.03 b | 1.01 ± 0.08 | 7.98 |
| Bicudo | 0.96 ± 0.03 c | 1.03 ± 0.02 b | 1.07 ± 0.02 a | 1.02 ± 0.05 | 5.29 | Z17 | 1.06 ± 0.02 a | 1.01 ± 0.01 b | 1.07 ± 0.01 a | 1.05 ± 0.03 | 3.06 |
| Alecrim | 0.88 ± 0.02 b | 0.95 ± 0.02 a | 0.83 ± 0.02 b c | 0.89 ± 0.06 | 6.77 | Z21 | 1.02 ± 0.01 a | 0.94 ± 0.02 b | 0.90 ± 0.03 c | 0.95 ± 0.06 | 6.71 |
| 700 | 0.97 ± 0.02 b | 1.01 ± 0.01 a | 0.91 ± 0.00 c | 0.96 ± 0.05 | 5.10 | Z36 | 1.02 ± 0.02 a | 0.96 ± 0.02 b | 0.88 ± 0.03 c | 0.95 ± 0.07 | 7.53 |
| CH1 | 0.91 ± 0.02 b | 0.96 ± 0.01 a | 0.93 ± 0.04 a b | 0.93 ± 0.03 | 2.70 | Ouro negro | 0.87 ± 0.01 a | 0.81 ± 0.01 b | 0.87 ± 0.02 a | 0.85 ± 0.03 | 3.97 |
| Imbigudinho | 0.94 ± 0.02 a | 0.94 ± 0.02 a | 0.88 ± 0.02 b | 0.92 ± 0.03 | 3.67 | 18 | 0.82 ± 0.03 b | 0.87 ± 0.04 a | 0.78 ± 0.03 c | 0.82 ± 0.05 | 5.86 |
| AT | 0.95 ± 0.04 b | 0.86 ± 0.01 c | 1.05 ± 0.03 a | 0.96 ± 0.10 | 9.95 | Tardio C | 0.96 ± 0.03 b | 0.89 ± 0.03 c | 1.01 ± 0.04 a | 0.95 ± 0.06 | 6.32 |
| Graudão HP | 1.03 ± 0.01 a | 0.87 ± 0.03 b | 1.01 ± 0.01 a | 0.97 ± 0.09 | 9.10 | A1 | 0.85 ± 0.02 b | 0.88 ± 0.01 a | 0.91 ± 0.01 a | 0.88 ± 0.03 | 3.60 |
| Valcir P | 1.03 ± 0.04 a | 1.02 ± 0.01 a | 1.03 ± 0.01 a | 1.03 ± 0.01 | 0.75 | Cheique | 1.05 ± 0.01 a | 1.00 ± 0.02 b | 1.03 ± 0.02 a b | 1.03 ± 0.03 | 2.48 |
| Beira Rio 8 | 1.03 ± 0.02 a | 0.93 ± 0.01 c | 1.00 ± 0.03 a b | 0.99 ± 0.05 | 5.17 | P2 | 1.03 ± 0.02 a | 0.98 ± 0.03 b | 0.86 ± 0.03 c | 0.96 ± 0.09 | 8.97 |
| Tardio V | 1.05 ± 0.01 a | 0.95 ± 0.02 c | 1.01 ± 0.02 b | 1.00 ± 0.05 | 5.36 | Emcapa 02 | 1.04 ± 0.02 a | 1.01 ± 0.02 b | 1.04 ± 0.01 a | 1.03 ± 0.02 | 1.97 |
| AP | 1.04 ± 0.02 a b | 1.01 ± 0.02 b | 1.07 ± 0.02 a | 1.04 ± 0.03 | 2.74 | Emcapa 153 | 1.06 ± 0.01 a | 1.04 ± 0.02 b | 1.07 ± 0.02 a | 1.06 ± 0.01 | 1.14 |
| L80 | 1.06 ± 0.02 a | 0.97 ± 0.03 b | 1.07 ± 0.01 a | 1.03 ± 0.06 | 5.45 | P1 | 1.11 ± 0.02 a | 1.06 ± 0.02 b | 1.12 ± 0.02 a | 1.09 ± 0.03 | 2.94 |
| Bamburral | 0.86 ± 0.04 c | 0.93 ± 0.04 b | 1.04 ± 0.01 a | 0.94 ± 0.09 | 9.41 | LB1 | 1.02 ± 0.01 b | 1.06 ± 0.03 a | 0.99 ± 0.02 b | 1.03 ± 0.04 | 3.42 |
| Pirata | 1.06 ± 0.04 a | 1.06 ± 0.03 a | 1.07 ± 0.02 a | 1.06 ± 0.01 | 0.79 | 122 | 1.03 ± 0.02 b | 1.02 ± 0.03 b | 1.07 ± 0.03 a | 1.04 ± 0.03 | 2.69 |
| Peneirão | 1.06 ± 0.02 a | 1.06 ± 0.03 a | 1.05 ± 0.02 a | 1.06 ± 0.01 | 0.48 | Verdim D | 1.05 ± 0.02 a | 1.05 ± 0.03 a | 1.05 ± 0.03 a | 1.05 ± 0.00 | 0.32 |
| Z39 | 1.04 ± 0.02 a | 1.04 ± 0.04 a | 1.03 ± 0.03 a | 1.04 ± 0.01 | 0.56 | Emcapa 143 | 1.06 ± 0.02 a | 0.95 ± 0.02 b | 0.98 ± 0.02 b | 0.99 ± 0.06 | 5.58 |
| Z35 | 1.09 ± 0.03 a | 0.96 ± 0.03 b | 0.96 ± 0.04 b | 1.00 ± 0.08 | 7.48 | Ouro negro 1 | 1.03 ± 0.02 a | 0.98 ± 0.05 b | 0.93 ± 0.03 c | 0.98 ± 0.05 | 5.09 |
| Z40 | 0.85 ± 0.01 a b | 0.85 ± 0.03 a b | 0.88 ± 0.02 a | 0.86 ± 0.02 | 2.14 | Ouro negro 2 | 0.95 ± 0.04 a | 0.81 ± 0.02 c | 0.86 ± 0.02 b | 0.87 ± 0.07 | 8.35 |
| Z29 | 0.94 ± 0.02 a | 0.83 ± 0.02 b | 0.92 ± 0.02 a | 0.90 ± 0.05 | 6.12 | Clementino | 1.03 ± 0.02 a | 0.93 ± 0.02 b | 0.95 ± 0.03 b | 0.97 ± 0.05 | 5.32 |
| Note (a): * Average trigonelline contents of the three consecutive crops (2018, 2019, and 2020). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold indicate genotypes that stood out for their consistently high contents of trigonelline (>1.0%, CV < 5%) in all crops evaluated, compared to average contents described in the literature [41] and with the remaining genotypes. | |||||||||||
| (b) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 0.90 ± 0.02 b | 1.04 ± 0.01 a | 1.06 ± 0.03 a | 1.00 ± 0.08 | 8.34 | Z38 | 1.03 ± 0.03 a | 0.95 ± 0.02 b | 0.86 ± 0.01 c | 0.95 ± 0.09 | 9.02 |
| B01 | 0.96 ± 0.02 a | 0.86 ± 0.01 b | 0.97 ± 0.05 a | 0.93 ± 0.06 | 6.50 | Z18 | 0.76 ± 0.04 c | 0.95 ± 0.02 a | 0.82 ± 0.02 b | 0.84 ± 0.10 | 11.46 |
| Bicudo | 0.92 ± 0.01 b | 0.95 ± 0.01 a | 0.93 ± 0.04 a b | 0.93 ± 0.01 | 1.49 | Z17 | 0.59 ± 0.02 c | 0.76 ± 0.03 a | 0.66 ± 0.01 b | 0.67 ± 0.08 | 12.26 |
| Alecrim | 0.96 ± 0.03 b | 1.05 ± 0.01 a | 0.92 ± 0.02 c | 0.98 ± 0.06 | 6.44 | Z21 | 1.12 ± 0.02 a | 1.08 ± 0.02 b | 1.01 ± 0.02 c | 1.07 ± 0.05 | 4.90 |
| 700 | 1.04 ± 0.01 c | 1.13 ± 0.02 b | 1.19 ± 0.02 a | 1.12 ± 0.08 | 6.76 | Z36 | 1.12 ± 0.02 a | 1.08 ± 0.02 b | 0.95 ± 0.03 c | 1.05 ± 0.09 | 8.49 |
| CH1 | 1.24 ± 0.02 a | 1.08 ± 0.02 b | 0.91 ± 0.02 c | 1.08 ± 0.16 | 15.02 | Ouro negro | 0.87 ± 0.02 b | 0.76 ± 0.03 b | 0.86 ± 0.04 a | 0.83 ± 0.06 | 7.13 |
| Imbigudinho | 1.21 ± 0.02 a | 1.16 ± 0.02 b | 1.09 ± 0.02 c | 1.15 ± 0.06 | 5.53 | 18 | 0.99 ± 0.03 a | 0.96 ± 0.02 a | 0.88 ± 0.02 b | 0.94 ± 0.06 | 6.10 |
| AT | 1.13 ± 0.01 a | 1.07 ± 0.02 b | 0.87 ± 0.03 c | 1.02 ± 0.14 | 13.50 | Tardio C | 0.74 ± 0.02 c | 0.84 ± 0.02 b | 1.00 ± 0.02 a | 0.86 ± 0.13 | 15.20 |
| Graudão HP | 1.05 ± 0.03 a | 1.05 ± 0.03 a | 0.94 ± 0.02 b | 1.01 ± 0.06 | 6.27 | A1 | 0.89 ± 0.02 b | 0.89 ± 0.02 b | 1.11 ± 0.01 a | 0.97 ± 0.13 | 12.96 |
| Valcir P | 1.04 ± 0.02 b | 1.03 ± 0.02 b | 1.11 ± 0.02 a | 1.06 ± 0.05 | 4.48 | Cheique | 1.02 ± 0.01 b | 1.06 ± 0.02 a | 1.06 ± 0.03 a | 1.05 ± 0.02 | 2.12 |
| Beira Rio 8 | 1.02 ± 0.02 a | 0.96 ± 0.03 b | 0.83 ± 0.02 c | 0.94 ± 0.10 | 10.69 | P2 | 0.76 ± 0.03 c | 0.81 ± 0.01 b | 0.97 ± 0.02 a | 0.85 ± 0.11 | 13.13 |
| Tardio V | 0.73 ± 0.02 c | 0.82 ± 0.03 b | 1.01 ± 0.02 a | 0.85 ± 0.14 | 16.56 | Emcapa 02 | 0.96 ± 0.02 c | 1.05 ± 0.02 b | 1.09 ± 0.04 a | 1.03 ± 0.06 | 6.17 |
| AP | 1.02 ± 0.01 a | 1.04 ± 0.03 a | 0.95 ± 0.03 b | 1.00 ± 0.05 | 4.52 | Emcapa 153 | 0.68 ± 0.02 b | 0.72 ± 0.03 a | 0.76 ± 0.02 a | 0.72 ± 0.04 | 5.33 |
| L80 | 0.84 ± 0.03 b | 0.92 ± 0.02 a | 0.83 ± 0.02 b | 0.87 ± 0.05 | 5.83 | P1 | 1.08 ± 0.02 b | 1.11 ± 0.02 b | 1.18 ± 0.02 a | 1.13 ± 0.05 | 4.60 |
| Bamburral | 0.65 ± 0.02 c | 0.72 ± 0.02 b | 0.82 ± 0.01 a | 0.73 ± 0.08 | 11.48 | LB1 | 0.86 ± 0.02 c | 0.91 ± 0.01 b | 1.12 ± 0.02 a | 0.96 ± 0.14 | 14.37 |
| Pirata | 0.70 ± 0.02 c | 0.84 ± 0.02 a | 0.76 ± 0.02 b | 0.77 ± 0.07 | 9.20 | 122 | 0.92 ± 0.02 a | 0.78 ± 0.02 c | 0.87 ± 0.02 b | 0.86 ± 0.07 | 8.64 |
| Peneirão | 0.82 ± 0.02 c | 0.90 ± 0.03 b | 0.93 ± 0.02 a | 0.88 ± 0.06 | 6.33 | Verdim D | 0.86 ± 0.03 c | 0.93 ± 0.03 b | 1.07 ± 0.02 a | 0.95 ± 0.11 | 11.26 |
| Z39 | 0.72 ± 0.02 b | 0.62 ± 0.01 c | 0.94 ± 0.01 a | 0.76 ± 0.16 | 21.32 | Emcapa 143 | 0.90 ± 0.03 c | 1.13 ± 0.03 a | 1.08 ± 0.04 b | 1.03 ± 0.12 | 11.75 |
| Z35 | 0.95 ± 0.03 b | 1.04 ± 0.01 a | 0.94 ± 0.02 b | 0.98 ± 0.06 | 5.83 | Ouro negro 1 | 0.98 ± 0.02 b | 1.10 ± 0.02 a | 1.12 ± 0.02 a | 1.07 ± 0.08 | 7.16 |
| Z40 | 0.67 ± 0.02 c | 0.94 ± 0.01 b | 1.05 ± 0.02 a | 0.88 ± 0.20 | 22.16 | Ouro negro 2 | 1.04 ± 0.02 b | 1.13 ± 0.03 a | 1.05 ± 0.03 b | 1.08 ± 0.05 | 4.58 |
| Z29 | 0.73 ± 0.02 c | 0.94 ± 0.02 b | 1.02 ± 0.02 a | 0.89 ± 0.15 | 16.90 | Clementino | 0.64 ± 0.02 b | 0.74 ± 0.04 a | 0.65 ± 0.03 b | 0.68 ± 0.05 | 7.93 |
| Note (b): * Average trigonelline contents of the three consecutive crops (2018, 2019, and 2020). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold were consistently outstanding compared to other genotypes. | |||||||||||
| (c) | |||||||||||
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
| Verdim R | 1.53 ± 0.03 a | 1.41 ± 0.03 b | 1.39 ± 0.03 b | 1.44 ± 0.08 | 5.30 | Z38 | 1.13 ± 0.03 b | 1.25 ± 0.02 a | 1.15 ± 0.03 b | 1.17 ± 0.06 | 5.48 |
| B01 | 0.93 ± 0.03 b | 0.88 ± 0.03 b | 1.22 ± 0.02 a | 1.01 ± 0.19 | 18.47 | Z18 | 1.55 ± 0.02 c | 1.72 ± 0.02 a | 1.66 ± 0.02 b | 1.64 ± 0.08 | 5.06 |
| Bicudo | 1.30 ± 0.01 b | 1.28 ± 0.02 b | 1.52 ± 0.02 a | 1.37 ± 0.13 | 9.63 | Z17 | 1.20 ± 0.03 b | 1.24 ± 0.02 a | 1.16 ± 0.02 c | 1.20 ± 0.04 | 3.31 |
| Alecrim | 1.54 ± 0.04 c | 1.64 ± 0.02 b | 1.75 ± 0.03 a | 1.65 ± 0.11 | 6.48 | Z21 | 1.65 ± 0.03 a | 1.39 ± 0.02 b | 1.11 ± 0.08 c | 1.38 ± 0.27 | 19.41 |
| 700 | 1.69 ± 0.03 c | 1.77 ± 0.02 b | 1.84 ± 0.02 a | 1.77 ± 0.07 | 4.16 | Z36 | 1.23 ± 0.02 b | 1.21 ± 0.04 b | 1.32 ± 0.03 a | 1.25 ± 0.06 | 4.83 |
| CH1 | 0.97 ± 0.02 c | 1.20 ± 0.01 a | 1.14 ± 0.02 b | 1.10 ± 0.12 | 10.84 | Ouro negro | 1.20 ± 0.02 a | 1.07 ± 0.02 c | 1.15 ± 0.03 b | 1.14 ± 0.06 | 5.68 |
| Imbigudinho | 1.17 ± 0.01 b | 1.06 ± 0.01 c | 1.22 ± 0.02 a | 1.15 ± 0.08 | 7.27 | 18 | 1.00 ± 0.04 a | 0.86 ± 0.03 b | 1.04 ± 0.03 a | 0.97 ± 0.09 | 9.70 |
| AT | 1.13 ± 0.02 b | 1.31 ± 0.02 a | 1.10 ± 0.01 b | 1.18 ± 0.11 | 9.50 | Tardio C | 1.02 ± 0.02 b | 1.04 ± 0.02 b | 1.10 ± 0.02 a | 1.05 ± 0.04 | 4.08 |
| Graudão HP | 1.08 ± 0.02 b | 1.12 ± 0.01 b | 1.20 ± 0.03 a | 1.13 ± 0.06 | 5.44 | A1 | 1.69 ± 0.04 c | 1.72 ± 0.02 b | 1.76 ± 0.09 a | 1.73 ± 0.04 | 2.05 |
| Valcir P | 1.35 ± 0.03 a | 1.22 ± 0.03 b | 1.32 ± 0.02 a | 1.30 ± 0.07 | 5.05 | Cheique | 1.50 ± 0.02 c | 1.54 ± 0.01 b | 1.69 ± 0.02 a | 1.57 ± 0.10 | 6.44 |
| Beira Rio 8 | 1.50 ± 0.03 c | 1.63 ± 0.07 b | 1.75 ± 0.02 a | 1.63 ± 0.12 | 7.56 | P2 | 1.69 ± 0.02 a | 1.69 ± 0.02 a | 1.43 ± 0.07 b | 1.60 ± 0.15 | 9.47 |
| Tardio V | 0.83 ± 0.02 c | 1.01 ± 0.02 b | 1.19 ± 0.02 a | 1.01 ± 0.18 | 17.37 | Emcapa 02 | 1.76 ± 0.03 a | 1.65 ± 0.02 b | 1.69 ± 0.02 b | 1.70 ± 0.05 | 3.10 |
| AP | 1.52 ± 0.02 b | 1.61 ± 0.04 a | 1.44 ± 0.01 c | 1.52 ± 0.08 | 5.50 | Emcapa 153 | 1.24 ± 0.02 b | 1.29 ± 0.02 a | 1.19 ± 0.02 c | 1.24 ± 0.05 | 4.31 |
| L80 | 1.55 ± 0.03 c | 1.76 ± 0.03 a | 1.60 ± 0.02 b | 1.64 ± 0.11 | 6.62 | P1 | 0.95 ± 0.01 c | 1.10 ± 0.01 b | 1.17 ± 0.02 a | 1.07 ± 0.11 | 10.36 |
| Bamburral | 1.09 ± 0.02 c | 1.23 ± 0.02 b | 1.35 ± 0.03 a | 1.23 ± 0.13 | 10.48 | LB1 | 1.33 ± 0.03 b | 1.45 ± 0.04 a | 1.33 ± 0.01 b | 1.37 ± 0.07 | 5.34 |
| Pirata | 1.02 ± 0.02 c | 1.09 ± 0.03 b | 1.24 ± 0.01 a | 1.12 ± 0.11 | 10.21 | 122 | 1.73 ± 0.04 a | 1.64 ± 0.04 b | 1.74 ± 0.06 a | 1.70 ± 0.06 | 3.30 |
| Peneirão | 0.98 ± 0.01 c | 1.12 ± 0.03 a | 1.10 ± 0.02 b | 1.07 ± 0.09 | 7.30 | Verdim D | 1.43 ± 0.03 a | 1.41 ± 0.03 a | 1.44 ± 0.01 a | 1.42 ± 0.02 | 1.15 |
| Z39 | 1.21 ± 0.01 b | 1.36 ± 0.02 a | 1.36 ± 0.03 a | 1.31 ± 0.08 | 6.64 | Emcapa 143 | 0.77 ± 0.02 c | 0.85 ± 0.02 b | 1.11 ± 0.02 a | 0.91 ± 0.17 | 19.04 |
| Z35 | 0.92 ± 0.01 b | 0.93 ± 0.02 b | 1.06 ± 0.03 a | 0.97 ± 0.08 | 8.10 | Ouro negro 1 | 1.28 ± 0.05 c | 1.38 ± 0.02 b | 1.56 ± 0.01 a | 1.41 ± 0.14 | 10.12 |
| Z40 | 0.96 ± 0.02 a | 0.91 ± 0.02 b | 0.74 ± 0.03 c | 0.87 ± 0.12 | 13.41 | Ouro negro 2 | 1.03 ± 0.03 b | 0.97 ± 0.01 c | 1.35 ± 0.02 a | 1.12 ± 0.21 | 18.52 |
| Z29 | 1.10 ± 0.03 a | 0.98 ± 0.01 b | 1.01 ± 0.02 b | 1.03 ± 0.06 | 5.85 | Clementino | 1.73 ± 0.02 b | 1.81 ± 0.01 a | 1.60 ± 0.03 c | 1.71 ± 0.11 | 6.31 |
| Note (c): * Average trigonelline contents of the three consecutive crops (2018, 2019, and 2020). CV: coefficient of variation. Different letters for the same genotype indicate statistical differences between crops by ANOVA (p < 0.05). Values in bold were consistently outstanding compared to other genotypes. | |||||||||||
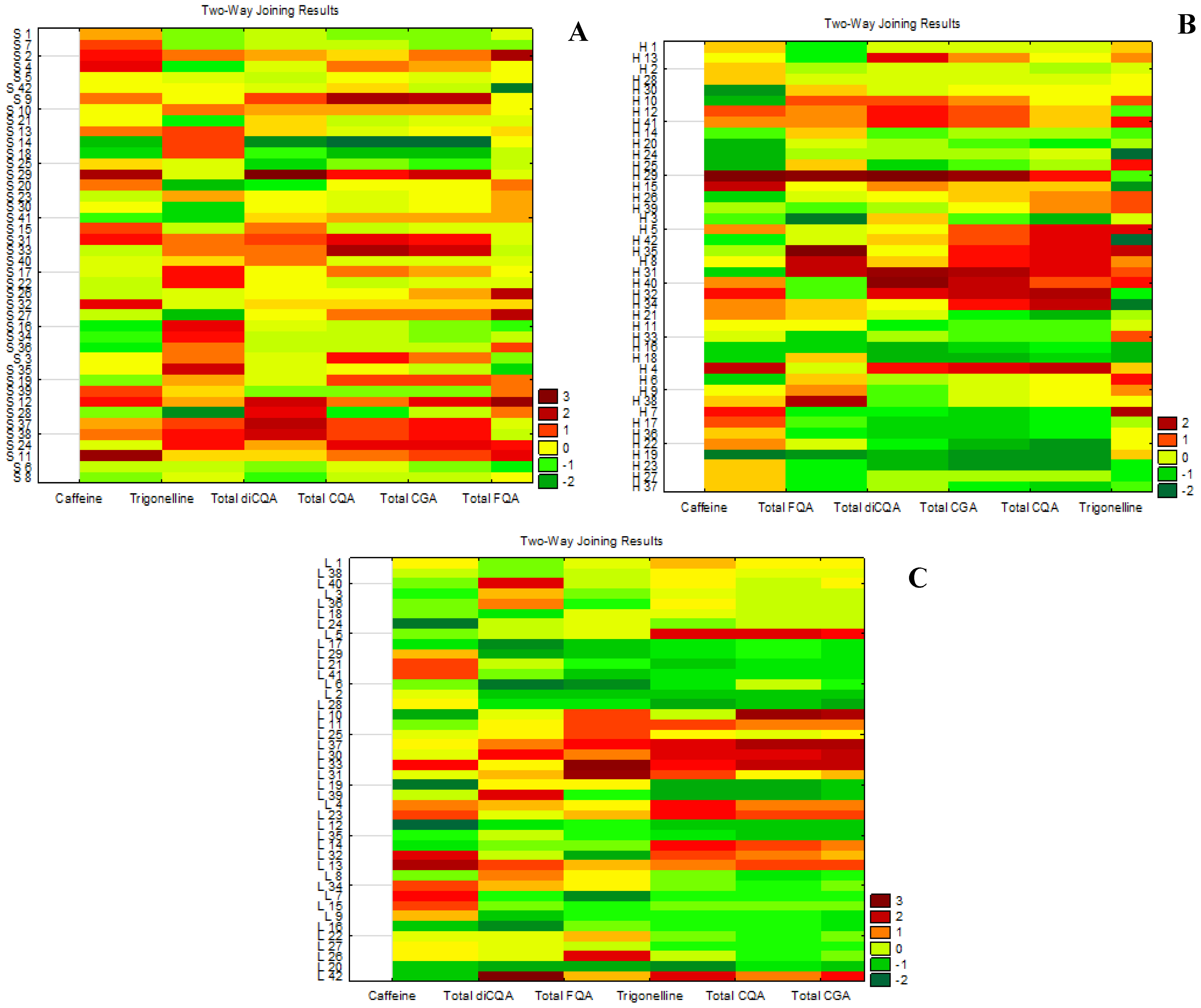
2.4. Correlations Among Bioactive Compounds in the Different Parts of the Plant
2.5. Selection of Promising Genotypes
3. Materials and Methods
3.1. Samples and Experiment Set Up
3.2. Harvest and Post-Harvest
3.3. Water Content
3.4. Soluble Solids of Green Seeds
3.5. Methanolic Extraction and Analysis of Bioactive Compounds
3.6. Statistical Analysis
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farah, A. Nutritional and Health Effects of Coffee. In Achieving Sustainable Cultivation of Coffee, 1st ed.; Lashermes, P., Ed.; Burleigh Dodds Science Publishing: London, UK, 2018; pp. 259–291. ISBN 9781351114363. [Google Scholar]
- ICO—International Coffee Organization. World Coffee Statistics Database. 2024. Available online: https://ico.org/what-we-do/world-coffee-statistics-database/ (accessed on 26 December 2024).
- Food and Drug Administration. Food Labeling: Nutrient Content Claims; Definition of Term “Healthy”. 2024. Available online: https://www.federalregister.gov/documents/2024/12/27/2024-29957/food-labeling-nutrient-content-claims-definition-of-term-healthy (accessed on 27 January 2025).
- Farah, A. Flavor development during roasting. In Drying and Roasting of Cocoa and Coffee; Lik, H.C., Borém, F., Eds.; CRC Press: NewYork, NY, USA, 2019; pp. 267–303. [Google Scholar]
- Fiorott, A.S.; Sturm, G.M. Café Canéfora: Em busca de Qualidade e Reconhecimento. In Comunicado Técnico; Embrapa: Brasília, Brazil, 2015; Volume 19, pp. 425–431. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1041013/cafe-canefora-em-busca-de-qualidade-e-reconhecimento (accessed on 27 January 2025).
- ICO—International Coffee Organization. Trade Statistics. 2023. Available online: https://icocoffee.org/documents/cy2023-24/Coffee_Report_and_Outlook_December_2023_ICO.pdf (accessed on 15 March 2025).
- CONAB (Companhia Nacional de Abastecimento). Acompanhamento da Safra Brasileira de Café. Segundo Levantamento. 2024. Available online: https://www.conab.gov.br/info-agro/safras/cafe (accessed on 19 January 2025).
- Alves, R.C.; Rodrigues, F.; Nunes, M.A.; Vinha, A.; Oliveira, M.B.P.P. State of the art in coffee processing by-products. In Handbook of Coffee Processing By-Products, 1st ed.; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–26. ISBN 9780128112908. [Google Scholar]
- Del Castillo, M.D.; Fernandez-Gomez, B.; Martinez-Saez, N.; Iriondo-DeHond, A.; Mesa, M.D. Coffee By-Products. In Coffee: Production, Quality and Chemistry, 1st ed.; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; Volume 1, pp. 309–334. [Google Scholar] [CrossRef]
- Matiello, J.B.; Almeida, S.R.; Aguiar, E.C.; Josino, V.; Araujo, R.A.; Oliveira, E.; Moulin, C. Quantificação do Processo de Reciclagem de Folhas em Cafezais. In 36° Congresso Brasileiro de Pesquisas Caffeiras; SBI Café Biblioteca do Café: Guarapari, Brazil, 2010. [Google Scholar]
- Beyene, A.; Kassahun, Y.; Addis, T.; Assefa, F.; Amsalu, A.; Legesse, W.; Kloos, H.; Triest, L. The impact of traditional coffee processing on river water quality in Ethiopia and the urgency of adopting sound environmental practices. Environmental 2012, 184, 7053–7063. [Google Scholar] [CrossRef] [PubMed]
- Awoke, A.; Beyene, A.; Kloos, H.; Goethals, P.L.; Triest, L. River water pollution status and water policy scenario in Ethiopia: Raising awareness for better implementation in developing countries. Environ. Manag. 2016, 58, 694–706. [Google Scholar] [CrossRef]
- Campa, C.; Petitvallet, A. Beneficial compounds from coffee leaves Monkey, Canada. In Achieving Sustainable Cultivation of Coffee, 1st ed.; Lashermes, P., Ed.; Burleigh Dodds Science Publishing: London, UK, 2018; pp. 237–258. ISBN 9781351114363. [Google Scholar]
- Chen, X. A review on coffee leaves: Phytochemicals, bioactivities, and applications. Crit. Rev. Food Sci. Nutr. 2018, 59, 1008–10025. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.T.S.; de Almeida, R.F.; Breuer, A.; Owen, R.W. Phytochemicals from Coffea leaves. In Coffee: Production, Quality and Chemistry, 1st ed.; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; Volume 1, pp. 771–787. [Google Scholar] [CrossRef]
- Mendonça, L.M.V.L.; Pereira, R.G.F.A.; Mendes, A.N.G. Parâmetros bromatológicos de grãos crus e torrados de cultivares de café (Coffea arabica L.). Ciênc. Tecnol. Aliment. 2005, 25, 239–243. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Monteiro, Â.; Colomban, S.; Azinheira, H.G.; Guerra-Guimarães, L.; Do Céu Silva, M.; Navarini, L.; Resmini, M. Dietary antioxidants in coffee leaves: Impact of botanical origin and maturity on chlorogenic acids and xanthones. Antioxidants 2019, 9, 6. [Google Scholar] [CrossRef]
- Farah, A.; dePaula, J. Consumption of chlorogenic acids through coffee and health implications. Beverages 2019, 5, 11. [Google Scholar] [CrossRef]
- Monteiro, M.C.; Farah, A. Chlorogenic acids in brazilian Coffea arabica cultivars from various consecutive crops. Food Chem. 2012, 134, 611–614. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic Acids. In Coffee: Volume 1: Chemistry; Clarke, R.J., Macrae, R., Eds.; Elsevier Applied Science: London, UK, 1985. [Google Scholar]
- Guerrero, G.; Suárez, M.; Moreno, G. Chlorogenic acids as a potential criterion in coffee genotype selections. J. Agric. Food Chem. 2001, 49, 2454–2458. [Google Scholar] [CrossRef]
- Perrone, D.; Farah, A.; Donangelo, C.M.; de Paulis, T.; Martin, P.R. Comprehensive analysis of major and minor chlorogenic acids and lactones in economically relevant Brazilian coffee cultivars. Food Chem. 2008, 106, 859–867. [Google Scholar] [CrossRef]
- Aerts, R.J.; Baumann, T.W. Distribution and utilization of chlorogenic acid in Coffea seedlings. J. Exp. Bot. 1994, 45, 497–503. [Google Scholar] [CrossRef]
- Ashihara, H.; Fujimura, T.; Crozier, A. Coffee plant biochemistry. In Coffee: Production, Quality and Chemistry, 1st ed.; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; Volume 1, pp. 100–162. [Google Scholar] [CrossRef]
- Mondolot, L.; LA Fisca, P.; Buatois, B.; Talansier, E.; De Kochko, A.; Campa, C. Evolution in caffeoylquinic acid content and histolocalization during Coffea canephora leaf development. Ann. Bot. 2006, 98, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Grace, S.C.; Logan, B.A. Acclimation of foliar antioxidant systems to growth irradiance in three broad-leaved evergreen species. Plant Physiol. 1996, 112, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Joët, T.; Salmona, J.; Laffargue, A.; Descroix, F.; Dussert, S. Use of the growing environment as a source of variation to identify the quantitative trait transcripts and modules of co-expressed genes that determine chlorogenic acid accumulation. Plant Cell Environ. 2010, 33, 1220–1233. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Trevisan, M.T.S.; Thomaziello, R.A.; Breuer, A.; Klika, K.D.; Ulrich, C.M.; Owen, R.W. Nutraceutical compounds: Echinoids, flavonoids, xanthones and caffeine identified and quantitated in the leaves of Coffea arabica treesfrom three regions of Brazil. Food Res. Int. 2019, 115, 493–503. [Google Scholar] [CrossRef]
- Campa, C.; Mondolot, L.; Rakotondravao, A.; Bidel, L.P.R.; Gargadennec, A.; Couturon, E.; La Fisca, P.; Rakotomalala, J.-J.; Jay-Allemand, C.; Davis, A.P. A survey of mangiferin and hydroxycinnamic acid ester accumulation in coffee (Coffea) leaves: Biological implications and uses. Ann. Bot. 2012, 110, 595–613. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005, 53, 1505–15013. [Google Scholar] [CrossRef]
- Lima, J. de P.; Farah, A.; King, B.; de Paulis, T.; Martin, P.R. Distribution of major chlorogenic acids and related compounds in Brazilian green and toasted Ilex paraguariensis (Maté) leaves. J. Agric. Food Chem. 2016, 64, 2361–2370. [Google Scholar] [CrossRef]
- Iriondo-DeHond, A.; García, N.A.; Fernandez-Gomez, B.; Guisantes-Batan, E.; Velázquez Escobar, F.; Blanch, G.P.; San Andres, M.I.; Sanchez-Fortun, S.; del Castillo, M.D. Validation of coffee by-products as novel food ingredientes. Innov. Food Sci. Emerg. Technol. 2019, 51, 194–204. [Google Scholar] [CrossRef]
- Mateos, R.; Baeza, G.; Sarriá, B.; Bravo, L. Improved LC-MSn characterization of hydroxycinnamic acid derivatives and flavonols in different commercial mate (Ilex paraguariensis) brands. Quantification of polyphenols, methylxanthines, and antioxidant activity. Food Chem. 2018, 241, 232–241. [Google Scholar] [CrossRef] [PubMed]
- DePaula, J.; Farah, A. Caffeine consumption through coffee: Content in the beverage, metabolism, health benefits and risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Harborne, B.J. The co-evolutionary arms Race: Plant defence and animal response. In Introduction to Ecological Biochemistry; Harborne, J.B., Ed.; Elsevier Academic Press: Cambridge, UK, 1993; pp. 186–210. ISBN 9780080918587. [Google Scholar]
- Hewavitharanage, P.; Karunaratne, S.; Kumar, N.S. Effect of caffeine on shot-hole borer beetle (Xyleborusfornicatus) of tea (Camellia sinensis). Phytochemistry 1999, 51, 35–41. [Google Scholar] [CrossRef]
- Waller, G.R. Biochemical frontiers of allelopathy. Biol. Plant. 1989, 31, 418–447. [Google Scholar] [CrossRef]
- Chaves, J.C.D.; Miyazawa, M.; Bloch, M.F.M.; Yamakami, J.K. Estimativa do teor de cafeína nas sementes de café baseada na sua concentração nas folhas de mudas e de plantas adultas. Acta Sci. Agron. 2004, 26, 287–292. [Google Scholar] [CrossRef]
- Farah, A.; Ferreira, T.; Vieira, A.C. Trigonelline and Derivatives. In Coffee: Production, Quality and Chemistry, 1st ed.; Farah, A., Ed.; Royal Society of Chemistry: London, UK, 2019; Volume 1, pp. 627–628. [Google Scholar] [CrossRef]
- Ashihara, H.; Ludwig, I.A.; Katahira, R.; Yokota, T.; Fujimura, T.; Crozier, A. Trigonelline and related nicotinic acid metabolites: Occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem. Rev. 2014, 14, 765–798. [Google Scholar] [CrossRef]
- Tatefuji, T.; Izumi, N.; Ohta, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Isolation and identification of compounds from Brazilian propolis which enhance macrophage spreading and mobility. Biol. Pharm. Bull. 1996, 19, 966–970. [Google Scholar] [CrossRef]
- Takemura, T.; Urushisaki, T.; Fukuoka, M.; Hosokawa-Muto, J.; Hata, T.; Okuda, Y.; Hori, S.; Tazawa, S.; Araki, Y.; Kuwata, K. 3,4-Dicaffeoylquinic acid, a major constituent of Brazilian propolis, increases TRAIL expression and extends the lifetimes ofmice infected with the Influenza A virus. Evid. Based Complement. Altern. Med. 2012, 2012, 946867. [Google Scholar] [CrossRef]
- Wan, P.; Xie, M.; Chen, G.; Dai, Z.; Hu, B.; Zeng, X.; Sun, Y. Anti-inflammatory effects of dicaffeoylquinic acids from Ilex kudingcha on lipopolysaccharide-treated RAW 264.7 macrophages and potential mechanisms. Food Chem. Toxicol. 2019, 126, 332–342. [Google Scholar] [CrossRef]
- Hufnagel, M.; Rademaekers, A.; Weisert, A.; Häberlein, H.; Franken, S. Pharmacological profile of dicaffeoylquinic acids and their role in the treatment of respiratory diseases. Front. Pharmacol. 2024, 15, 1371613. [Google Scholar] [CrossRef]
- Robinson Jr, W.E.; Cordeiro, M.; Abdel-Malek, S.; Jia, Q.; Chow, S.A.; Reinecke, M.G.; Mitchell, W.M. Dicaffeoylquinic acid inhibitors of human immunodeficiency virus integrase: Inhibition of the core catalytic domain of human immunodeficiency virus integrase. Mol. Pharmacol. 1996, 50, 846–855. [Google Scholar] [PubMed]
- Boulebd, H.; Carmena-Bargueño, M.; Pérez-Sánchez, H. Exploring the antioxidant properties of caffeoylquinic and feruloylquinic acids: A computational study on hydroperoxyl radical scavenging and xanthine oxidase inhibition. Antioxidants 2023, 12, 1669. [Google Scholar] [CrossRef] [PubMed]
- Marques, V.; Farah, A. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chem. 2009, 113, 1370–1376. [Google Scholar] [CrossRef]
- Horman, I.; Viani, R. The nature and conformation of the caffeine-chlorogenate complex of coffee. J. Food Sci. 1972, 37, 925–927. [Google Scholar] [CrossRef]
- D’amelio, N.; Fontanive, L.; Uggeri, F.; Suggi-Liverani, F.; Navarini, L. NMR Reinvestigation of the caffeine–chlorogenate complex in cqueous solution and in coffee Brews. Food Biophys. 2009, 4, 321–330. [Google Scholar] [CrossRef]
- Smith, R.F. A History of Coffee. In Coffee: Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Wilson, K.C., Eds.; AVI Pub Co.: London, UK, 1985; pp. 1–12. [Google Scholar]
- Covre, A.M.; Partelli, F.L.; Mauri, A.L.; Dias, M.A. Initial growth and development of Conilon coffee genotypes. Rev. Agroambiente 2013, 7, 193–202. [Google Scholar]
- Charr, J.-C.; Garavito, A.; Guyeux, C.; Crouzillat, D.; Descombes, P.; Fournier, C.; Ly, S.N.; Raharimalala, E.N.; Rakotomalala, J.-J.; Stoffelen, P.; et al. Complex evolutionary history of coffees revealed by full plastidgenomes and 28,800 nuclear SNP analyses, with particular emphasis on Coffea canephora (Robusta coffee). Mol. Phylogenet. Evol. 2020, 151, 106906. [Google Scholar] [CrossRef]
- Salvador, H.P.; Berilli, A.P.C.G.; Rodrigues, W.P.; Mazzafera, P.; Partelli, F.L. A Climate Change Perspective on the Selection, Development, and Management of Coffea canephora Genotypes. In Coffee—A Glimpse into the Future; Damatta, F.M., Ramalho, J.C., Eds.; Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2025; ISBN 9780443222948. [Google Scholar]
- Charrier, A.; Berthaud, J. Variation de la teneur en caféine dans le genre Coffea. Café Cacao Thé 1991, 11, 251–264. [Google Scholar]
- Partelli, F.L.; Oliosi, G.; Farah, A.; de Paula, J.; de Oliveira, H.F.; Salvador, H.P. Salutar: First cultivar bred for soluble coffeeproduction and health. Funct. Plant Breed. J. 2022, 4, 59–65. [Google Scholar] [CrossRef]
- Associação Brasileira da Indústria de Café Solúvel (ABICS). Relatório do Café Solúvel do Brasil. 2024. Available online: https://www.abics.com.br/wp-content/uploads/2024/01/Relatorio-Cafe-Soluvel-Brasil-Janeiro-2024-1.pdf (accessed on 28 January 2025).
- European Food Safety Authority. Scientific Opinion on the Safety of Caffeine. 2015. Available online: http://www.efsa.europa.eu/en/efsajournal/pub/4102 (accessed on 28 January 2025).
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Moraes, G.J.L.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- AOAC International Publications. Official Methods of Analysis of AOAC International, 22nd ed.; Latimer, G.W., Jr., Ed.; AOAC: New York, NY, USA, 2023. [Google Scholar] [CrossRef]
- Trugo, L.C.; Macrae, R. Chlorogenic acid composition of instant coffees. Analyst 1984, 109, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Farah, A.; Monteiro, M.; Calado, V.; Franca, A.; Trugo, L. Correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Huynh-Ba, T. Preparation of Quinic Acid Derivatives. U.S. Patent 5,401,858, 28 March 1995. [Google Scholar]
- Industry ARC—Analytics, Research and Consulting. Caffeine Market Forecast (2025–2031). 2024. Available online: https://www.industryarc.com/Research/Caffeine-Market-Research-504527 (accessed on 28 January 2025).

| Seeds | Husks | Leaves | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Genotype | Crop 1 | Crop 2 | Crop 3 | Crop 1 | Crop 2 | Crop 3 | Crop 1 | Crop 2 | Crop 3 |
| Verdim R | 8.9 ± 0.2 | 8.4 ± 0.2 | 8.54 ± 0.3 | 11.2 ± 0.2 | 11.0 ± 0.2 | 11.1 ± 0.3 | 10.1 ± 0.2 | 9.6 ± 0.1 | 9.8 ± 0.1 |
| B01 | 8.7 ± 0.2 | 8.5 ± 0.2 | 8.6 ± 0.2 | 10.3 ± 0.2 | 10.6 ± 0.1 | 10.6 ± 0.1 | 10.3 ± 0.1 | 10.5 ± 0.2 | 10.2 ± 0.2 |
| Bicudo | 8.6 ± 0.1 | 8.4 ± 0.2 | 8.4 ± 0.1 | 11.1 ± 0.3 | 11.0 ± 0.1 | 10.9 ± 0.3 | 10.4 ± 0.2 | 10.1 ± 0.1 | 10.0 ± 0.2 |
| Alecrim | 9.0 ± 0.1 | 8.7 ± 0.2 | 8.8 ± 0.1 | 11.1 ± 0.3 | 10.7 ± 0.0 | 10.9 ± 0.2 | 9.9 ± 0.1 | 9.7 ± 0.1 | 9.5 ± 0.2 |
| 700 | 9.1 ± 0.0 | 9.0 ± 0.1 | 9.2 ± 0.2 | 11.2 ± 0.3 | 10.6 ± 0.0 | 10.5 ± 0.1 | 10.8 ± 0.1 | 10.5 ± 0.1 | 10.1 ± 0.3 |
| CH1 | 8.9 ± 0.2 | 9.0 ± 0.2 | 8.9 ± 0.1 | 11.3 ± 0.2 | 10.7 ± 0.0 | 10.9 ± 0.2 | 11.0 ± 0.2 | 10.3 ± 0.1 | 10.2 ± 0.2 |
| Imbigudinho | 8.7 ± 0.1 | 9.0 ± 0.3 | 9.2 ± 0.3 | 11.2 ± 0.2 | 11.2 ± 0.1 | 11.0 ± 0.1 | 11.0 ± 0.3 | 10.2 ± 0.1 | 10.1 ± 0.3 |
| AT | 8.8 ± 0.1 | 8.5 ± 0.2 | 8.7 ± 0.3 | 11.8 ± 0.2 | 11.6 ± 0.2 | 11.4 ± 0.2 | 10.1 ± 0.2 | 10.0 ± 0.2 | 10.5 ± 0.3 |
| Graudão HP | 9.1 ± 0.1 | 9.2 ± 0.3 | 9.0 ± 0.2 | 11.2 ± 0.1 | 10.4 ± 0.2 | 10.2 ± 0.2 | 10.1 ± 0.2 | 10.0 ± 0.0 | 9.7 ± 0.4 |
| Valcir P | 9.2 ± 0.3 | 9.0 ± 0.1 | 8.9 ± 0.3 | 11.2 ± 0.1 | 10.5 ± 0.2 | 10.4 ± 0.3 | 10.4 ± 0.1 | 10.1 ± 0.0 | 10.2 ± 0.0 |
| Beira Rio 8 | 8.6 ± 0.1 | 8.3 ± 0.1 | 8.5 ± 0.1 | 11.4 ± 0.1 | 10.3 ± 0.2 | 10.2 ± 0.3 | 10.2 ± 0.1 | 10.1 ± 0.2 | 10.0 ± 0.3 |
| Tardio V | 8.7 ± 0.1 | 8.4 ± 0.1 | 8.3 ± 0.1 | 11.3 ± 0.1 | 11.3 ± 0.1 | 11.0 ± 0.2 | 10.6 ± 0.1 | 10.8 ± 0.1 | 10.5 ± 0.2 |
| AP | 8.8 ± 0.0 | 8.9 ± 0.1 | 9.0 ± 0.1 | 10.8 ± 0.2 | 11.3 ± 0.2 | 11.5 ± 0.2 | 11.1 ± 0.3 | 10.7 ± 0.1 | 10.6 ± 0.1 |
| L80 | 8.9 ± 0.0 | 8.9 ± 0.1 | 9.2 ± 0.3 | 11.3 ± 0.2 | 11.5 ± 0.3 | 11.1 ± 0.2 | 10.7 ± 0.3 | 10.5 ± 0.1 | 10.5 ± 0.1 |
| Bamburral | 9.2 ± 0.1 | 9.0 ± 0.0 | 9.2 ± 0.0 | 10.7 ± 0.1 | 11.3 ± 0.2 | 11.0 ± 0.3 | 10.8 ± 0.2 | 10.9 ± 0.2 | 11.0 ± 0.1 |
| Pirata | 9.5 ± 0.1 | 9.4 ± 0.3 | 9.3 ± 0.2 | 12.7 ± 0.2 | 12.0 ± 0.2 | 12.5 ± 0.2 | 9.9 ± 0.1 | 10.0 ± 0.0 | 10.3 ± 0.2 |
| Peneirão | 8.4 ± 0.1 | 8.7 ± 0.1 | 8.9 ± 0.2 | 10.7 ± 0.1 | 11.4 ± 0.2 | 11.0 ± 0.1 | 9.9 ± 0.1 | 10.0 ± 0.1 | 10.2 ± 0.2 |
| Z39 | 8.2 ± 0.1 | 8.0 ± 0.2 | 8.4 ± 0.3 | 11.4 ± 0.1 | 11.0 ± 0.1 | 11.2 ± 0.3 | 10.1 ± 0.0 | 10.4 ± 0.1 | 10.5 ± 0.3 |
| Z35 | 8.0 ± 0.0 | 8.2 ± 0.1 | 8.0 ± 0.4 | 10.9 ± 0.0 | 10.8 ± 0.1 | 10.9 ± 0.2 | 10.5 ± 0.0 | 10.3 ± 0.1 | 10.1 ± 0.4 |
| Z40 | 8.5 ± 0.2 | 8.7 ± 0.1 | 8.5 ± 0.2 | 10.7 ± 0.0 | 11.1 ± 0.2 | 11.0 ± 0.3 | 11.2 ± 0.0 | 11.0 ± 0.3 | 11.1 ± 0.2 |
| Z29 | 8.6 ± 0.2 | 8.9 ± 0.1 | 9.0 ± 0.4 | 11.4 ± 0.1 | 11.0 ± 0.1 | 11.2 ± 0.3 | 10.1 ± 0.0 | 10.4 ± 0.1 | 10.5 ± 0.3 |
| Z38 | 8.8 ± 0.1 | 9.1 ± 0.2 | 9.2 ± 0.3 | 11.2 ± 0.1 | 11.7 ± 0.2 | 11.1 ± 0.4 | 10.9 ± 0.2 | 11.0 ± 0.2 | 11.2 ± 0.1 |
| Z18 | 9.2 ± 0.2 | 9.1 ± 0.1 | 9.3 ± 0.3 | 11.3 ± 0.1 | 11.5 ± 0.2 | 11.2 ± 0.1 | 10.5 ± 0.3 | 10.7 ± 0.2 | 10.4 ± 0.1 |
| Z37 | 8.2 ± 0.1 | 8.4 ± 0.1 | 8.5 ± 0.3 | 11.2 ± 0.1 | 11.6 ± 0.2 | 11.4 ± 0.0 | 10.1 ± 0.2 | 10.3 ± 0.1 | 10.3 ± 0.2 |
| Z21 | 9.1 ± 0.1 | 9.1 ± 0.2 | 9.0 ± 0.4 | 10.8 ± 0.0 | 11.3 ± 0.3 | 11.2 ± 0.4 | 11.0 ± 0.4 | 10.7 ± 0.1 | 11.0 ± 0.2 |
| Z36 | 8.3 ± 0.1 | 8.3 ± 0.2 | 8.6 ± 0.2 | 10.7 ± 0.2 | 11.2 ± 0.2 | 11.1 ± 0.2 | 9.8 ± 0.2 | 10.0 ± 0.0 | 10.2 ± 0.1 |
| Ouro negro | 8.2 ± 0.1 | 8.2 ± 0.1 | 8.3 ± 0.3 | 12.7 ± 0.2 | 12.5 ± 0.3 | 12.2 ± 0.4 | 9.5 ± 0.2 | 10.2 ± 0.1 | 10.3 ± 0.1 |
| 18 | 8.4 ± 0.2 | 8.4 ± 0.2 | 8.5 ± 0.4 | 11.0 ± 0.1 | 11.3 ± 0.1 | 11.1 ± 0.3 | 9.9 ± 0.2 | 10.2 ± 0.2 | 10.3 ± 0.2 |
| Tardio C | 9.0 ± 0.2 | 9.1 ± 0.3 | 9.2 ± 0.4 | 10.8 ± 0.1 | 11.0 ± 0.1 | 11.3 ± 0.3 | 10.3 ± 0.1 | 10.5 ± 0.1 | 10.1 ± 0.2 |
| A1 | 8.5 ± 0.1 | 8.5 ± 0.3 | 8.3 ± 0.2 | 13.4 ± 0.2 | 12.5 ± 0.1 | 12.3 ± 0.4 | 10.1 ± 0.1 | 10.1 ± 0.1 | 10.2 ± 0.1 |
| Cheique | 9.2 ± 0.1 | 9.0 ± 0.0 | 9.4 ± 0.2 | 10.9 ± 0.1 | 11.1 ± 0.1 | 10.8 ± 0.2 | 10.4 ± 0.1 | 10.4 ± 0.1 | 10.5 ± 0.1 |
| P2 | 9.3 ± 0.2 | 9.4 ± 0.4 | 9.0 ± 0.4 | 10.6 ± 0.1 | 10.7 ± 0.1 | 10.5 ± 0.4 | 10.6 ± 0.2 | 10.5 ± 0.1 | 10.4 ± 0.2 |
| Emcapa 02 | 8.7 ± 0.0 | 8.5 ± 0.2 | 8.4 ± 0.3 | 10.8 ± 0.2 | 10.9 ± 0.2 | 11.1 ± 0.1 | 10.2 ± 0.2 | 10.2 ± 0.1 | 10.1 ± 0.3 |
| Emcapa 153 | 8.5 ± 0.0 | 8.6 ± 0.1 | 8.3 ± 0.3 | 10.6 ± 0.2 | 10.4 ± 0.2 | 10.5 ± 0.3 | 10.4 ± 0.1 | 10.4 ± 0.1 | 10.2 ± 0.1 |
| P1 | 8.2 ± 0.1 | 8.0 ± 0.2 | 8.5 ± 0.3 | 10.9 ± 0.2 | 11.2 ± 0.2 | 11.4 ± 0.2 | 9.8 ± 0.0 | 10.1 ± 0.1 | 10.3 ± 0.1 |
| LB1 | 8.1 ± 0.0 | 8.1 ± 0.2 | 8.5 ± 0.4 | 10.7 ± 0.1 | 10.5 ± 0.1 | 10.3 ± 0.2 | 10.9 ± 0.1 | 10.3 ± 0.1 | 10.4 ± 0.3 |
| 122 | 9.2 ± 0.2 | 8.7 ± 0.0 | 9.2 ± 0.3 | 11.1 ± 0.1 | 11.6 ± 0.3 | 11.1 ± 0.2 | 11.1 ± 0.1 | 11.0 ± 0.1 | 11.1 ± 0.2 |
| Verdim D | 9.2 ± 0.2 | 9.2 ± 0.3 | 9.3 ± 0.3 | 11.1 ± 0.1 | 11.6 ± 0.3 | 11.5 ± 0.2 | 10.9 ± 0.1 | 11.1 ± 0.1 | 11.2 ± 0.1 |
| Emcapa 143 | 8.5 ± 0.2 | 8.6 ± 0.1 | 8.5 ± 0.3 | 10.6 ± 0.1 | 10.9 ± 0.3 | 10.5 ± 0.3 | 10.3 ± 0.1 | 10.4 ± 0.0 | 10.4 ± 0.3 |
| Ouro negro 1 | 9.0 ± 0.1 | 9.2 ± 0.0 | 9.0 ± 0.4 | 11.4 ± 0.1 | 11.5 ± 0.1 | 11.3 ± 0.0 | 10.8 ± 0.1 | 10.7 ± 0.0 | 10.5 ± 0.3 |
| Ouro negro 2 | 8.4 ± 0.1 | 8.7 ± 0.1 | 9.0 ± 0.3 | 11.3 ± 0.1 | 11.5 ± 0.1 | 11.1 ± 0.2 | 11.0 ± 0.2 | 10.7 ± 0.1 | 10.8 ± 0.1 |
| Clementino | 8.7 ± 0.1 | 8.5 ± 0.0 | 8.8 ± 0.3 | 12.3 ± 0.2 | 12.0 ± 0.2 | 11.7 ± 0.4 | 11.2 ± 0.2 | 10.7 ± 0.1 | 11.0 ± 0.3 |
| Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) | Genotype | Crop 1 | Crop 2 | Crop 3 | Mean * | CV (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Verdim R | 3.6 ± 0.2 a | 3.8 ± 0.1 a | 3.3 ± 0.2 b | 3.6 ± 0.3 | 7.28 | Z38 | 3.9 ± 0.0 b | 4.0 ± 0.1 a | 3.5 ± 0.1 c | 3.8 ± 0.3 | 6.77 |
| B01 | 4.1 ± 0.1 b | 4.4 ± 0.1 a | 4.1 ± 0.1 b | 4.2 ± 0.2 | 4.12 | Z18 | 3.8 ± 0.0 a | 4.1 ± 0.2 a | 4.1 ± 0.2 a | 4.0 ± 0.2 | 4.78 |
| Bicudo | 4.1 ± 0.1 a | 4.3 ± 0.0 a | 3.9 ± 0.2 b | 4.1 ± 0.3 | 5.33 | Z17 | 4.2 ± 0.1 b | 4.6 ± 0.2 a | 4.5 ± 0.1 a | 4.5 ± 0.2 | 4.66 |
| Alecrim | 4.0 ± 0.0 a | 4.1 ± 0.1 a | 3.6 ± 0.2 b | 3.9 ± 0.3 | 7.27 | Z21 | 3.9 ± 0.1 a | 3.8 ± 0.1 a | 3.9 ± 0.1 a | 3.9 ± 0.1 | 1.31 |
| 700 | 3.6 ± 0.1 a | 3.7 ± 0.0 a | 3.5 ± 0.2 a | 3.6 ± 0.1 | 2.45 | Z36 | 4.5 ± 0.1 a | 4.4 ± 0.0 a | 4.5 ± 0.1 a | 4.5 ± 0.1 | 1.55 |
| CH1 | 3.2 ± 0.0 b | 3.5 ± 0.1 a | 3.1 ± 0.1 b | 3.3 ± 0.2 | 7.07 | Ouro negro | 4.0 ± 0.1 a | 4.2 ± 0.0 a | 3.9 ± 0.3 a | 4.1 ± 0.1 | 3.32 |
| Imbigudinho | 3.5 ± 0.1 a | 3.7 ± 0.1 a | 3.5 ± 0.2 a | 3.5 ± 0.1 | 3.02 | 18 | 3.5 ± 0.1 b | 3.9 ± 0.0 a | 3.9 ± 0.1 a | 3.8 ± 0.2 | 6.13 |
| AT | 3.2 ± 0.1 a | 3.3 ± 0.0 a | 2.9 ± 0.1 b | 3.1 ± 0.2 | 7.45 | Tardio C | 4.4 ± 0.1 b | 4.9 ± 0.0 a | 4.9 ± 0.0 a | 4.7 ± 0.3 | 6.52 |
| Graudão HP | 4.2 ± 0.1 b | 4.7 ± 0.1 a | 4.3 ± 0.2 b | 4.4 ± 0.3 | 5.97 | A1 | 4.3 ± 0.1 a | 3.7 ± 0.1 b | 3.8 ± 0.0 b | 3.9 ± 0.3 | 7.85 |
| Valcir P | 3.8 ± 0.0 b | 4.2 ± 0.2 a | 3.9 ± 0.1 ab | 4.0 ± 0.2 | 4.84 | Cheique | 4.1 ± 0.1 a | 4.2 ± 0.0 a | 3.7 ± 0.2 b | 4.0 ± 0.2 | 6.01 |
| Beira Rio 8 | 3.8 ± 0.1 b | 4.1 ± 0.1 a | 3.9 ± 0.0 ab | 3.9 ± 0.2 | 4.28 | P2 | 3.9 ± 0.2 b | 4.1 ± 0.0 ab | 4.3 ± 0.1 a | 4.1 ± 0.2 | 4.47 |
| Tardio V | 4.4 ± 0.1 a | 4.4 ± 0.1 a | 4.3 ± 0.1 a | 4.4 ± 0.1 | 1.93 | Emcapa 02 | 4.7 ± 0.1 b | 5.0 ± 0.2 a | 4.4 ± 0.1 b | 4.7 ± 0.3 | 6.42 |
| AP | 4.2 ± 0.2 a | 4.3 ± 0.1 a | 4.0 ± 0.1 b | 4.2 ± 0.2 | 4.54 | Emcapa 153 | 3.7 ± 0.1 b | 3.8 ± 0.1 a | 3.6 ± 0.0 b | 3.7 ± 0.1 | 3.25 |
| L80 | 3.4 ± 0.1 a | 3.3 ± 0.1 a | 3.0 ± 0.2 b | 3.2 ± 0.2 | 5.84 | P1 | 4.0 ± 0.2 a | 3.7 ± 0.1 a | 3.9 ± 0.2 a | 3.9 ± 0.2 | 3.92 |
| Bamburral | 3.4 ± 0.1 a | 3.3 ± 0.0 b | 3.0 ± 0.2 c | 3.2 ± 0.2 | 7.43 | LB1 | 3.4 ± 0.2 ab | 3.2 ± 0.1 b | 3.7 ± 0.2 a | 3.5 ± 0.3 | 7.26 |
| Pirata | 3.8 ± 0.1 a | 3.9 ± 0.0 a | 3.5 ± 0.3 a | 3.7 ± 0.2 | 4.97 | 122 | 4.1 ± 0.2 a | 4.0 ± 0.0 a | 4.0 ± 0.3 a | 4.0 ± 0.1 | 2.19 |
| Peneirão | 4.2 ± 0.1 b | 4.6 ± 0.1 a | 4.1 ± 0.3 b | 4.3 ± 0.3 | 6.76 | Verdim D | 4.2 ± 0.3 a | 4.2 ± 0.0 a | 3.8 ± 0.2 a | 4.1 ± 0.3 | 6.17 |
| Z39 | 3.7 ± 0.1 a | 3.8 ± 0.1 a | 3.3 ± 0.2 b | 3.6 ± 0.2 | 6.32 | Emcapa 143 | 4.0 ± 0.2 a | 4.0 ± 0.1 a | 3.6 ± 0.2 b | 3.9 ± 0.2 | 5.53 |
| Z35 | 4.2 ± 0.2 a | 4.2 ± 0.2 a | 3.9 ± 0.3 a | 4.1 ± 0.2 | 4.26 | Ouro negro 1 | 3.9 ± 0.2 a | 3.5 ± 0.0 a | 3.5 ± 0.5 a | 3.6 ± 0.3 | 6.86 |
| Z40 | 4.0 ± 0.1 a | 4.1 ± 0.1 a | 3.7 ± 0.1 b | 3.9 ± 0.2 | 5.29 | Ouro negro 2 | 3.7 ± 0.2 b | 3.8 ± 0.0 b | 4.1 ± 0.1 a | 3.9 ± 0.2 | 5.51 |
| Z29 | 4.2 ± 0.1 a | 4.0 ± 0.1 a | 3.6 ± 0.1 b | 3.9 ± 0.3 | 6.87 | Clementino | 3.9 ± 0.2 a | 3.9 ± 0.0 a | 3.6 ± 0.1 b | 3.8 ± 0.2 | 4.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DePaula, J.; Partelli, F.L.; Batista, A.M.; Calado, V.; Farah, A. Major Bioactive Compounds in Seeds, Husks, and Leaves of Selected Genotypes of Coffea canephora cv. Conilon from Three Consecutive Crops. Plants 2025, 14, 1040. https://doi.org/10.3390/plants14071040
DePaula J, Partelli FL, Batista AM, Calado V, Farah A. Major Bioactive Compounds in Seeds, Husks, and Leaves of Selected Genotypes of Coffea canephora cv. Conilon from Three Consecutive Crops. Plants. 2025; 14(7):1040. https://doi.org/10.3390/plants14071040
Chicago/Turabian StyleDePaula, Juliana, Fábio Luiz Partelli, Alessandro M. Batista, Veronica Calado, and Adriana Farah. 2025. "Major Bioactive Compounds in Seeds, Husks, and Leaves of Selected Genotypes of Coffea canephora cv. Conilon from Three Consecutive Crops" Plants 14, no. 7: 1040. https://doi.org/10.3390/plants14071040
APA StyleDePaula, J., Partelli, F. L., Batista, A. M., Calado, V., & Farah, A. (2025). Major Bioactive Compounds in Seeds, Husks, and Leaves of Selected Genotypes of Coffea canephora cv. Conilon from Three Consecutive Crops. Plants, 14(7), 1040. https://doi.org/10.3390/plants14071040








