Harnessing Light Quality for Potato Production: Red and Blue Light as Key Regulators of Growth and Yield
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Light Quality Settings
2.3. Measurement of Morphological and Yield Trait Profiling
2.4. Measurement of Photosynthetic Parameters
2.5. Measurement of Stomatal Parameters
2.6. Measurement of Chlorophyll Content
2.7. Leaf Anatomical Structure Observation
2.8. Data Analysis
3. Results
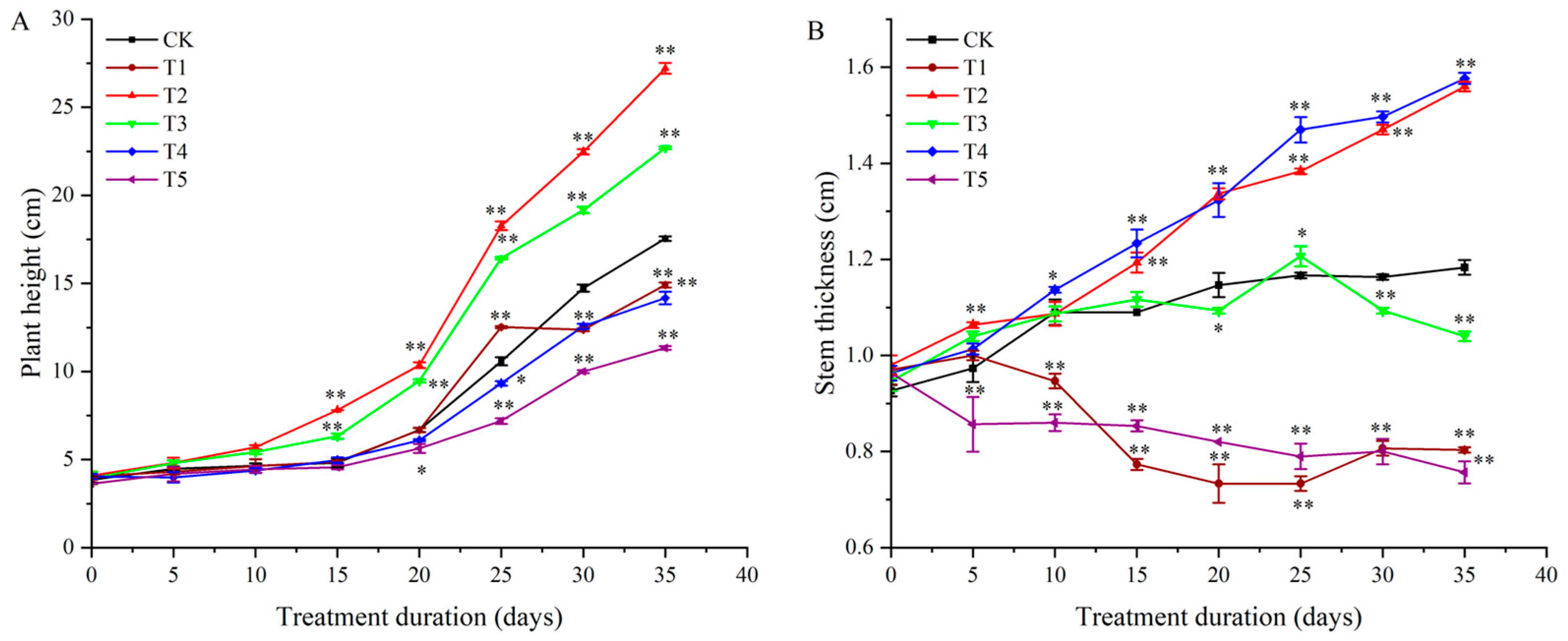
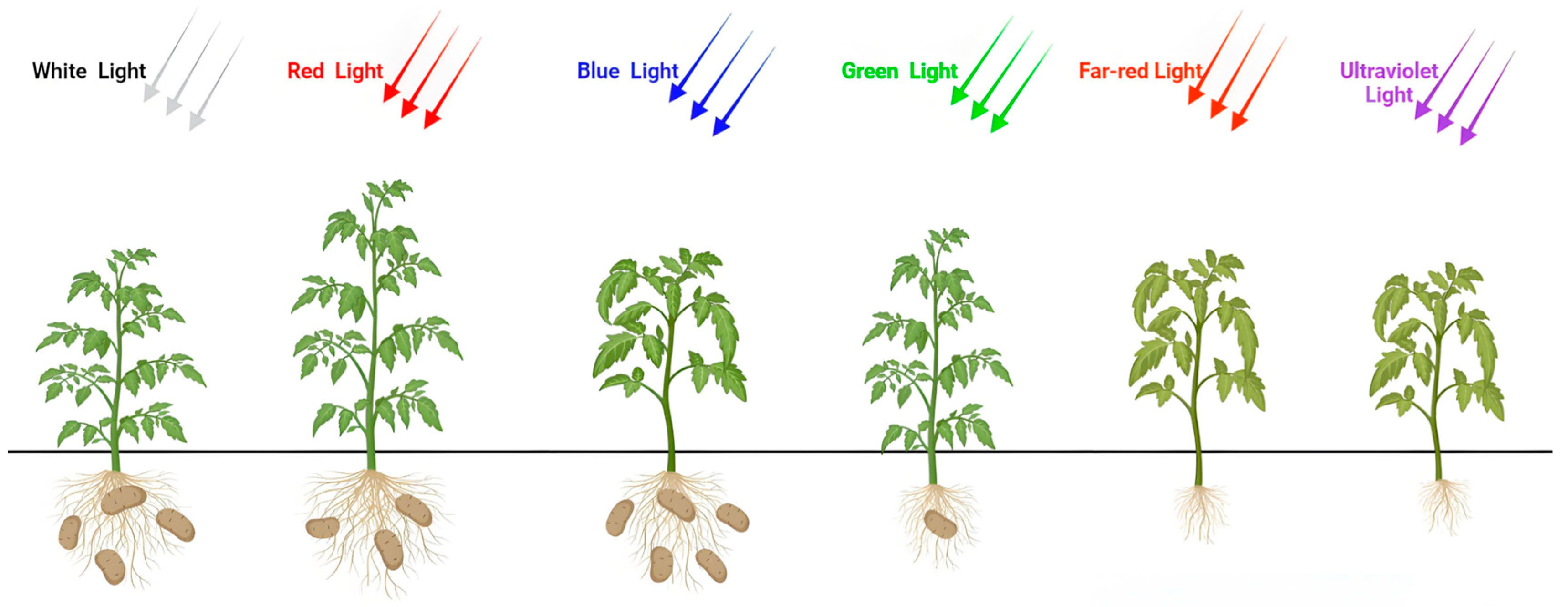
3.1. Effects of Light Quality on Potato Plant Growth
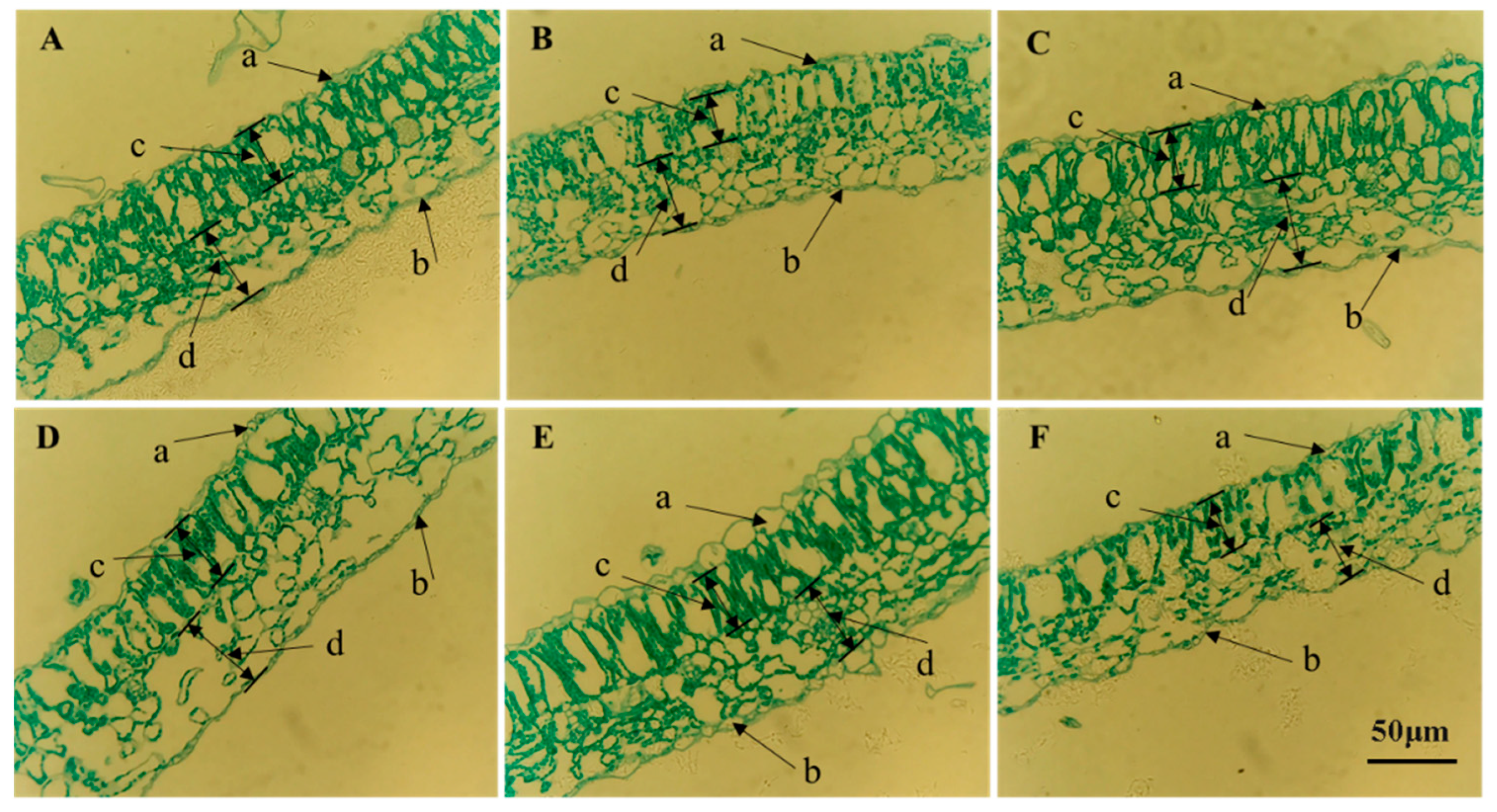
3.2. Effects of Light Quality on Anatomical Structure of Potato Leaves
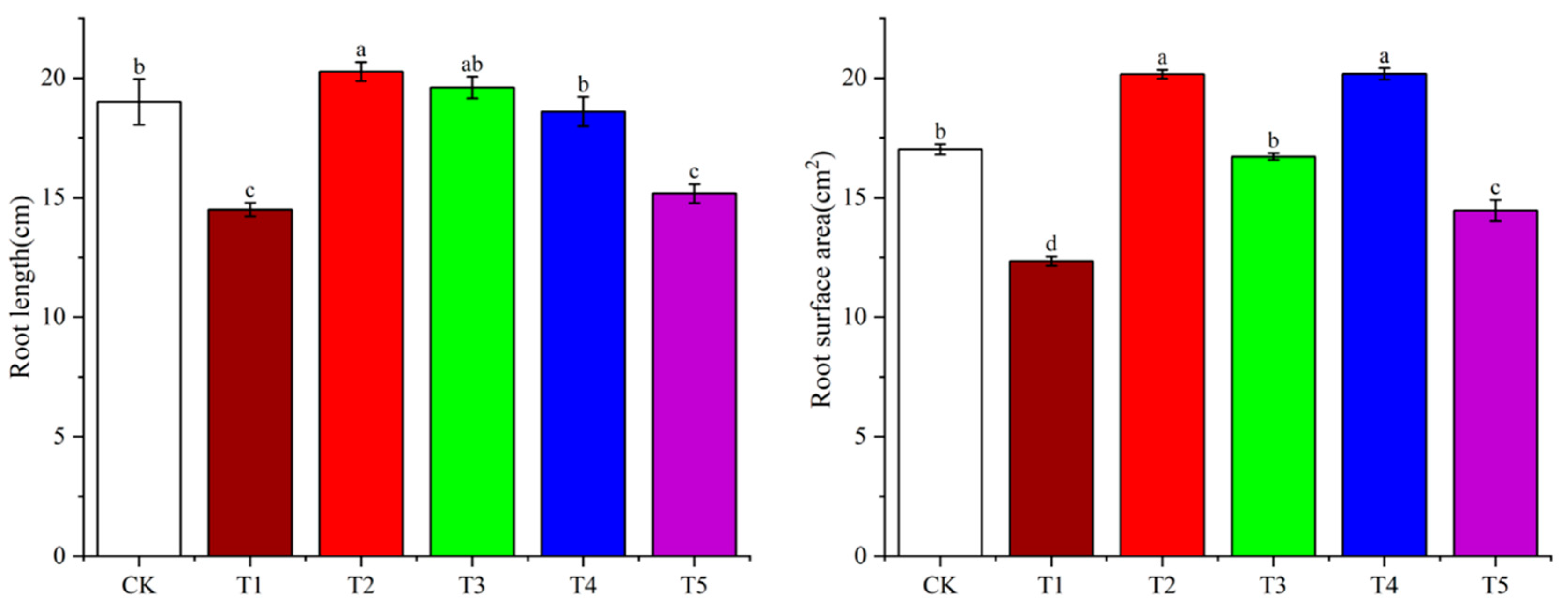
3.3. Effects of Light Quality on Potato Root Growth
3.4. Effects of Light Quality on Photosynthetic Characteristics of Potato Leaves
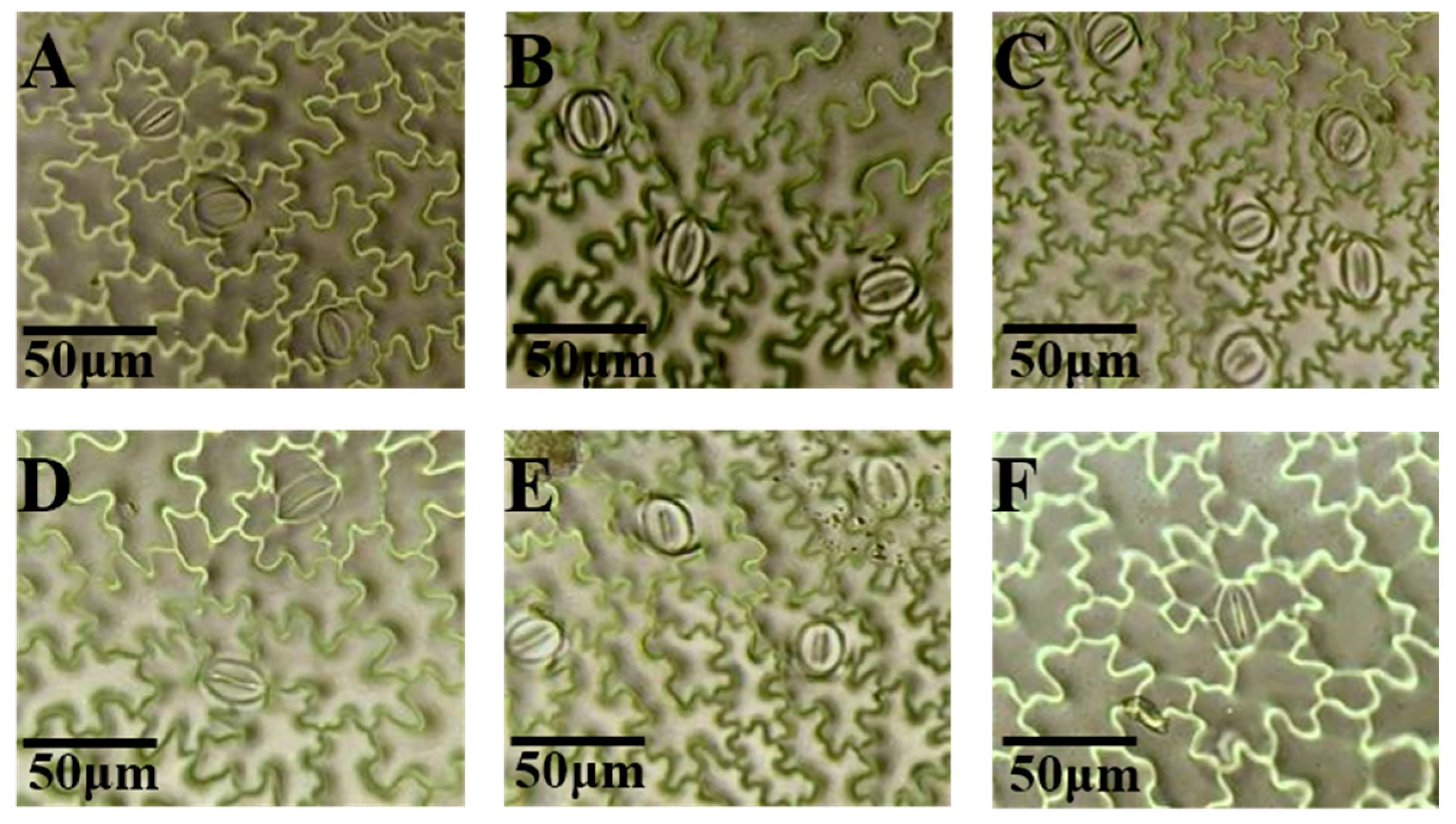
3.5. Effects of Light Quality on Stomatal Characteristics of Potato Leaves
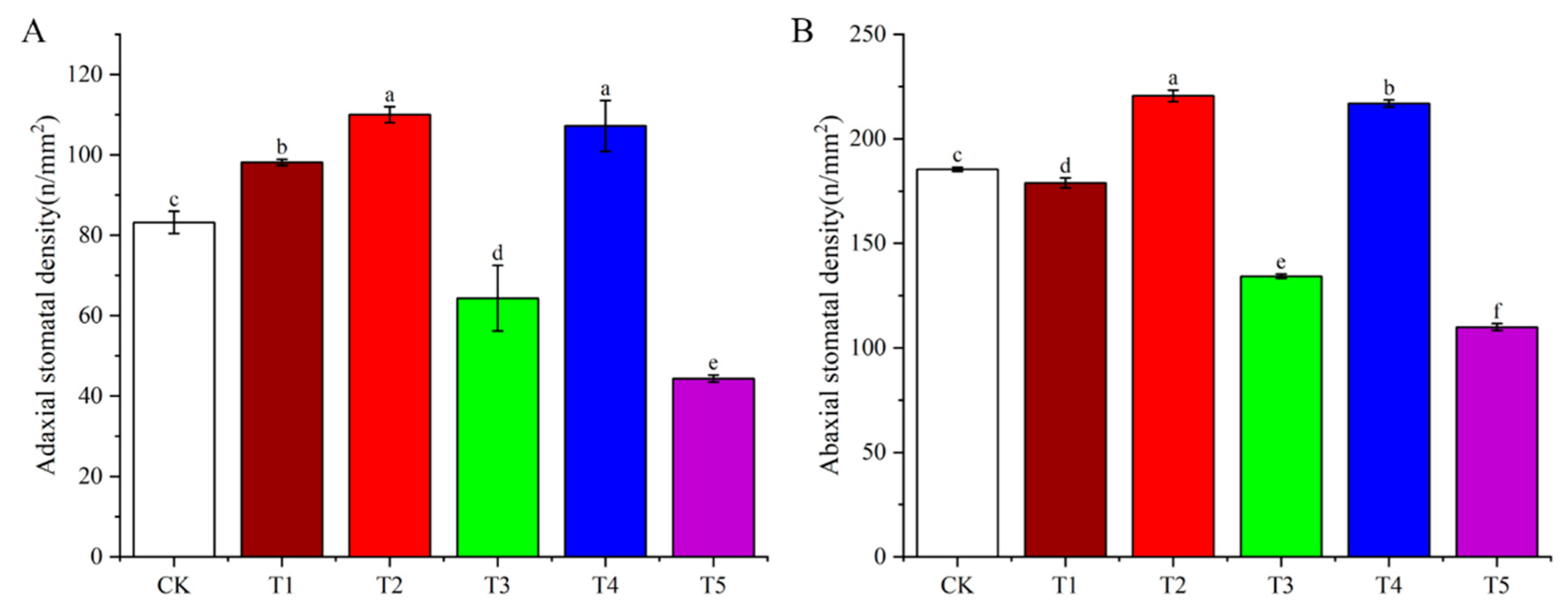
3.6. Effects of Light Quality on Stomatal Density of Potato Leaves
3.7. Effects of Light Quality on Tuber Yield Traits
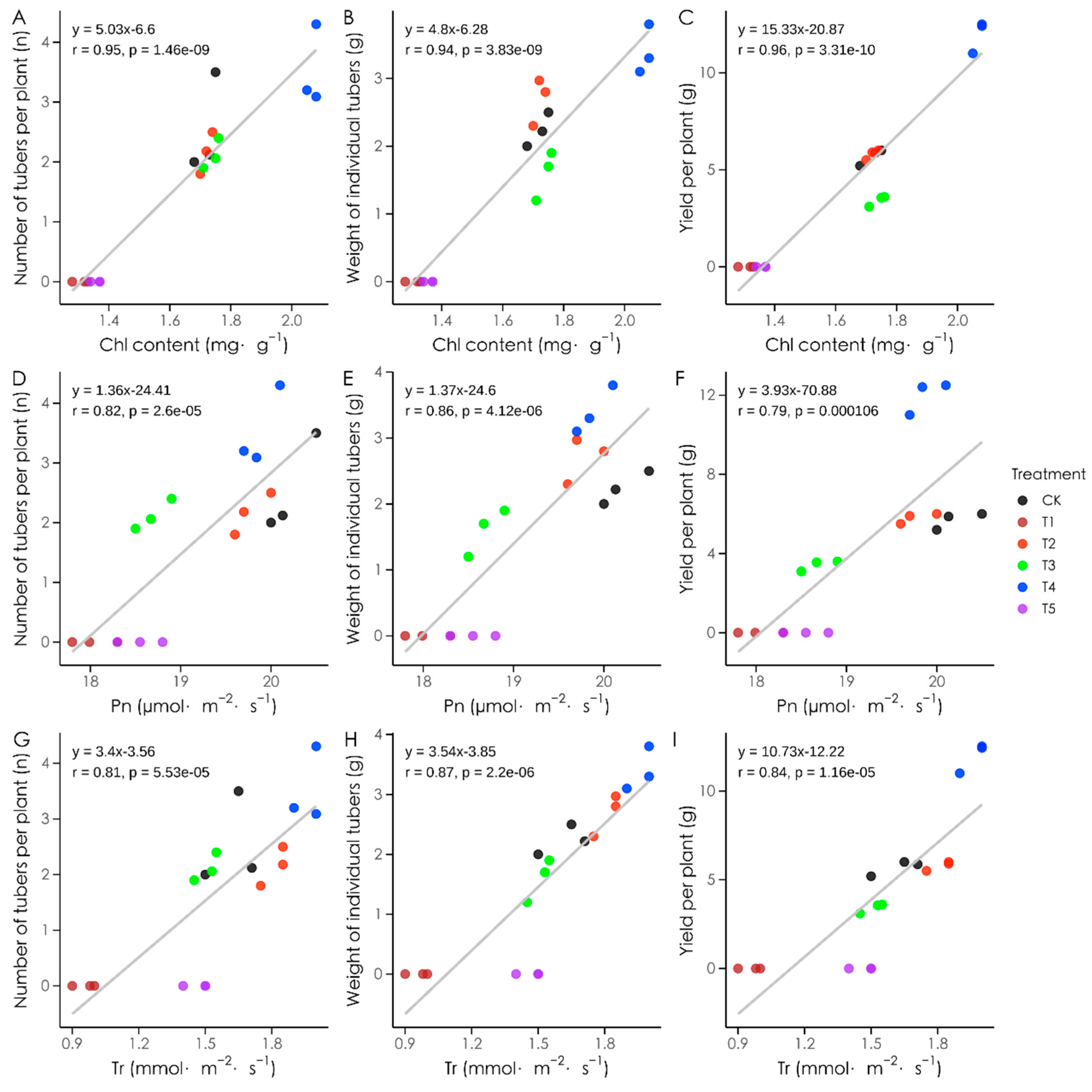
3.8. Correlations Between Photosynthetic Characteristics and Yield Traits Under Different Light Qualities
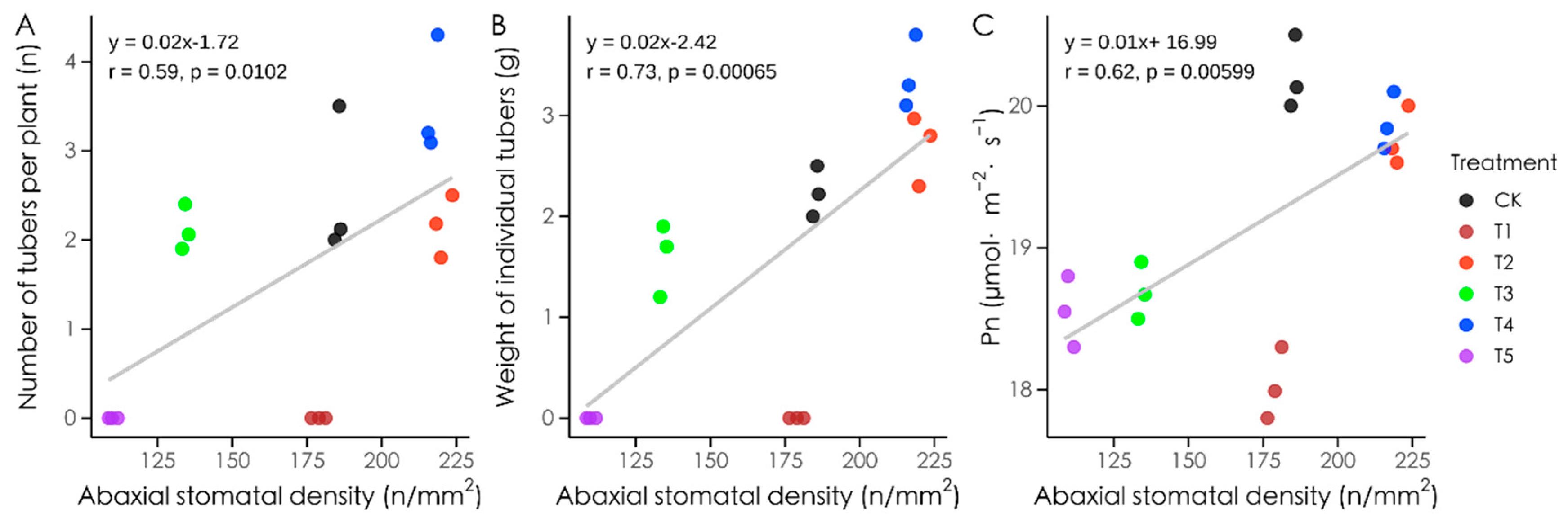
3.9. Correlations Between Stomatal Density and Yield Traits Under Different Light Qualities
4. Discussion
4.1. Effects of Light Quality on Morphological Development of Potatoes
4.2. Effects of Light Quality on Photosynthetic Properties of Potatoes
4.3. Effects of Light Quality on Leaf Structure and Stomatal Characteristics of Potatoes
4.4. Effects of Light Quality on Yield Traits of Potato
4.5. Correlations Between Photosynthetic Characteristics and Yield Traits Under Different Light Qualities
4.6. Future Research Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Kaiser, E.; Zhang, Y.; Yang, Q.; Li, T. Red/blue light ratio strongly affects steady-state photosynthesis, but hardly affects photosynthetic induction in tomato (Solanum lycopersicum). Physiol. Plant. 2019, 167, 144–158. [Google Scholar] [CrossRef]
- Westbrook, A.S.; McAdam, S.A.M. Atavistic Stomatal Responses to Blue Light in Marsileaceae. Plant Physiol. 2020, 184, 1378–1388. [Google Scholar] [CrossRef]
- Khoshimkhujaev, B.; Kwon, J.K.; Park, K.S.; Choi, H.G.; Lee, S.Y. Effect of monochromatic UV-A LED irradiation on the growth of tomato seedlings. Hortic. Environ. Biotechnol. 2014, 55, 287–292. [Google Scholar]
- Liu, M.; Sun, W.; Ma, Z.; Guo, C.; Chen, J.; Wu, Q.; Wang, X.; Chen, H. Integrated network analyses identify MYB4R1 neofunctionalization in the UV-B adaptation of Tartary buckwheat. Plant Commun. 2022, 3, 100414. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Bakshi, S.; Yadukrishnan, P.; Lingwan, M.; Dolde, U.; Wenkel, S.; Masakapalli, S.K.; Datta, S. The B-Box-Containing MicroProtein miP1a/BBX31 Regulates Photomorphogenesis and UV-B Protection. Plant Physiol. 2019, 179, 1876–1892. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, L.; Jin, D.; Nezames, C.D.; Terzaghi, W.; Deng, X.W. UV-B-induced photomorphogenesis in Arabidopsis. Protein Cell 2013, 4, 485–492. [Google Scholar]
- Lanoue, J.; Little, C.; Hawley, D.; Hao, X.M. Addition of green light improves fruit weight and dry matter content in sweet pepper due to greater light penetration within the canopy. Sci. Hortic. 2022, 304, 111350. [Google Scholar] [CrossRef]
- Silvia, F.; Talbott, L.D.; Bogomolni, R.A.; Eduardo, Z. Reversal of Blue Light-Stimulated Stomatal Opening by Green Light. Plant Cell Physiol. 2000, 41, 171–176. [Google Scholar] [CrossRef]
- Aasamaa, K.T.; Aphalo, P.; Botany, E. Effect of vegetational shade and its components on stomatal responses to red, blue and green light in two deciduous tree species with different shade tolerance. Environ. Exp. Bot. 2016, 121, 94–101. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic Quantum Yield Dynamics: From Photosystems to Leaves. Plant Cell 2012, 24, 1921–1935. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, H.; Ma, M.; Li, Q.; Wang, H. Arabidopsis FHY3 and FAR1 Proteins Regulate the Balance between Growth and Defense Responses Under Shade Conditions. Plant Cell 2019, 31, 2089–2106. [Google Scholar] [CrossRef] [PubMed]
- Smith, H. Phytochromes and light signal perception by plants—An emerging synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, M.; Yabe, N.; Ichikawa, T.; Yamamoto, Y.Y.; Matsui, M. DFL1, an auxin-responsive GH3 gene homologue, negatively regulates shoot cell elongation and lateral root formation, and positively regulates the light response of hypocotyl length. Plant J. 2010, 25, 213–221. [Google Scholar] [CrossRef]
- Sperling, U.; Cleve, B.V.; Frick, G.; Apel, K.; Armstrong, G. Overexpression of light-dependent PORA or PORB in plants depleted of endogenous POR by far-red light enhances seedling survival in white light and protects against photooxidative damage. Plant J. 2010, 12, 649–658. [Google Scholar] [CrossRef]
- Lyu, X.; Cheng, Q.; Qin, C.; Li, Y.; Xu, X.; Ji, R.; Mu, R.; Li, H.; Zhao, T.; Liu, J.; et al. GmCRY1s modulate gibberellin metabolism to regulate soybean shade avoidance in response to reduced blue light. Mol. Plant 2021, 14, 298–314. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Kobayashi, K.; Fujii, S.; Fukaki, H.; Mitsuda, N.; Ohme-Takagi, M. Blue Light Regulates Phosphate Deficiency-Dependent Primary Root Growth Inhibition in Arabidopsis. Front. Plant Sci. 2020, 10, 1803. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, W.; Zhao, D.; Song, Y.; Lin, X.; Shen, M.; Chi, C.; Xu, B.; Zhao, J.; Deng, X.W.; et al. Light-induced remodeling of phytochrome B enables signal transduction by phytochrome-interacting factor. Cell 2024, 187, 6235–6250. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kong, X.; Hu, K.; Cao, M.; Liu, J.; Ma, C.; Guo, S.; Yuan, X.; Zhao, S.; Robert, H.S.; et al. PIFs coordinate shade avoidance by inhibiting auxin repressor ARF18 and metabolic regulator QQS. New Phytol. 2020, 228, 609–621. [Google Scholar] [CrossRef]
- Bi, X.; Xu, H.; Yang, C.; Zhang, H.; Li, W.; Su, W.; Zheng, M.; Lei, B. Investigating the influence of varied ratios of red and far-red light on lettuce (Lactuca sativa): Effects on growth, photosynthetic characteristics and chlorophyll fluorescence. Front. Plant Sci. 2024, 15, 1430241. [Google Scholar] [CrossRef]
- Li, L.; Tong, Y.X.; Lu, J.L.; Li, Y.M.; Liu, X.; Cheng, R.F. Morphology, Photosynthetic Traits, and Nutritional Quality of Lettuce Plants as Affected by Green Light Substituting Proportion of Blue and Red Light. Front. Plant Sci. 2021, 12, 627311. [Google Scholar] [CrossRef]
- Wissanee, P.; Sumiko, S.; Songsin, P. Color Development and Phytochemical Changes in Mature Green Chili (Capsicum annuum L.) Exposed to Red and Blue Light-Emitting Diodes. J. Agric. Food Chem. 2020, 68, 59–66. [Google Scholar]
- Miao, Y.; Chen, Q.; Qu, M.; Gao, L.; Hou, L. Blue light alleviates ‘red light syndrome’ by regulating chloroplast ultrastructure, photosynthetic traits and nutrient accumulation in cucumber plants. Sci. Hortic. 2019, 257, 108680. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, L.; Wu, Y.; Tang, Z.; Xiao, X.; Lyu, J.; Xie, J.; Yu, J. Green Light Partial Replacement of Red and Blue Light Improved Drought Tolerance by Regulating Water Use Efficiency in Cucumber Seedlings. Front. Plant Sci. 2022, 13, 878932. [Google Scholar] [CrossRef]
- Wassenaar, M.L.J.; van Ieperen, W.; Driever, S.M. Low Red to Far-red ratio increases resistance to CO2 diffusion and reduces photosynthetic efficiency in low light grown tomato plants. Environ. Exp. Bot. 2022, 200, 104918. [Google Scholar] [CrossRef]
- Tang, D.; Jia, Y.; Zhang, J.; Li, H.; Cheng, L.; Wang, P.; Bao, Z.; Liu, Z.; Feng, S.; Zhu, X.; et al. Genome evolution and diversity of wild and cultivated potatoes. Nature 2022, 606, 535–541. [Google Scholar] [PubMed]
- Chen, L.; Zhang, K.; Gong, X.; Wang, H.; Gao, Y.; Wang, X.; Zeng, Z.; Hu, Y. Effects of different LEDs light spectrum on the growth, leaf anatomy, and chloroplast ultrastructure of potato plantlets in vitro and minituber production after transplanting in the greenhouse. J. Integr. Agric. 2020, 19, 108–119. [Google Scholar]
- Lee, S.H.; Won, H.J.; Ban, S.; Choi, H.; Jung, J.H. Tomato Fruit Growth and Nutrient Accumulation in Response to Blue and Red Light Treatments during the Reproductive Growth Stage. Horticulturae 2023, 9, 1113. [Google Scholar] [CrossRef]
- Jishi, T.; Matsuda, R.; Fujiwara, K. Manipulation of Intraday Durations of Blue- and Red-Light Irradiation to Improve Cos Lettuce Growth. Front. Plant Sci. 2021, 12, 778205. [Google Scholar] [CrossRef]
- Li, X.; Zhao, S.; Cao, Q.; Qiu, C.; Yang, Y.; Zhang, G.; Wu, Y.; Yang, Z. Effect of Green Light Replacing Some Red and Blue Light on Cucumis melo under Drought Stress. Int. J. Mol. Sci. 2024, 25, 7561. [Google Scholar] [CrossRef]
- Jeong, S.J.; Niu, G.; Zhen, S. Far-red light and temperature interactively regulate plant growth and morphology of lettuce and basil. Environ. Exp. Bot. 2024, 218, 105589. [Google Scholar] [CrossRef]
- Hayes, S.; Velanis, C.N.; Jenkins, G.I.; Franklin, K.A. UV-B detected by the UVR8 photoreceptor antagonizes auxin signaling and plant shade avoidance. Proc. Natl. Acad. Sci. USA 2014, 111, 11894–11899. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A. Shade avoidance. New Phytol. 2010, 179, 930–944. [Google Scholar] [CrossRef]
- Skinner, R.H. Modulation of leaf elongation, tiller appearance and tiller senescence in spring barley by far-red light. Plant Cell Environ. 2010, 16, 555–562. [Google Scholar] [CrossRef]
- Casal, J.J. Photoreceptor Signaling Networks in Plant Responses to Shade. Annu. Rev. Plant Biol. 2013, 64, 403. [Google Scholar] [CrossRef]
- Beall, F.D.; Yeung, E.C.; Pharis, R.P. Far-red light stimulates internode elongation, cell division, cell elongation, and gibberellin levels in bean. Rev. Can. Bot. 1996, 74, 743–752. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Z.; Chang, T. Growth and Photosynthesis of Cherry Tomato Seedling Exposed to Different Low Light of LED Light Quality. Acta Bot. Boreali-Occident. Sin. 2010, 30, 725–732. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, K.; Wang, Y.; Wang, H.; Ding, S.; Song, D.; Shen, J.; Li, H.; Song, Y.; Han, X.; et al. Red and Blue Light Affect the Formation of Adventitious Roots of Tea Cuttings (Camellia sinensis) by Regulating Hormone Synthesis and Signal Transduction Pathways of Mature Leaves. Front. Plant Sci. 2022, 13, 943662. [Google Scholar] [CrossRef]
- Vlcko, T.; Tarkowska, D.; Siroka, J.; Pencik, A.; Simersky, R.; Chamrad, I.; Lenobel, R.; Novak, O.; Ohnoutkova, L. Hormone profiling and the root proteome analysis of itpk1 mutant seedlings of barley (Hordeum vulgare) during the red-light induced. Environ. Exp. Bot. 2023, 213, 105428. [Google Scholar] [CrossRef]
- Li, R.; You, J.; Miao, C.; Kong, L.; Long, J.; Yan, Y.; Xu, Z.; Liu, X. Monochromatic lights regulate the formation, growth, and dormancy of in vitro-grown Solanum tuberosum L. microtubers. Sci. Hortic. 2020, 261, 108947. [Google Scholar] [CrossRef]
- Liu, X.; Yang, M.; Xie, X.; Khaldun AB, M.; Atak, A.; Zhong, C.; Li, D. Effect of light on growth and chlorophyll development in kiwifruit ex vitro and in vitro. Sci. Hortic. 2022, 291, 110599. [Google Scholar] [CrossRef]
- Yamazaki, J.Y. Is light quality involved in the regulation of the photosynthetic apparatus in attached rice leaves? Photosynth. Res. 2010, 105, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Su, X.; Li, Y.; Shi, M.; Yang, B.; Wan, W.; Wen, X.; Yang, S.; Ding, X.; Zou, J. Effect of Red and Blue Light on Cucumber Seedlings Grown in a Plant Factory. Horticulturae 2023, 9, 124. [Google Scholar] [CrossRef]
- Ji, T.; Du, Y.; Wei, M.; Gu, D.; Li, J.; Wang, H.; Yang, F. Adding different proportions of red/blue = 3/1 to white light affects eggplant seedling quality by regulating leaf morphology and photosynthetic system Plant Growth Regulation. Plant Growth Regul. 2023, 100, 593–607. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.; Xu, Z.; Jiao, X.; Tezuka, T. Regulation of Chloroplast Ultrastructure, Cross-section Anatomy of Leaves, and Morphology of Stomata of Cherry Tomato by Different Light Irradiations of Light-emitting Diodes. Hortscience 2011, 46, 217–221. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Vereshchagin, M.; Kreslavski, V.; Ivanov, Y.; Kumachova, T.; Ryabchenko, A.; Voronkov, A.; Kosobryukhov, A.; Kuznetsov, V.; Allakhverdiev, S.I. Effect of Phytochrome Deficiency on Photosynthesis, Light-Related Genes Expression and Flavonoid Accumulation in Solanum lycopersicum under Red and Blue Light. Cells 2022, 11, 3437. [Google Scholar] [CrossRef]
- Matthews, J.S.A.; Vialet-Chabrand, S.; Lawson, T. Role of blue and red light in stomatal dynamic behaviour. J. Exp. Bot. 2020, 71, 2253–2269. [Google Scholar] [CrossRef]
- Bernardo, E.L.; Sales, C.R.G.; Cubas, L.A.; Vath, R.L.; Kromdijk, J. A comparison of stomatal conductance responses to blue and red light between C3 and C4 photosynthetic species in three phylogenetically-controlled experiments. Front. Plant Sci. 2023, 14, 1253976. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kim, J. Light Quality Affects Water Use of Sweet Basil by Changing Its Stomatal Development. Agronomy 2021, 11, 303. [Google Scholar] [CrossRef]
- Saeedi, S.A.; Vahdati, K.; Sarikhani, S.; Daylami, S.D.; Davarzani, M.; Gruda, N.S.; Aliniaeifard, S. Growth, photosynthetic function, and stomatal characteristics of Persian walnut explants in vitro under different light spectra. Front. Plant Sci. 2023, 14, 1292045. [Google Scholar] [CrossRef]
- Kang, C.Y.; Lian, H.L.; Wang, F.F.; Huang, J.R.; Yang, H.Q. Cryptochromes, Phytochromes, and COP1 Regulate Light-Controlled Stomatal Development in Arabidopsis. Plant Cell 2009, 21, 2624–2641. [Google Scholar] [CrossRef]
- Terfa, M.T.; Olsen, J.E.; Torre, S. Blue Light Improves Stomatal Function and Dark-Induced Closure of Rose Leaves (Rosa hybrida) Developed at High Air Humidity. Front. Plant Sci. 2020, 11, 1036. [Google Scholar] [CrossRef]
- Trojak, M.; Skowron, E.; Sobala, T.; Kocurek, M.; Palyga, J. Effects of partial replacement of red by green light in the growth spectrum on photomorphogenesis and photosynthesis in tomato plants. Photosynth. Res. 2022, 151, 295–312. [Google Scholar] [PubMed]
- He, W.; Chai, Q.; Zhang, D.; Li, W.; Zhao, C.; Yin, W.; Fan, H.; Yu, A.; Hu, F.; Fan, Z. Beneficial effects of red and blue light on potato leaf antioxidant capacity and tuber bulking. Physiol. Mol. Biol. Plants 2023, 29, 513–523. [Google Scholar]
- Rahman, M.H.; Azad, M.O.K.; Islam, M.J.; Rana, M.S.; Li, K.-H.; Lim, Y.S. Production of Potato (Solanum tuberosum L.) Seed Tuber under Artificial LED Light Irradiation in Plant Factory. Plants 2021, 10, 297. [Google Scholar] [CrossRef]
- He, Z.G. Effects of Red and Blue Light with Supplemental White Light on Growth, Carbohydrate Metabolism, and Yield of Virus-Free Potato in Plant Factories. Am. J. Potato Res. 2020, 97, 554–564. [Google Scholar]
- Navarro, C.; Abelenda, J.A.; Cruz-Oró, E.; Cuéllar, C.A.; Tamaki, S.; Silva, J.; Shimamoto, K.; Prat, S. Control of flowering and storage organ formation in potato by FLOWERING LOCUS T. Nature 2011, 478, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Falcón, M.; Bou, J.; Biology, S. Seasonal Control of Tuberization in Potato: Conserved Elements with the Flowering Response. Annu. Rev. Plant Biol. 2005, 57, 151–180. [Google Scholar] [CrossRef]
- Jutta, B.; Edwards, G.E. Photosynthetic Efficiency, and Photodamage by UV and Visible Radiation, in Red versus Green Leaf Coleus Varieties. Plant Cell Physiol. 1996, 37, 395–399. [Google Scholar] [CrossRef]
- Walters, M.B.; Reich, P.B. Low-light carbon balance and shade tolerance in the seedlings of woody plants: Do winter deciduous and broad-leaved evergreen species differ? New Phytol. 2010, 143, 143–154. [Google Scholar] [CrossRef]
- Jin, Y.J.; Zhu, H.S.; Wang, J.Y.; Li, C.D.; Tang, Z.S.; Yang, Q.H.; Zhang, L.; Liang, L.; Liu, J.; Yu, B. Effects of different light qualities on the growth and photosynthetic properties of potatoes. J. Gansu Agric. Univ. 2024, 59, 45–53. [Google Scholar] [CrossRef]
- Trebst, A. Electron Transfer in Photosynthetic Systems: Energy Conservation in Photosynthetic Electron Transport of Chloroplasts—ScienceDirect. Living Syst. Energy Convert. 1977, 209–220. [Google Scholar] [CrossRef]
- Sun, W.; Berry, J.A.; Yakir, D.; Seibt, U. Leaf relative uptake of carbonyl sulfide to CO2 seen through the lens of stomatal conductance–photosynthesis coupling. New Phytol. 2022, 235, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Moshelion, M.; Halperin, O.; Wallach, R.; Oren, R.; Way, D.A. Role of aquaporins in determining transpiration and photosynthesis in water-stressed plants: Crop water-use efficiency, growth and yield. Plant Cell Environ. 2015, 38, 1785–1793. [Google Scholar] [CrossRef] [PubMed]



| Treatment Number | CK | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| Different light qualities | White light | Far-red light | Red light | Green light | Blue light | Ultraviolet light |
| Wavelengths (nm) | 400–760 | 740 | 660 | 520 | 450 | 390 |
| FWHM (nm) | 21–150 | 100 | 24 | 30 | 26 | 30 |
| Variety | Treatment | Chl (mg/g) | Pn (μmol·m−2·s−1) | Tr (mmol·m−2·s−1) |
|---|---|---|---|---|
| Atlantic | CK | 1.72 ± 0.01 b | 20.21 ± 0.22 a | 1.62 ± 0.02 bc |
| T1 | 1.31 ± 0.04 c | 18.03 ± 0.40 d | 0.96 ± 0.05 d | |
| T2 | 1.72 ± 0.05 b | 19.77 ± 0.16 b | 1.82 ± 0.04 ab | |
| T3 | 1.74 ± 0.04 b | 18.69 ± 0.02 c | 1.51 ± 0.05 c | |
| T4 | 2.07 ± 0.05 a | 19.88 ± 0.02 ab | 1.97 ± 0.25 a | |
| T5 | 1.36 ± 0.01 c | 18.55 ± 0.16 c | 1.47 ± 0.06 c |
| Variety | Treatment | Adaxial Stomatal Length (μm) | Adaxial Stomatal Width (μm) | Abaxial Stomatal Length (μm) | Abaxial Stomatal Width (μm) |
|---|---|---|---|---|---|
| Atlantic | CK | 36.11 ± 1.94 ab | 20.35 ± 2.55 ab | 25.89 ± 2.67 bc | 16.15 ± 1.80 b |
| T1 | 35.75 ± 3.13 b | 22.53 ± 1.50 ab | 28.74 ± 3.61 ab | 17.28 ± 1.76 ab | |
| T2 | 34.10 ± 3.62 b | 21.99 ± 2.16 ab | 24.73 ± 3.00 bc | 16.36 ± 2.32 ab | |
| T3 | 34.45 ± 3.47 b | 24.57 ± 2.99 a | 22.42 ± 2.11 c | 17.30 ± 2.43 ab | |
| T4 | 42.09 ± 3.50 a | 23.74 ± 2.81 a | 33.13 ± 2.47 a | 20.92 ± 2.43 a | |
| T5 | 30.12 ± 2.01 b | 18.50 ± 1.25 b | 25.23 ± 3.00 bc | 15.48 ± 2.39 b |
| Variety | Treatment | Number of Tubers per Plant (n) | Weight of Individual Tubers (g) | Yield per Plant (g) |
|---|---|---|---|---|
| Atlantic | CK | 2.54 ± 0.12 b | 2.24 ± 0.07 b | 5.69 ± 0.09 b |
| T1 | —— | —— | —— | |
| T2 | 2.16 ± 0.14 c | 2.69 ± 0.12 b | 5.80 ± 0.20 b | |
| T3 | 2.12 ± 0.26 c | 1.6 ± 0.24 c | 3.42 ± 0.76 c | |
| T4 | 3.53 ± 0.12 a | 3.40 ± 0.53 a | 11.97 ± 1.72 a | |
| T5 | —— | —— | —— |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Jin, Y.; Liu, J.; Yang, H.; Cheng, L.; Yu, B. Harnessing Light Quality for Potato Production: Red and Blue Light as Key Regulators of Growth and Yield. Plants 2025, 14, 1039. https://doi.org/10.3390/plants14071039
Guo R, Jin Y, Liu J, Yang H, Cheng L, Yu B. Harnessing Light Quality for Potato Production: Red and Blue Light as Key Regulators of Growth and Yield. Plants. 2025; 14(7):1039. https://doi.org/10.3390/plants14071039
Chicago/Turabian StyleGuo, Rong, Yanjun Jin, Juan Liu, Hongyu Yang, Lixiang Cheng, and Bin Yu. 2025. "Harnessing Light Quality for Potato Production: Red and Blue Light as Key Regulators of Growth and Yield" Plants 14, no. 7: 1039. https://doi.org/10.3390/plants14071039
APA StyleGuo, R., Jin, Y., Liu, J., Yang, H., Cheng, L., & Yu, B. (2025). Harnessing Light Quality for Potato Production: Red and Blue Light as Key Regulators of Growth and Yield. Plants, 14(7), 1039. https://doi.org/10.3390/plants14071039






