The Extended Synaptotagmins of Physcomitrium patens
Abstract
1. Introduction
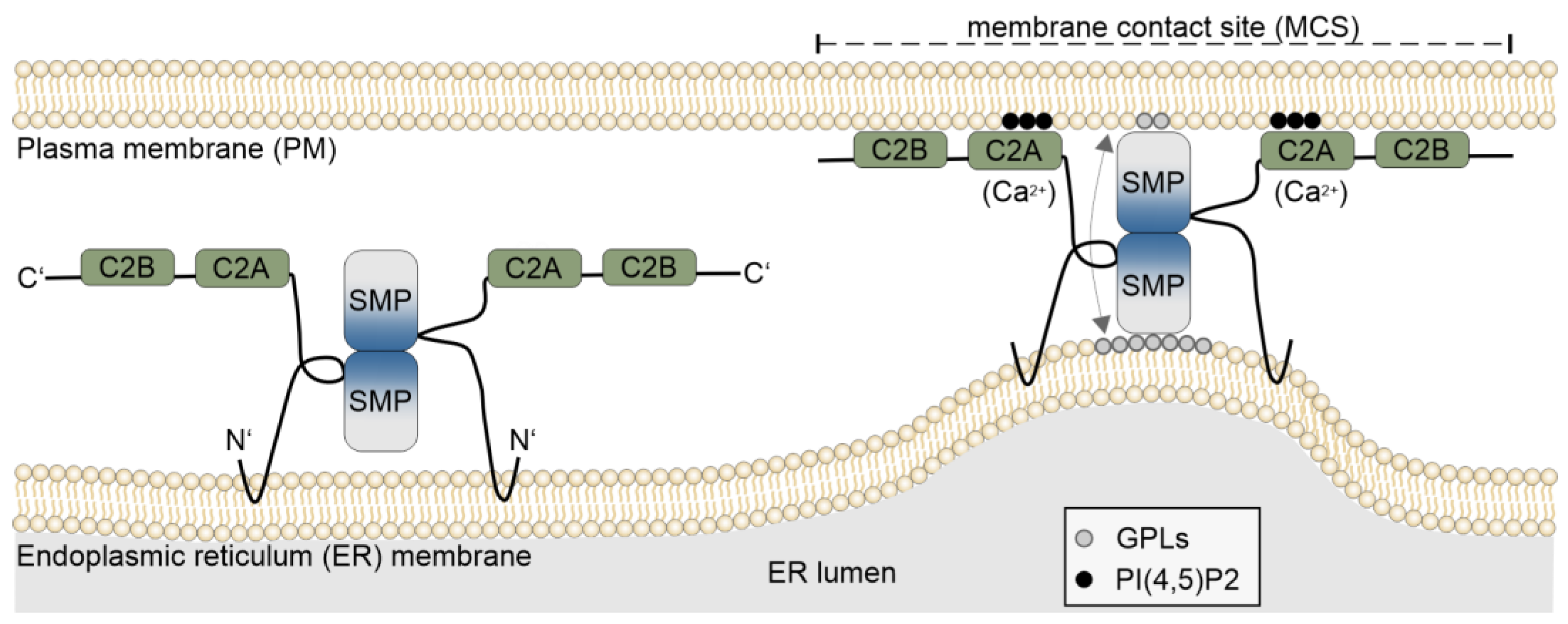
2. ESYTs Function as Tethers at Membrane Contact Sites
2.1. Membrane Contact Sites
2.2. Domain Structure and Functions of ESYTs
2.3. ESYTs in Arabidopsis thaliana
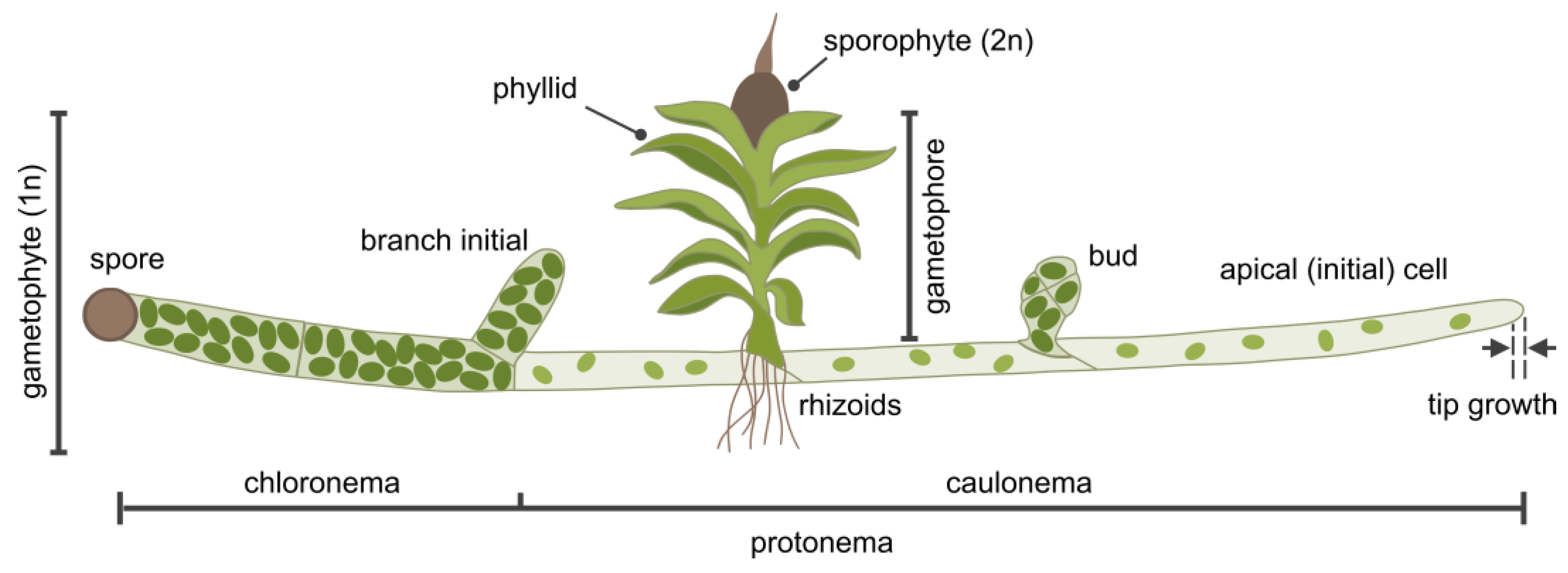
2.4. P. patens as a Model Organism for Membrane Dynamics
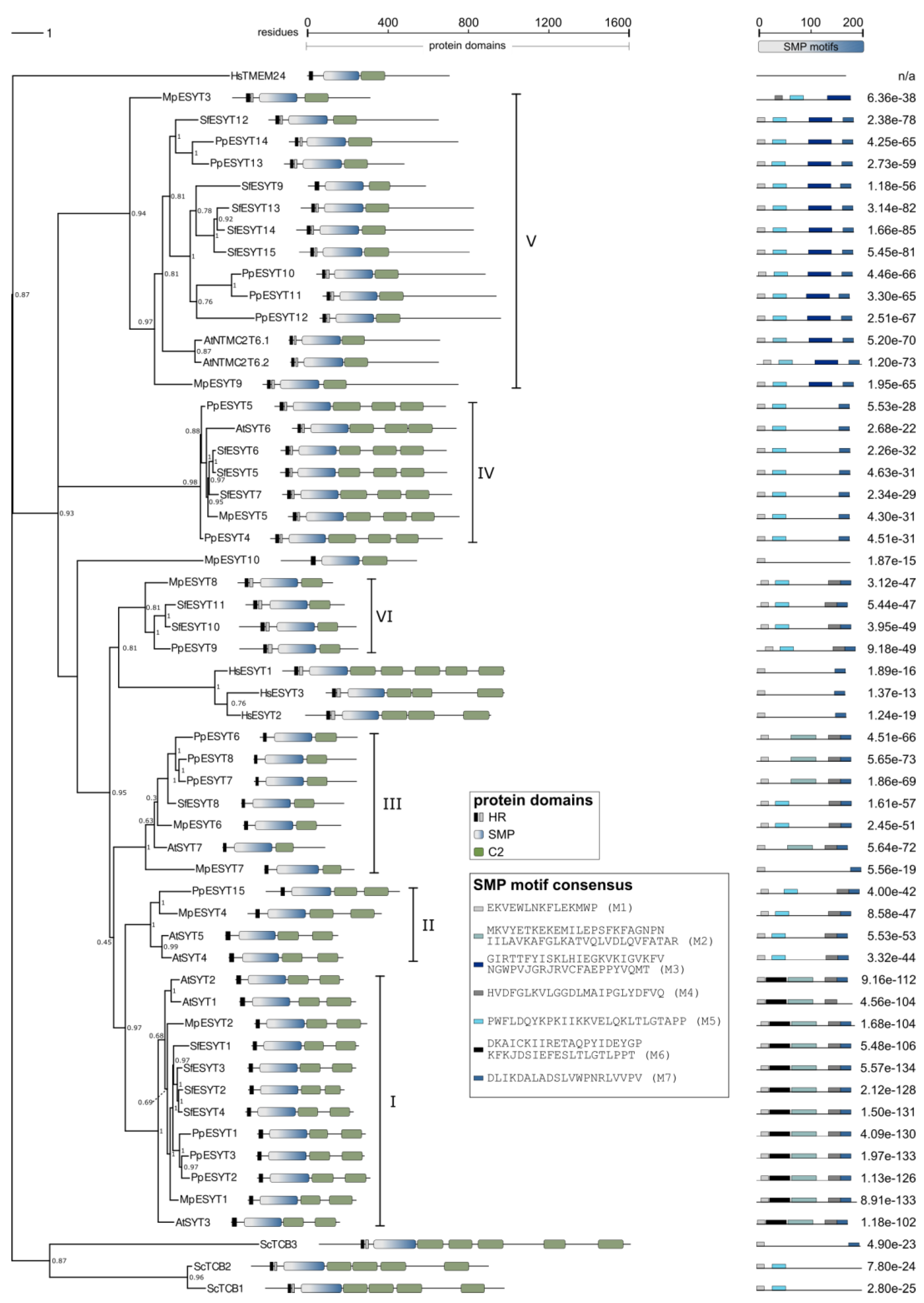
2.5. ESYTs in Bryophytes
| Class I | Class II | Class III | Class IV | Class V | Class VI | Total | |
|---|---|---|---|---|---|---|---|
| A. thaliana | 3 | 2 | 1 | 1 | 2 | 0 | 9 |
| M. polymorpha | 2 | 1 | 2 | 1 | 2 | 1 | 9 1 |
| S. fallax | 4 | 0 | 1 | 3 | 5 | 2 | 15 |
| P. patens | 3 | 1 | 3 | 2 | 5 | 1 | 15 |
3. Conclusions
4. Methods
4.1. Genome and Protein Database Search
4.2. Protein Domains and Motif Analysis
4.3. Multiple Sequence Alignment and Phylogenetic Tree Construction
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saheki, Y.; De Camilli, P. Endoplasmic Reticulum-Plasma Membrane Contact Sites. Annu. Rev. Biochem. 2017, 86, 659–684. [Google Scholar] [CrossRef] [PubMed]
- Toulmay, A.; Prinz, W.A. A conserved membrane-binding domain targets proteins to organelle contact sites. J. Cell Sci. 2012, 125, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Saheki, Y.; Idevall-Hagren, O.; Colombo, S.F.; Pirruccello, M.; Milosevic, I.; Gracheva, E.O.; Bagriantsev, S.N.; Borgese, N.; De Camilli, P. PI(4,5)P2-Dependent and Ca2+-Regulated ER-PM Interactions Mediated by the Extended Synaptotagmins. Cell 2013, 153, 1494–1509. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; De Camilli, P. The Extended-Synaptotagmins. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 1490–1493. [Google Scholar] [CrossRef]
- Schapire, A.L.; Voigt, B.; Jasik, J.; Rosado, A.; Lopez-Cobollo, R.; Menzel, D.; Salinas, J.; Mancuso, S.; Valpuesta, V.; Baluska, F.; et al. Arabidopsis synaptotagmin 1 is required for the maintenance of plasma membrane integrity and cell viability. Plant Cell 2008, 20, 3374–3388. [Google Scholar] [CrossRef]
- Benitez-Fuente, F.; Botella, M.A. Biological roles of plant synaptotagmins. Eur. J. Cell Biol. 2023, 102, 151335. [Google Scholar] [CrossRef]
- Rensing, S.A.; Goffinet, B.; Meyberg, R.; Wu, S.-Z.; Bezanilla, M. The Moss Physcomitrium (Physcomitrella) patens: A Model Organism for Non-Seed Plants. Plant Cell 2020, 32, 1361–1376. [Google Scholar] [CrossRef]
- Vidali, L.; Bezanilla, M. Physcomitrella patens: A model for tip cell growth and differentiation. Curr. Opin. Plant Biol. 2012, 15, 625–631. [Google Scholar] [CrossRef]
- Bi, G.; Zhao, S.; Yao, J.; Wang, H.; Zhao, M.; Sun, Y.; Hou, X.; Haas, F.B.; Varshney, D.; Prigge, M.; et al. Near telomere-to-telomere genome of the model plant Physcomitrium patens. Nat. Plants 2024, 10, 327–343. [Google Scholar] [CrossRef]
- Cove, D.J.; Perroud, P.-F.; Charron, A.J.; McDaniel, S.F.; Khandelwal, A.; Quatrano, R.S. The moss Physcomitrella patens: A novel model system for plant development and genomic studies. Cold Spring Harb. Protoc. 2009, 2009, pdb.emo115. [Google Scholar] [CrossRef]
- Grebnev, G.; Cvitkovic, M.; Fritz, C.; Cai, G.; Smith, A.-S.; Kost, B. Quantitative Structural Organization of Bulk Apical Membrane Traffic in Pollen Tubes. Plant Physiol. 2020, 183, 1559–1585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xing, J.; Lin, J. At the intersection of exocytosis and endocytosis in plants. New Phytol. 2019, 224, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Voeltz, G.K.; Sawyer, E.M.; Hajnóczky, G.; Prinz, W.A. Making the connection: How membrane contact sites have changed our view of organelle biology. Cell 2024, 187, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, W.; Rouiller, C. Close topographical relationship between mitochondria and ergastoplasm of liver cells in a definite phase of cellular activity. J. Biophys. Biochem. Cytol. 1956, 2, 73–78. [Google Scholar] [CrossRef]
- Rosenbluth, J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J. Cell Biol. 1962, 13, 405–421. [Google Scholar] [CrossRef]
- Lang, A.; John Peter, A.T.; Kornmann, B. ER–mitochondria contact sites in yeast: Beyond the myths of ERMES. Curr. Opin. Cell Biol. 2015, 35, 7–12. [Google Scholar] [CrossRef]
- Phillips, M.J.; Voeltz, G.K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 2016, 17, 69–82. [Google Scholar] [CrossRef]
- Venditti, R.; Masone, M.C.; De Matteis, M.A. ER-Golgi membrane contact sites. Biochem. Soc. Trans. 2020, 48, 187–197. [Google Scholar] [CrossRef]
- Renna, L.; Stefano, G.; Puggioni, M.P.; Kim, S.-J.; Lavell, A.; Froehlich, J.E.; Burkart, G.; Mancuso, S.; Benning, C.; Brandizzi, F. ER-associated VAP27-1 and VAP27-3 proteins functionally link the lipid-binding ORP2A at the ER-chloroplast contact sites. Nat. Commun. 2024, 15, 6008. [Google Scholar] [CrossRef]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming together to define membrane contact sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef]
- Bohnert, M. Tether Me, Tether Me Not—Dynamic Organelle Contact Sites in Metabolic Rewiring. Dev. Cell 2020, 54, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Tamura, K.; Fukao, Y.; Shimada, T. Structural and functional relationships between plasmodesmata and plant endoplasmic reticulum–plasma membrane contact sites consisting of three synaptotagmins. New Phytol. 2020, 226, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.C.; Bharat, T.A.M.; Wozny, M.R.; Boulanger, J.; Miller, E.A.; Kukulski, W. Tricalbins Contribute to Cellular Lipid Flux and Form Curved ER-PM Contacts that Are Bridged by Rod-Shaped Structures. Dev. Cell 2019, 51, 488–502.e8. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hawkins, T.J.; Richardson, C.; Cummins, I.; Deeks, M.J.; Sparkes, I.; Hawes, C.; Hussey, P.J. The Plant Cytoskeleton, NET3C, and VAP27 Mediate the Link between the Plasma Membrane and Endoplasmic Reticulum. Curr. Biol. 2014, 24, 1397–1405. [Google Scholar] [CrossRef]
- Peretti, D.; Dahan, N.; Shimoni, E.; Hirschberg, K.; Lev, S. Coordinated Lipid Transfer between the Endoplasmic Reticulum and the Golgi Complex Requires the VAP Proteins and Is Essential for Golgi-mediated Transport. Mol. Biol. Cell 2008, 19, 3871–3884. [Google Scholar] [CrossRef]
- Ye, H.; Gao, J.; Liang, Z.; Lin, Y.; Yu, Q.; Huang, S.; Jiang, L. Arabidopsis ORP2A mediates ER–autophagosomal membrane contact sites and regulates PI3P in plant autophagy. Proc. Natl. Acad. Sci. USA 2022, 119, e2205314119. [Google Scholar] [CrossRef]
- Rocha, N.; Kuijl, C.; van der Kant, R.; Janssen, L.; Houben, D.; Janssen, H.; Zwart, W.; Neefjes, J. Cholesterol sensor ORP1L contacts the ER protein VAP to control Rab7–RILP–p150Glued and late endosome positioning. J. Cell Biol. 2009, 185, 1209–1225. [Google Scholar] [CrossRef]
- Pérez-Sancho, J.; Smokvarska, M.; Dubois, G.; Glavier, M.; Sritharan, S.; Moraes, T.S.; Moreau, H.; Dietrich, V.; Platre, M.P.; Paterlini, A.; et al. Plasmodesmata act as unconventional membrane contact sites regulating intercellular molecular exchange in plants. Cell 2025, 188, 958–977.e3. [Google Scholar] [CrossRef]
- Zang, J.; Klemm, S.; Pain, C.; Duckney, P.; Bao, Z.; Stamm, G.; Kriechbaumer, V.; Bürstenbinder, K.; Hussey, P.J.; Wang, P. A novel plant actin-microtubule bridging complex regulates cytoskeletal and ER structure at ER-PM contact sites. Curr. Biol. 2021, 31, 1251–1260.e4. [Google Scholar] [CrossRef]
- Friedman, J.R.; Lackner, L.L.; West, M.; DiBenedetto, J.R.; Nunnari, J.; Voeltz, G.K. ER tubules mark sites of mitochondrial division. Science 2011, 334, 358–362. [Google Scholar] [CrossRef]
- Ji, W.-K.; Chakrabarti, R.; Fan, X.; Schoenfeld, L.; Strack, S.; Higgs, H.N. Receptor-mediated Drp1 oligomerization on endoplasmic reticulum. J. Cell Biol. 2017, 216, 4123–4139. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Duckney, P.; Zhang, T.; Fu, Y.; Li, X.; Kroon, J.; De Jaeger, G.; Cheng, Y.; Hussey, P.J.; Wang, P. TraB family proteins are components of ER-mitochondrial contact sites and regulate ER-mitochondrial interactions and mitophagy. Nat. Commun. 2022, 13, 5658. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lopez, N.; Pérez-Sancho, J.; del Valle, A.E.; Haslam, R.P.; Vanneste, S.; Catalá, R.; Perea-Resa, C.; Damme, D.V.; García-Hernández, S.; Albert, A.; et al. Synaptotagmins at the endoplasmic reticulum–plasma membrane contact sites maintain diacylglycerol homeostasis during abiotic stress. Plant Cell 2021, 33, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, K.M.; Prinz, W.A. Mechanisms of nonvesicular lipid transport. J. Cell Biol. 2021, 220, e202012058. [Google Scholar] [CrossRef]
- Roos, J.; DiGregorio, P.J.; Yeromin, A.V.; Ohlsen, K.; Lioudyno, M.; Zhang, S.; Safrina, O.; Kozak, J.A.; Wagner, S.L.; Cahalan, M.D.; et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005, 169, 435–445. [Google Scholar] [CrossRef]
- Yeromin, A.V.; Zhang, S.L.; Jiang, W.; Yu, Y.; Safrina, O.; Cahalan, M.D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 2006, 443, 226–229. [Google Scholar] [CrossRef]
- Vig, M.; Peinelt, C.; Beck, A.; Koomoa, D.L.; Rabah, D.; Koblan-Huberson, M.; Kraft, S.; Turner, H.; Fleig, A.; Penner, R.; et al. CRACM1 Is a Plasma Membrane Protein Essential for Store-Operated Ca2+ Entry. Science 2006, 312, 1220–1223. [Google Scholar] [CrossRef]
- Prakriya, M.; Feske, S.; Gwack, Y.; Srikanth, S.; Rao, A.; Hogan, P.G. Orai1 is an essential pore subunit of the CRAC channel. Nature 2006, 443, 230–233. [Google Scholar] [CrossRef]
- Wu, M.M.; Buchanan, J.; Luik, R.M.; Lewis, R.S. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006, 174, 803–813. [Google Scholar] [CrossRef]
- Putney, J.W. A model for receptor-regulated calcium entry. Cell Calcium 1986, 7, 1–12. [Google Scholar] [CrossRef]
- Coussens, L.; Parker, P.J.; Rhee, L.; Yang-Feng, T.L.; Chen, E.; Waterfield, M.D.; Francke, U.; Ullrich, A. Multiple, Distinct Forms of Bovine and Human Protein Kinase C Suggest Diversity in Cellular Signaling Pathways. Science 1986, 233, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Matthew, W.D.; Tsavaler, L.; Reichardt, L.F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J. Cell Biol. 1981, 91, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.R. How Does Synaptotagmin Trigger Neurotransmitter Release? Annu. Rev. Biochem. 2008, 77, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Maximov, A.; Shin, O.-H.; Dai, H.; Rizo, J.; Südhof, T.C. A Complexin/Synaptotagmin 1 Switch Controls Fast Synaptic Vesicle Exocytosis. Cell 2006, 126, 1175–1187. [Google Scholar] [CrossRef]
- Brose, N.; Petrenko, A.G.; Südhof, T.C.; Jahn, R. Synaptotagmin: A Calcium Sensor on the Synaptic Vesicle Surface. Science 1992, 256, 1021–1025. [Google Scholar] [CrossRef]
- Perin, M.S.; Fried, V.A.; Mignery, G.A.; Jahn, R.; Südhof, T.C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature 1990, 345, 260–263. [Google Scholar] [CrossRef]
- Sugita, S.; Shin, O.H.; Han, W.; Lao, Y.; Südhof, T.C. Synaptotagmins form a hierarchy of exocytotic Ca(2+) sensors with distinct Ca(2+) affinities. Embo J. 2002, 21, 270–280. [Google Scholar] [CrossRef]
- Min, S.-W.; Chang, W.-P.; Südhof, T.C. E-Syts, a family of membranous Ca2+-sensor proteins with multiple C2 domains. Proc. Natl. Acad. Sci. USA 2007, 104, 3823–3828. [Google Scholar] [CrossRef]
- Lee, I.; Hong, W. Diverse membrane-associated proteins contain a novel SMP domain. FASEB J. 2006, 20, 202–206. [Google Scholar] [CrossRef]
- Schauder, C.M.; Wu, X.; Saheki, Y.; Narayanaswamy, P.; Torta, F.; Wenk, M.R.; De Camilli, P.; Reinisch, K.M. Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature 2014, 510, 552–555. [Google Scholar] [CrossRef]
- Levine, T.P. Remote homology searches identify bacterial homologues of eukaryotic lipid transfer proteins, including Chorein-N domains in TamB and AsmA and Mdm31p. BMC Mol. Cell Biol. 2019, 20, 43. [Google Scholar] [CrossRef]
- Ge, J.; Bian, X.; Ma, L.; Cai, Y.; Li, Y.; Yang, J.; Karatekin, E.; De Camilli, P.; Zhang, Y. Stepwise membrane binding of extended synaptotagmins revealed by optical tweezers. Nat. Chem. Biol. 2022, 18, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Saheki, Y.; Bian, X.; Schauder, C.M.; Sawaki, Y.; Surma, M.A.; Klose, C.; Pincet, F.; Reinisch, K.M.; De Camilli, P. Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nat. Cell Biol. 2016, 18, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Z.; Wang, X.; Zhao, Z.; Jiao, L.; Liu, R.; Wang, K.; Ma, R.; Yang, Y.; Chen, G.; et al. Insights into membrane association of the SMP domain of extended synaptotagmin. Nat. Commun. 2023, 14, 1504. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tamura, K.; Ueda, H.; Ito, Y.; Nakano, A.; Hara-Nishimura, I.; Shimada, T. Synaptotagmin-Associated Endoplasmic Reticulum-Plasma Membrane Contact Sites Are Localized to Immobile ER Tubules. Plant Physiol. 2018, 178, 641–653. [Google Scholar] [CrossRef]
- Manford, A.G.; Stefan, C.J.; Yuan, H.L.; MacGurn, J.A.; Emr, S.D. ER-to-Plasma Membrane Tethering Proteins Regulate Cell Signaling and ER Morphology. Dev. Cell 2012, 23, 1129–1140. [Google Scholar] [CrossRef]
- Benavente, J.L.; Siliqi, D.; Infantes, L.; Lagartera, L.; Mills, A.; Gago, F.; Ruiz-López, N.; Botella, M.A.; Sánchez-Barrena, M.J.; Albert, A. The structure and flexibility analysis of the Arabidopsis synaptotagmin 1 reveal the basis of its regulation at membrane contact sites. Life Sci. Alliance 2021, 4, e202101152. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Gulbranson, D.R.; Paine, A.; Rathore, S.S.; Shen, J. Extended synaptotagmins are Ca2+-dependent lipid transfer proteins at membrane contact sites. Proc. Natl. Acad. Sci. USA 2016, 113, 4362–4367. [Google Scholar] [CrossRef]
- Fernández-Busnadiego, R.; Saheki, Y.; De Camilli, P. Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum–plasma membrane contact sites. Proc. Natl. Acad. Sci. USA 2015, 112, E2004–E2013. [Google Scholar] [CrossRef]
- Yamazaki, T.; Takata, N.; Uemura, M.; Kawamura, Y. Arabidopsis synaptotagmin SYT1, a type I signal-anchor protein, requires tandem C2 domains for delivery to the plasma membrane. J. Biol. Chem. 2010, 285, 23165–23176. [Google Scholar] [CrossRef]
- Idevall-Hagren, O.; Lü, A.; Xie, B.; De Camilli, P. Triggered Ca2+ influx is required for extended synaptotagmin 1-induced ER-plasma membrane tethering. EMBO J. 2015, 34, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bacaj, T.; Zhou, A.; Tomchick, D.R.; Südhof, T.C.; Rizo, J. Structure and Ca2+ Binding Properties of the Tandem C2 Domains of E-Syt2. Structure 2014, 22, 269–280. [Google Scholar] [CrossRef] [PubMed]
- von Poser, C.; Ichtchenko, K.; Shao, X.; Rizo, J.; Südhof, T.C. The evolutionary pressure to inactivate: A subclass of Synaptotagmins with an amino acid substitution that abolishes Ca2+ binding. J. Biol. Chem. 1997, 272, 14314–14319. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Kawamura, Y.; Minami, A.; Uemura, M. Calcium-dependent freezing tolerance in Arabidopsis involves membrane resealing via synaptotagmin SYT1. Plant Cell 2008, 20, 3389–3404. [Google Scholar] [CrossRef]
- Bian, X.; Saheki, Y.; De Camilli, P. Ca(2+) releases E-Syt1 autoinhibition to couple ER-plasma membrane tethering with lipid transport. Embo J. 2018, 37, 219–234. [Google Scholar] [CrossRef]
- Davletov, B.A.; Südhof, T.C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J. Biol. Chem. 1993, 268, 26386–26390. [Google Scholar]
- Gruget, C.; Bello, O.; Coleman, J.; Krishnakumar, S.S.; Perez, E.; Rothman, J.E.; Pincet, F.; Donaldson, S.H. Synaptotagmin-1 membrane binding is driven by the C2B domain and assisted cooperatively by the C2A domain. Sci. Rep. 2020, 10, 18011. [Google Scholar] [CrossRef]
- Lees, J.A.; Messa, M.; Sun, E.W.; Wheeler, H.; Torta, F.; Wenk, M.R.; De Camilli, P.; Reinisch, K.M. Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science 2017, 355, eaah6171. [Google Scholar] [CrossRef]
- Pottekat, A.; Becker, S.; Spencer, K.R.; Yates, J.R.; Manning, G.; Itkin-Ansari, P.; Balch, W.E. Insulin Biosynthetic Interaction Network Component, TMEM24, Facilitates Insulin Reserve Pool Release. Cell Rep. 2013, 4, 921–930. [Google Scholar] [CrossRef]
- Sun, E.W.; Guillén-Samander, A.; Bian, X.; Wu, Y.; Cai, Y.; Messa, M.; De Camilli, P. Lipid transporter TMEM24/C2CD2L is a Ca(2+)-regulated component of ER-plasma membrane contacts in mammalian neurons. Proc. Natl. Acad. Sci. USA 2019, 116, 5775–5784. [Google Scholar] [CrossRef]
- Xie, B.; Panagiotou, S.; Cen, J.; Gilon, P.; Bergsten, P.; Idevall-Hagren, O. The endoplasmic reticulum-plasma membrane tethering protein TMEM24 is a regulator of cellular Ca2+ homeostasis. J. Cell Sci. 2021, 135, jcs259073. [Google Scholar] [CrossRef] [PubMed]
- Huercano, C.; Percio, F.; Sanchez-Vera, V.; Morello-López, J.; Botella, M.A.; Ruiz-Lopez, N. Identification of plant exclusive lipid transfer SMP proteins at membrane contact sites in Arabidopsis and Tomato. bioRxiv 2022, bioRxiv: 2022.2012.2014.520452. [Google Scholar] [CrossRef]
- Lee, E.; Santana, B.V.N.; Samuels, E.; Benitez-Fuente, F.; Corsi, E.; Botella, M.A.; Perez-Sancho, J.; Vanneste, S.; Friml, J.; Macho, A.; et al. Rare earth elements induce cytoskeleton-dependent and PI4P-associated rearrangement of SYT1/SYT5 endoplasmic reticulum–plasma membrane contact site complexes in Arabidopsis. J. Exp. Bot. 2020, 71, 3986–3998. [Google Scholar] [CrossRef] [PubMed]
- Craxton, M. Evolutionary genomics of plant genes encoding N-terminal-TM-C2 domain proteins and the similar FAM62 genes and synaptotagmin genes of metazoans. BMC Genom. 2007, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sancho, J.; Vanneste, S.; Lee, E.; McFarlane, H.E.; Esteban del Valle, A.; Valpuesta, V.; Friml, J.; Botella, M.A.; Rosado, A. The Arabidopsis Synaptotagmin1 Is Enriched in Endoplasmic Reticulum-Plasma Membrane Contact Sites and Confers Cellular Resistance to Mechanical Stresses. Plant Physiol. 2015, 168, 132–143. [Google Scholar] [CrossRef]
- Lee, E.; Vanneste, S.; Pérez-Sancho, J.; Benitez-Fuente, F.; Strelau, M.; Macho, A.P.; Botella, M.A.; Friml, J.; Rosado, A. Ionic stress enhances ER-PM connectivity via phosphoinositide-associated SYT1 contact site expansion in Arabidopsis. Proc. Natl. Acad. Sci. USA 2019, 116, 1420–1429. [Google Scholar] [CrossRef]
- García-Hernández, S.; Rubio, L.; Pérez-Sancho, J.; Esteban del Valle, A.; Benítez-Fuente, F.; Beuzón, C.R.; Macho, A.P.; Ruiz-López, N.; Albert, A.; Botella, M.A. Unravelling Different Biological Roles of Plant Synaptotagmins. bioRxiv 2024, bioRxiv: 2024.2001.2021.576508. [Google Scholar] [CrossRef]
- Kumar, A.; Krausko, M.; Jásik, J. SYNAPTOTAGMIN 4 is expressed mainly in the phloem and participates in abiotic stress tolerance in Arabidopsis. Front. Plant Sci. 2024, 15, 1363555. [Google Scholar] [CrossRef]
- Wang, H.; Han, S.; Siao, W.; Song, C.; Xiang, Y.; Wu, X.; Cheng, P.; Li, H.; Jásik, J.; Mičieta, K.; et al. Arabidopsis Synaptotagmin 2 Participates in Pollen Germination and Tube Growth and Is Delivered to Plasma Membrane via Conventional Secretion. Mol. Plant 2015, 8, 1737–1750. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Gao, B.; Fan, H.; Jin, J.; Botella, M.A.; Jiang, L.; Lin, J. Golgi Apparatus-Localized Synaptotagmin 2 Is Required for Unconventional Secretion in Arabidopsis. PLoS ONE 2011, 6, e26477. [Google Scholar] [CrossRef]
- Kwon, C.; Neu, C.; Pajonk, S.; Yun, H.S.; Lipka, U.; Humphry, M.; Bau, S.; Straus, M.; Kwaaitaal, M.; Rampelt, H.; et al. Co-option of a default secretory pathway for plant immune responses. Nature 2008, 451, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, D.G.; Jiang, J.; Movahed, N.; Germain, H.; Yamaji, Y.; Zheng, H.; Laliberté, J.F. Turnip Mosaic Virus Uses the SNARE Protein VTI11 in an Unconventional Route for Replication Vesicle Trafficking. Plant Cell 2018, 30, 2594–2615. [Google Scholar] [CrossRef] [PubMed]
- Siao, W.; Wang, P.; Voigt, B.; Hussey, P.J.; Baluska, F. Arabidopsis SYT1 maintains stability of cortical endoplasmic reticulum networks and VAP27-1-enriched endoplasmic reticulum-plasma membrane contact sites. J. Exp. Bot. 2016, 67, 6161–6171. [Google Scholar] [CrossRef] [PubMed]
- Creutz, C.E.; Snyder, S.L.; Schulz, T.A. Characterization of the yeast tricalbins: Membrane-bound multi-C2-domain proteins that form complexes involved in membrane trafficking. Cell. Mol. Life Sci. 2004, 61, 1208–1220. [Google Scholar] [CrossRef]
- Codjoe, J.M.; Richardson, R.A.; McLoughlin, F.; Vierstra, R.D.; Haswell, E.S. Unbiased proteomic and forward genetic screens reveal that mechanosensitive ion channel MSL10 functions at ER–plasma membrane contact sites in Arabidopsis thaliana. eLife 2022, 11, e80501. [Google Scholar] [CrossRef]
- Basu, D.; Haswell, E.S. The Mechanosensitive Ion Channel MSL10 Potentiates Responses to Cell Swelling in Arabidopsis Seedlings. Curr. Biol. 2020, 30, 2716–2728.e6. [Google Scholar] [CrossRef]
- Kang, F.; Zhou, M.; Huang, X.; Fan, J.; Wei, L.; Boulanger, J.; Liu, Z.; Salamero, J.; Liu, Y.; Chen, L. E-syt1 Re-arranges STIM1 Clusters to Stabilize Ring-shaped ER-PM Contact Sites and Accelerate Ca(2+) Store Replenishment. Sci. Rep. 2019, 9, 3975. [Google Scholar] [CrossRef]
- Qian, T.; Li, C.; Liu, F.; Xu, K.; Wan, C.; Liu, Y.; Yu, H. Arabidopsis synaptotagmin 1 mediates lipid transport in a lipid composition-dependent manner. Traffic 2022, 23, 346–356. [Google Scholar] [CrossRef]
- Bell, K.; Oparka, K. Imaging plasmodesmata. Protoplasma 2011, 248, 9–25. [Google Scholar] [CrossRef]
- Bayer, E.M.; Benitez-Alfonso, Y. Plasmodesmata: Channels Under Pressure. Annu. Rev. Plant Biol. 2024, 75, 291–317. [Google Scholar] [CrossRef]
- Robertson, J.D.; Locke, M. Cellular Membranes in Development; Locke, M., Ed.; Academic Press: New York, NY, USA, 1964; p. 1. [Google Scholar]
- Brault, M.L.; Petit, J.D.; Immel, F.; Nicolas, W.J.; Glavier, M.; Brocard, L.; Gaston, A.; Fouché, M.; Hawkins, T.J.; Crowet, J.M.; et al. Multiple C2 domains and transmembrane region proteins (MCTPs) tether membranes at plasmodesmata. EMBO Rep. 2019, 20, e47182. [Google Scholar] [CrossRef] [PubMed]
- Kriechbaumer, V.; Botchway, S.W.; Slade, S.E.; Knox, K.; Frigerio, L.; Oparka, K.; Hawes, C. Reticulomics: Protein-Protein Interaction Studies with Two Plasmodesmata-Localized Reticulon Family Proteins Identify Binding Partners Enriched at Plasmodesmata, Endoplasmic Reticulum, and the Plasma Membrane. Plant Physiol. 2015, 169, 1933–1945. [Google Scholar] [CrossRef]
- Kim, S.; Park, K.; Kwon, C.; Yun, H.S. Synaptotagmin 4 and 5 additively contribute to Arabidopsis immunity to Pseudomonas syringae DC3000. Plant Signal Behav. 2022, 17, 2025323. [Google Scholar] [CrossRef] [PubMed]
- Menand, B.; Calder, G.; Dolan, L. Both chloronemal and caulonemal cells expand by tip growth in the moss Physcomitrella patens. J. Exp. Bot. 2007, 58, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Furt, F.; Lemoi, K.; Tüzel, E.; Vidali, L. Quantitative analysis of organelle distribution and dynamics in Physcomitrella patens protonemal cells. BMC Plant Biol. 2012, 12, 70. [Google Scholar] [CrossRef]
- Jang, G.; Dolan, L. Auxin promotes the transition from chloronema to caulonema in moss protonema by positively regulating PpRSL1and PpRSL2 in Physcomitrella patens. New Phytol. 2011, 192, 319–327. [Google Scholar] [CrossRef]
- Thelander, M.; Landberg, K.; Sundberg, E. Auxin-mediated developmental control in the moss Physcomitrella patens. J. Exp. Bot. 2018, 69, 277–290. [Google Scholar]
- Ntefidou, M.; Eklund, D.M.; Le Bail, A.; Schulmeister, S.; Scherbel, F.; Brandl, L.; Dörfler, W.; Eichstädt, C.; Bannmüller, A.; Ljung, K.; et al. Physcomitrium patens PpRIC, an ancestral CRIB-domain ROP effector, inhibits auxin-induced differentiation of apical initial cells. Cell Rep. 2023, 42, 112130. [Google Scholar] [CrossRef]
- Jaeger, R.; Moody, L.A. A fundamental developmental transition in Physcomitrium patens is regulated by evolutionarily conserved mechanisms. Evol. Devel. 2021, 23, 123–136. [Google Scholar] [CrossRef]
- Pressel, S.; Ligrone, R.; Duckett, J.G. Cellular Differentiation in Moss Protonemata: A Morphological and Experimental Study. Ann. Bot. 2008, 102, 227–245. [Google Scholar]
- Thelander, M.; Olsson, T.; Ronne, H. Effect of the energy supply on filamentous growth and development in Physcomitrella patens. J. Exp. Bot. 2005, 56, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Schumaker, K.S.; Dietrich, M.A. Programmed Changes in Form during Moss Development. Plant Cell 1997, 9, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y.; Ohtsuka, J.; Hiwatashi, Y.; Naramoto, S.; Kyozuka, J. Cytokinin and ALOG proteins regulate pluripotent stem cell identity in the moss Physcomitrium patens. Sci. Adv. 2024, 10, eadq6082. [Google Scholar] [CrossRef]
- Schulz, P.; Reski, R.; Maldiney, R.; Laloue, M.; Schwartzenberg, K.v. Kinetics of Cytokinin Production and Bud Formation in Physcomitrella: Analysis of Wild Type, a Developmental Mutant and Two of Its ipt Transgenics. J. Plant Physiol. 2000, 156, 768–774. [Google Scholar] [CrossRef]
- Moody, L.A.; Kelly, S.; Rabbinowitsch, E.; Langdale, J.A. Genetic Regulation of the 2D to 3D Growth Transition in the Moss Physcomitrella patens. Curr. Biol. 2018, 28, 473–478.e5. [Google Scholar] [CrossRef]
- Jones, V.A.S.; Dolan, L. The evolution of root hairs and rhizoids. Ann. Bot. 2012, 110, 205–212. [Google Scholar] [CrossRef]
- Sakakibara, K.; Nishiyama, T.; Sumikawa, N.; Kofuji, R.; Murata, T.; Hasebe, M. Involvement of auxin and a homeodomain-leucine zipper I gene in rhizoid development of the moss Physcomitrella patens. Development 2003, 130, 4835–4846. [Google Scholar] [CrossRef]
- Perroud, P.-F.; Quatrano, R.S. The role of ARPC4 in tip growth and alignment of the polar axis in filaments of Physcomitrella patens. Cell Motil. Cytoskelet. 2006, 63, 162–171. [Google Scholar] [CrossRef]
- Schaefer, D.G.; Zrÿd, J.-P. Efficient gene targeting in the moss Physcomitrella patens. Plant J. 1997, 11, 1195–1206. [Google Scholar] [CrossRef]
- Collonnier, C.; Epert, A.; Mara, K.; Maclot, F.; Guyon-Debast, A.; Charlot, F.; White, C.; Schaefer, D.G.; Nogué, F. CRISPR-Cas9-mediated efficient directed mutagenesis and RAD51-dependent and RAD51-independent gene targeting in the moss Physcomitrella patens. Plant Biotechnol. J. 2017, 15, 122–131. [Google Scholar] [CrossRef]
- Brunkard, J.O.; Zambryski, P.C. Plasmodesmata enable multicellularity: New insights into their evolution, biogenesis, and functions in development and immunity. Curr. Opin. Plant Biol. 2017, 35, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.E.; Graham, L.E.; Botha, C.E.J.; Lavin, C.A. Comparative ultrastructure of plasmodesmata of Chara and selected bryophytes: Toward an elucidation of the evolutionary origin of plant plasmodesmata. Am. J. Bot. 1997, 84, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.; Ehlers, K. Plasmodesmata dynamics in bryophyte model organisms: Secondary formation and developmental modifications of structure and function. Planta 2024, 260, 45. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, M.; Fujita, T. Quantitative imaging of directional transport through plasmodesmata in moss protonemata via single-cell photoconversion of Dendra2. J. Plant Res. 2013, 126, 577–585. [Google Scholar] [CrossRef]
- Olsson, T.; Thelander, M.; Ronne, H. A Novel Type of Chloroplast Stromal Hexokinase Is the Major Glucose-phosphorylating Enzyme in the Moss Physcomitrella patens. J. Biol. Chem. 2003, 278, 44439–44447. [Google Scholar] [CrossRef]
- Augustine, R.C.; Pattavina, K.A.; Tüzel, E.; Vidali, L.; Bezanilla, M. Actin interacting protein1 and actin depolymerizing factor drive rapid actin dynamics in Physcomitrella patens. Plant Cell 2011, 23, 3696–3710. [Google Scholar] [CrossRef]
- Gombos, S.; Miras, M.; Howe, V.; Xi, L.; Pottier, M.; Kazemein Jasemi, N.S.; Schladt, M.; Ejike, J.O.; Neumann, U.; Hänsch, S.; et al. A high-confidence Physcomitrium patens plasmodesmata proteome by iterative scoring and validation reveals diversification of cell wall proteins during evolution. New Phytol. 2023, 238, 637–653. [Google Scholar] [CrossRef]
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Rensing, S.A.; Lang, D.; Zimmer, A.D.; Terry, A.; Salamov, A.; Shapiro, H.; Nishiyama, T.; Perroud, P.-F.O.; Lindquist, E.A.; Kamisugi, Y.; et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science 2008, 319, 64–69. [Google Scholar] [CrossRef]
- Schreiber, M.; Rensing, S.A.; Gould, S.B. The greening ashore. Trends Plant Sci. 2022, 27, 847–857. [Google Scholar] [CrossRef]
- Hori, K.; Maruyama, F.; Fujisawa, T.; Togashi, T.; Yamamoto, N.; Seo, M.; Sato, S.; Yamada, T.; Mori, H.; Tajima, N.; et al. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat. Commun. 2014, 5, 3978. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Schliep, K.P. phangorn: Phylogenetic analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef]
- Inkscape Project. Available online: https://inkscape.org/ (accessed on 13 January 2025).
- Blum, M.; Andreeva, A.; Florentino, L.C.; Chuguransky, S.R.; Grego, T.; Hobbs, E.; Pinto, B.L.; Orr, A.; Paysan-Lafosse, T.; Ponamareva, I.; et al. InterPro: The protein sequence classification resource in 2025. Nucleic Acids Res. 2025, 53, D444–D456. [Google Scholar] [CrossRef]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef]
- Ishikawa, K.; Konno, R.; Hirano, S.; Fujii, Y.; Fujiwara, M.; Fukao, Y.; Kodama, Y. The endoplasmic reticulum membrane–bending protein RETICULON facilitates chloroplast relocation movement in Marchantia polymorpha. Plant J. 2022, 111, 205–216. [Google Scholar] [CrossRef]
- Kasahara, M.; Kagawa, T.; Sato, Y.; Kiyosue, T.; Wada, M. Phototropins mediate blue and red light-induced chloroplast movements in Physcomitrella patens. Plant Physiol. 2004, 135, 1388–1397. [Google Scholar] [CrossRef]
- Wang, X.Q.; Yang, P.F.; Liu, Z.; Liu, W.Z.; Hu, Y.; Chen, H.; Kuang, T.Y.; Pei, Z.M.; Shen, S.H.; He, Y.K. Exploring the mechanism of Physcomitrella patens desiccation tolerance through a proteomic strategy. Plant Physiol. 2009, 149, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Beike, A.K.; Lang, D.; Zimmer, A.D.; Wüst, F.; Trautmann, D.; Wiedemann, G.; Beyer, P.; Decker, E.L.; Reski, R. Insights from the cold transcriptome of Physcomitrella patens: Global specialization pattern of conserved transcriptional regulators and identification of orphan genes involved in cold acclimation. New Phytol. 2015, 205, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Chater, C.C.; Caine, R.S.; Tomek, M.; Wallace, S.; Kamisugi, Y.; Cuming, A.C.; Lang, D.; MacAlister, C.A.; Casson, S.; Bergmann, D.C.; et al. Origin and function of stomata in the moss Physcomitrella patens. Nat. Plants 2016, 2, 16179. [Google Scholar] [PubMed]
- The UniProt Consortium. UniProt: The Universal Protein Knowledgebase in 2025. Nucleic Acids Res. 2024, 53, gkae1010. [Google Scholar] [CrossRef]
- Berardini, T.Z.; Reiser, L.; Li, D.; Mezheritsky, Y.; Muller, R.; Strait, E.; Huala, E. The arabidopsis information resource: Making and mining the “gold standard” annotated reference plant genome. Genesis 2015, 53, 474–485. [Google Scholar] [CrossRef]
- Healey, A.L.; Piatkowski, B.; Lovell, J.T.; Sreedasyam, A.; Carey, S.B.; Mamidi, S.; Shu, S.; Plott, C.; Jenkins, J.; Lawrence, T.; et al. Newly identified sex chromosomes in the Sphagnum (peat moss) genome alter carbon sequestration and ecosystem dynamics. Nat. Plants 2023, 9, 238–254. [Google Scholar] [CrossRef]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into Land Plant Evolution Garnered from the Marchantia polymorpha Genome. Cell 2017, 171, 287–304.e5. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Hallgren, J.; Tsirigos, K.; Pedersen, M.D.; Almagro Armenteros, J.J.; Marcatili, P.; Nielsen, H.; Krogh, A.; Winther, O. DeepTMHMM predicts alpha and beta transmembrane proteins using deep neural networks. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bodenhofer, U.; Bonatesta, E.; Horejš-Kainrath, C.; Hochreiter, S. msa: An R package for multiple sequence alignment. Bioinformatics 2015, 31, 3997–3999. [Google Scholar] [CrossRef]
- Henikoff, S.; Henikoff, J.G. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. USA 1992, 89, 10915–10919. [Google Scholar] [CrossRef] [PubMed]
- Yu, G. Data Integration, Manipulation and Visualization of Phylogenetic Trees; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: New York, NY, USA, 2022. [Google Scholar]
- Zhou, L.; Feng, T.; Xu, S.; Gao, F.; Lam, T.T.; Wang, Q.; Wu, T.; Huang, H.; Zhan, L.; Li, L.; et al. ggmsa: A visual exploration tool for multiple sequence alignment and associated data. Brief. Bioinform. 2022, 23, bbac222. [Google Scholar] [CrossRef]
- Charif, D.; Lobry, J.R. Structural Approaches to Sequence Evolution: Molecules, Networks, Populations; Bastolla, U., Porto, M., Roman, H.E., Vendruscolo, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Comput. Appl. Biosci. 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaier, A.; Ntefidou, M. The Extended Synaptotagmins of Physcomitrium patens. Plants 2025, 14, 1027. https://doi.org/10.3390/plants14071027
Kaier A, Ntefidou M. The Extended Synaptotagmins of Physcomitrium patens. Plants. 2025; 14(7):1027. https://doi.org/10.3390/plants14071027
Chicago/Turabian StyleKaier, Alexander, and Maria Ntefidou. 2025. "The Extended Synaptotagmins of Physcomitrium patens" Plants 14, no. 7: 1027. https://doi.org/10.3390/plants14071027
APA StyleKaier, A., & Ntefidou, M. (2025). The Extended Synaptotagmins of Physcomitrium patens. Plants, 14(7), 1027. https://doi.org/10.3390/plants14071027






