Core Mycorrhizal Fungi Promote Seedling Growth in Dendrobium officinale: An Important Medicinal Orchid
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Species
2.2. Fungal Strains and Plant Materials
2.3. Symbiotic Cultures
2.4. Testing the Ability of Synthetic Fungal Combinations to Promote Seedling Growth
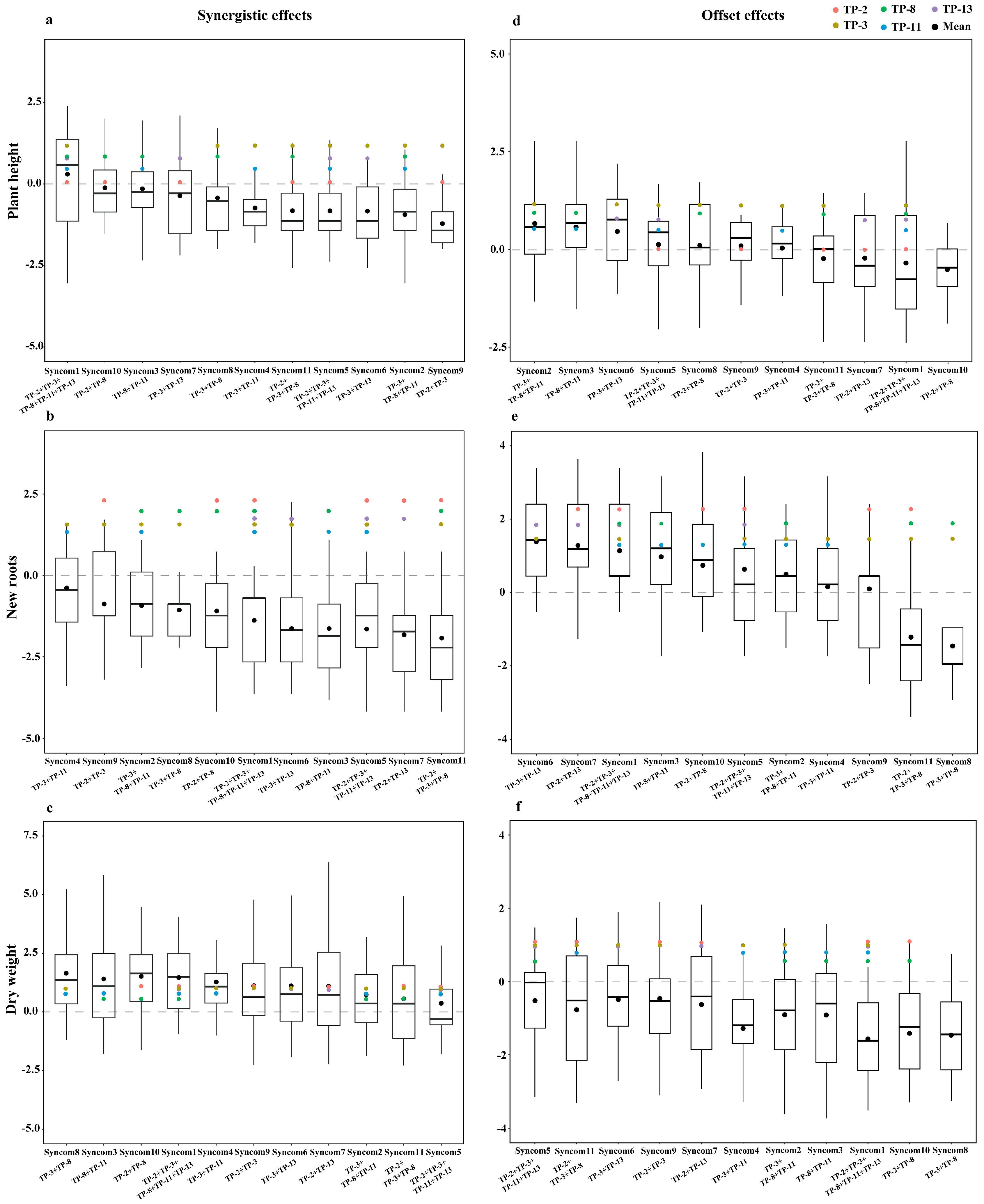
2.5. Synergistic and Offset Effects
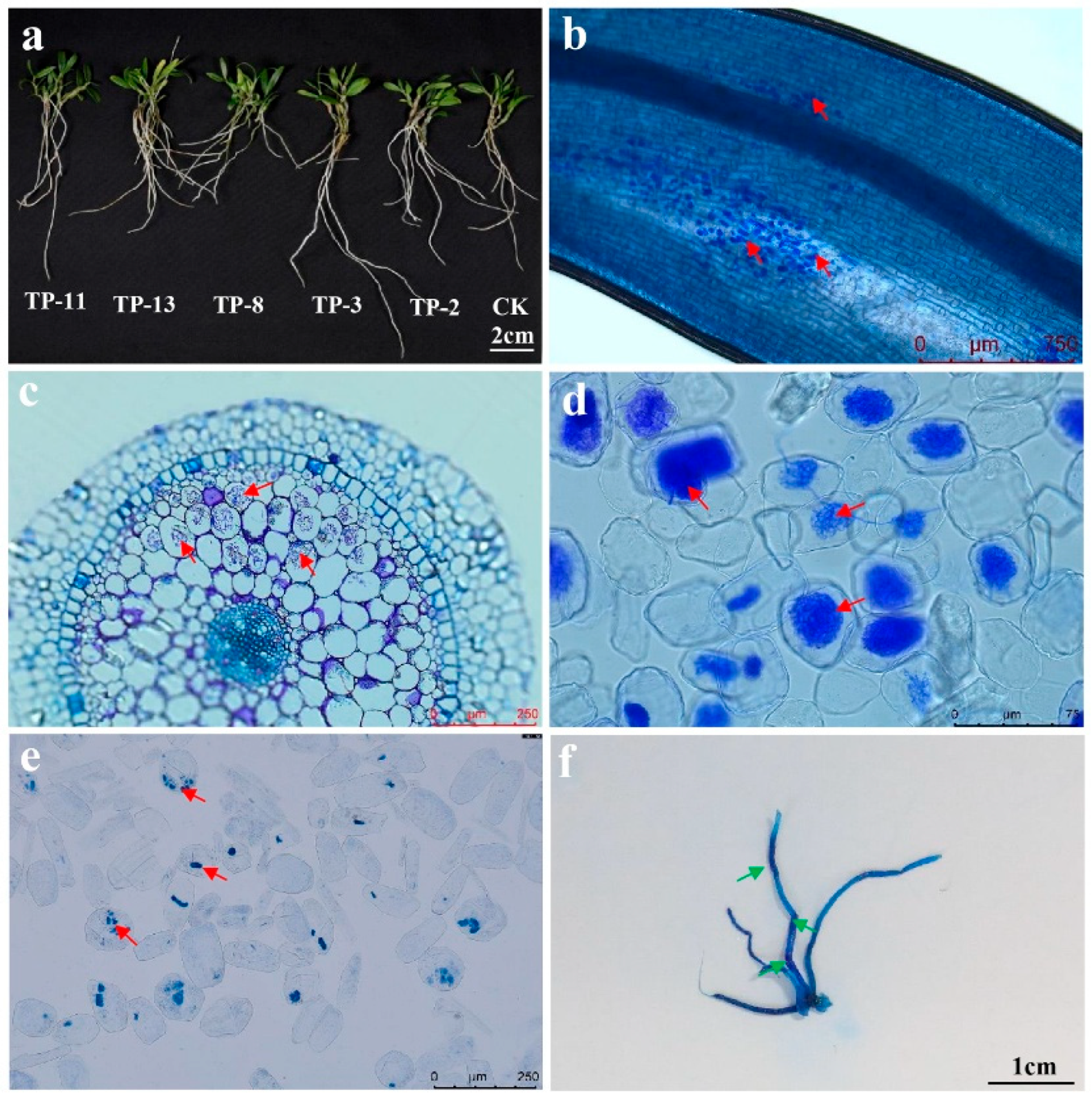
2.6. Detection of Fungal Colonization
2.7. Statistical Analysis
3. Results
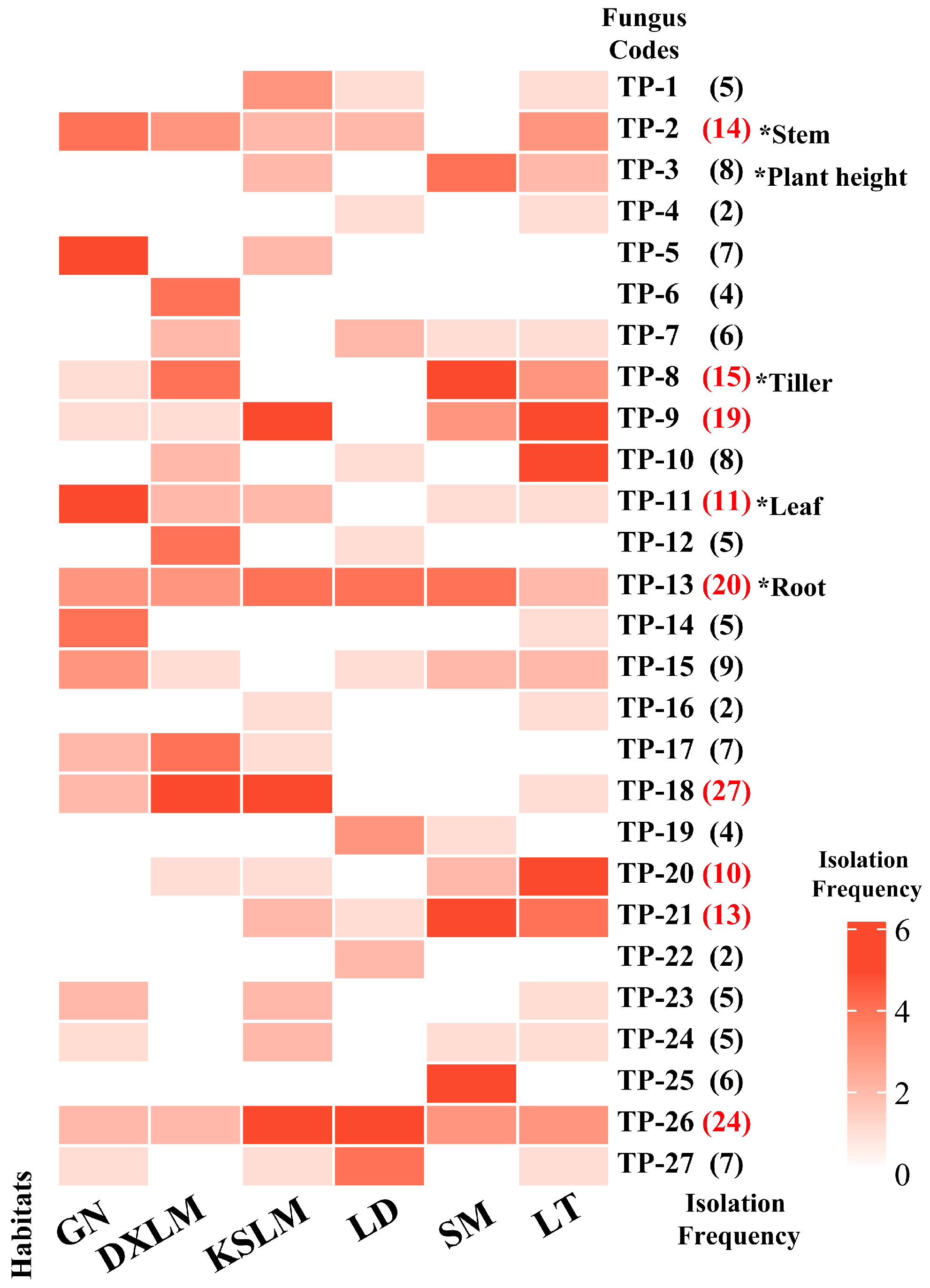
3.1. The Capacity of Fungi to Support Seedling Growth
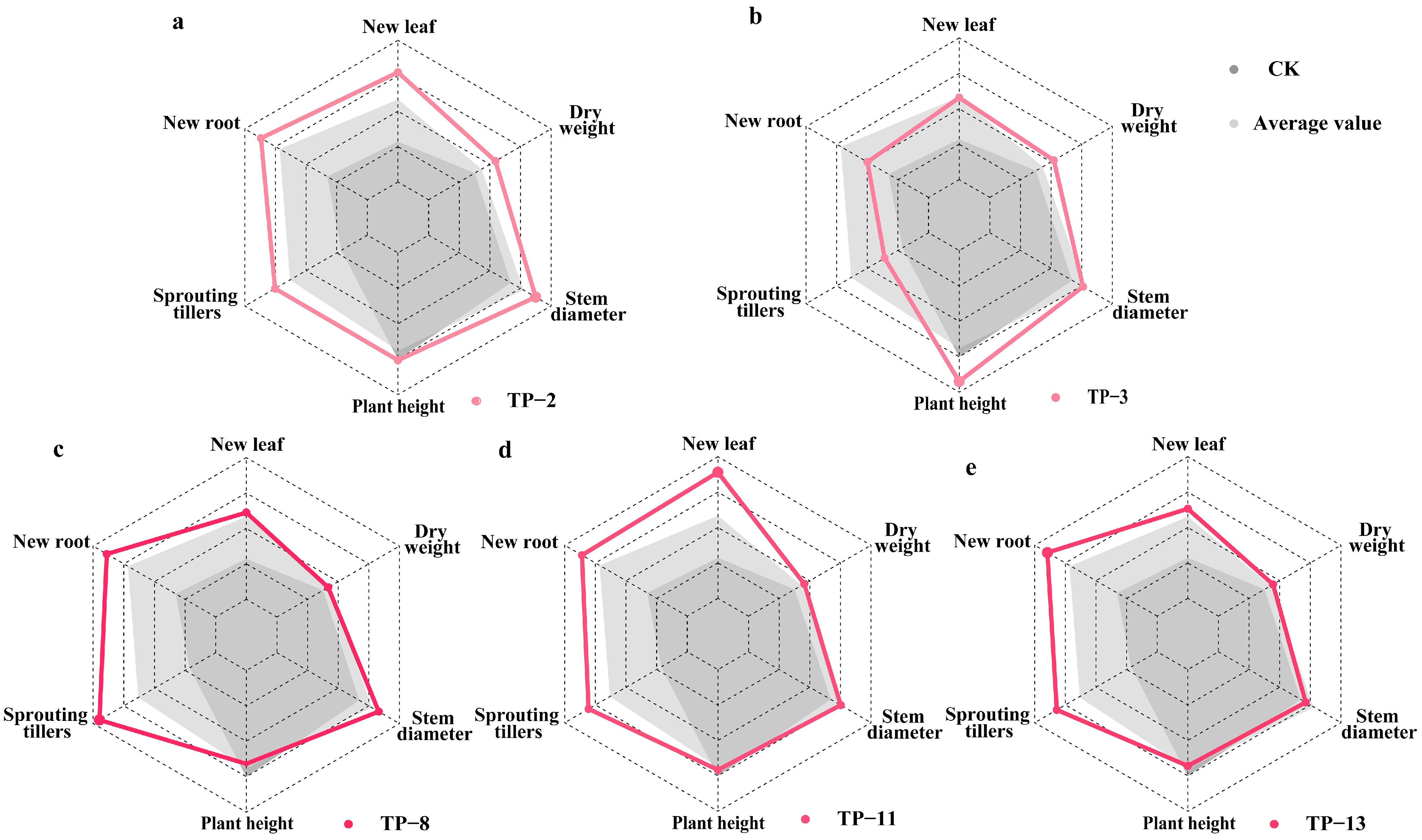
3.2. The Effect of Mycorrhizal Fungi on the Growth of New Leaves, Roots, and Sprouting Tillers of Seedlings
3.3. The Effect of Mycorrhizal Fungi on Plant Height, Stem Diameter Growth, and Dry Weight of Seedlings
3.4. The Ability of Synthetic Fungal Combinations to Promote Seedling Growth
4. Discussion
4.1. “Core + Stochastic” Fungal Recruitment
4.2. Functional Diversification of Mycorrhizal Symbiosis
4.3. Synthetic Fungal Combinations: Offset vs. Synergistic Effects
4.4. Perspectives on the Application of Mycorrhizal Fungi
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Wang, X.; Wang, E. Mycorrhizal symbiosis in plant growth and stress adaptation: From genes to ecosystems. Annu. Rev. Plant Biol. 2023, 74, 569–607. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Verma, J.P. Does plant—Microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Ventre Lespiaucq, A.; Jacquemyn, H.; Rasmussen, H.N.; Méndez, M. Temporal turnover in mycorrhizal interactions: A proof of concept with orchids. New Phytol. 2021, 230, 1690–1699. [Google Scholar] [CrossRef]
- Favre-Godal, Q.; Gourguillon, L.; Lordel-Madeleine, S.; Gindro, K.; Choisy, P. Orchids and their mycorrhizal fungi: An insufficiently explored relationship. Mycorrhiza 2020, 30, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Q.; Wu, S.M.; Yang, W.K.; Selosse, M.A.; Gao, J.Y. How mycorrhizal associations influence orchid distribution and population dynamics. Front. Plant Sci. 2021, 12, 647114. [Google Scholar] [CrossRef]
- Freestone, M.; Reiter, N.; Swarts, N.D.; Linde, C.C. Temporal turnover of ceratobasidiaceae orchid mycorrhizal fungal communities with ontogenetic and phenological development in Prasophyllum (Orchidaceae). Ann. Bot. 2024, 134, 933–948. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, X.H.; Liu, J.; Zhao, X.; Zhou, J.H.; Wang, X.; Wang, D.Y.; Lai, C.J.S.; Xu, W.; Huang, J.W.; et al. The Gastrodia Elata genome provides insights into plant adaptation to heterotrophy. Nat. Commun. 2018, 9, 1615. [Google Scholar] [CrossRef]
- Calevo, J.; Duffy, K.J. Interactions among mycorrhizal fungi enhance the early development of a mediterranean orchid. Mycorrhiza 2023, 33, 229–240. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Dixon, K.W.; Jersáková, J.; Těšitelová, T. Germination and seedling establishment in orchids: A complex of requirements. Ann. Bot. 2015, 116, 391–402. [Google Scholar] [CrossRef]
- McCormick, M.K.; Taylor, D.L.; Whigham, D.F.; Burnett, R.K., Jr. Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi. J. Ecol. 2016, 104, 744–754. [Google Scholar] [CrossRef]
- Wang, X.J.; Wu, Y.H.; Ming, X.J.; Wang, G.; Gao, J.Y. Isolating ecological-specific fungi and creating fungus-seed bags for epiphytic orchid conservation. Glob. Ecol. Conserv. 2021, 28, e01714. [Google Scholar] [CrossRef]
- Ma, G.H.; Chen, X.G.; Selosse, M.A.; Gao, J.Y. Compatible and incompatible mycorrhizal fungi with seeds of Dendrobium species: The colonization process and effects of coculture on germination and seedling development. Front. Plant Sci. 2022, 13, 823794. [Google Scholar] [CrossRef]
- Shan, T.T.; Chen, T.Y.; Chen, X.M.; Guo, S.X.; Wang, A.R. Advance on the association between mycorrhizal fungi and Orchidaceae in nitrogen nutrition. Chin. J. Plant Ecol. 2022, 46, 516–528. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Huo, W.W.; Hou, J.Y.; Liu, L.; Yu, X.Y.; Xu, L. Effects and benefits of orchid mycorrhizal symbionts on Dendrobium officinale. Horticulturae 2022, 8, 861. [Google Scholar] [CrossRef]
- Chen, D.Y.; Wang, X.J.; Li, T.Q.; Li, N.Q.; Gao, J.Y. In Situ seedling baiting to isolate plant growth-promoting fungi from Dendrobium officinale, an over-collected medicinal orchid in China. Glob. Ecol. Conserv. 2021, 28, e01659. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Rammitsu, K.; Kinoshita, A.; Tokuhara, K.; Yukawa, T.; Ogura-Tsujita, Y. Symbiotic culture of three closely related Dendrobium species reveals a growth bottleneck and differences in mycorrhizal specificity at early developmental stages. Diversity. 2022, 14, 1119. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Rammitsu, K.; Tetsuka, K.; Yukawa, T.; Ogura-Tsujita, Y. Dominant Dendrobium officinale mycorrhizal partners vary among habitats and strongly induce seed germination in vitro. Front. Ecol. Evol. 2022, 10, 994641. [Google Scholar] [CrossRef]
- Oja, J.; Kohout, P.; Tedersoo, L.; Kull, T.; Kõljalg, U. Temporal patterns of orchid mycorrhizal fungi in meadows and forests as revealed by 454 pyrosequencing. New Phytol. 2015, 205, 1608–1618. [Google Scholar] [CrossRef]
- Deveautour, C.; Power, S.A.; Barnett, K.L.; Ochoa-Hueso, R.; Donn, S.; Bennett, A.E.; Powell, J.R. Temporal dynamics of mycorrhizal fungal communities and co-associations with grassland plant communities following experimental manipulation of rainfall. J. Ecol. 2020, 108, 515–527. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Liu, N.; Xing, X.K. Research advances in mechanisms of interaction between mycorrhizal fungi and Orchidaceae plants by using omics techniques. Mycosystema 2021, 40, 423–435. [Google Scholar] [CrossRef]
- Teste, F.P.; Jones, M.D.; Dickie, I.A. Dual-mycorrhizal plants: Their ecology and relevance. New Phytol. 2020, 225, 1835–1851. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, S.; Declerck, S.; Suárez, J.P. In situ orchid seedling-trap experiment shows few keystone and many randomly associated mycorrhizal fungal species during early plant colonization. Front. Plant Sci. 2018, 9, 1664. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chen, D.Y.; Wang, X.J.; Li, N.Q.; Gao, J.Y. Using ex situ seedling baiting to capture seedling-associated mycorrhizal fungi in medicinal orchid Dendrobium officinale. J. Fungi. 2022, 8, 1036. [Google Scholar] [CrossRef]
- Busby, P.E.; Soman, C.; Wagner, M.R.; Friesen, M.L.; Kremer, J.; Bennett, A.; Morsy, M.; Eisen, J.A.; Leach, J.E.; Dangl, J.L. Research priorities for harnessing plant microbiomes in sustainable agriculture. PLoS Biol. 2017, 15, e2001793. [Google Scholar] [CrossRef]
- Toju, H.; Tanabe, A.S.; Sato, H. Network hubs in root-associated fungal metacommunities. Microbiome. 2018, 6, 116. [Google Scholar] [CrossRef]
- Hori, Y.; Fujita, H.; Hiruma, K.; Narisawa, K.; Toju, H. Synergistic and offset effects of fungal species combinations on plant performance. Front. Microbiol. 2021, 12, 713180. [Google Scholar] [CrossRef]
- Wu, L.S.; Dong, W.G.; Si, J.P.; Liu, J.J.; Zhu, Y.Q. Endophytic fungi, host genotype, and their interaction influence the growth and production of key chemical components of Dendrobium catenatum. Fungal Biol. 2020, 124, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Batstone, R.T.; Carscadden, K.A.; Afkhami, M.E.; Frederickson, M.E. Using niche breadth theory to explain generalization in mutualisms. Ecology. 2018, 99, 1039–1050. [Google Scholar] [CrossRef]
- Vogt-Schilb, H.; Těšitelová, T.; Kotilínek, M.; Sucháček, P.; Kohout, P.; Jersáková, J. Altered rhizoctonia assemblages in grasslands on ex-arable land support germination of mycorrhizal generalist, not specialist orchids. New Phytol. 2020, 227, 1200–1212. [Google Scholar] [CrossRef]
- Mennicken, S.; Vogt-Schilb, H.; Těšitelová, T.; Kotilínek, M.; Alomía, Y.A.; Schatz, B.; Jersáková, J. Orchid–mycorrhizal fungi interactions reveal a duality in their network structure in two European regions differing in climate. Mol. Ecol. 2023, 32, 3308–3321. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.Y.; Chen, X.M.; Guo, S.X.; Lee, Y.I. Effect of different mycobionts on symbiotic germination and seedling growth of Dendrobium officinale, an important medicinal orchid. Bot. Stud. 2020, 61, 2. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Feng, C.L.; Luo, Y.B.; Chen, B.S.; Wang, Z.S.; Gu, H.Y. Potential challenges of climate change to orchid conservation in a wild orchid hotspot in southwestern China. Bot. Rev. 2010, 76, 174–192. [Google Scholar] [CrossRef]
- Zi, X.M.; Sheng, C.L.; Goodale, U.M.; Shao, S.C.; Gao, J.Y. In situ seed baiting to isolate germination-enhancing fungi for an epiphytic orchid, Dendrobium aphyllum (Orchidaceae). Mycorrhiza 2014, 24, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Miura, C.; Fuji, M.; Nagata, S.; Otani, Y.; Yagame, T.; Yamato, M.; Kaminaka, H. Quantitative evaluation of protocorm growth and fungal colonization in Bletilla Striata (Orchidaceae) reveals less-productive symbiosis with a non-native symbiotic fungus. BMC Plant Biol. 2017, 17, 50. [Google Scholar] [CrossRef]
- Yokoya, K.; Zettler, L.W.; Bell, J.; Kendon, J.P.; Jacob, A.S.; Schofield, E.; Rajaovelona, L.; Sarasan, V. The diverse assemblage of fungal endophytes from orchids in Madagascar linked to abiotic factors and seasonality. Diversity 2021, 13, 96. [Google Scholar] [CrossRef]
- Clemmensen, K.E.; Finlay, R.D.; Dahlberg, A.; Stenlid, J.; Wardle, D.A.; Lindahl, B.D. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 2015, 205, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Wang, J.C.; Bai, X.X.; Yang, Y.B.; Huang, L.; Liao, X.F. Symbiotic seed germination and seedling growth of mycorrhizal fungi in Paphiopedilum hirsutissimun (Lindl.Ex Hook.) stein from China. Plant Signal. Behav. 2023, 18, 2293405. [Google Scholar] [CrossRef]
- Cevallos, S.; Sánchez-Rodríguez, A.; Decock, C.; Declerck, S.; Suárez, J.P. Are there keystone mycorrhizal fungi associated to tropical epiphytic orchids? Mycorrhiza 2017, 27, 225–232. [Google Scholar] [CrossRef]
- Zahn, F.E.; Jiang, H.; Lee, Y.I.; Gebauer, G. Mode of carbon gain and fungal associations of Neuwiedia malipoensis within the evolutionarily early-diverging orchid subfamily Apostasioideae. Ann. Bot. 2024, 134, 511–520. [Google Scholar] [CrossRef]
- Sathiyadash, K.; Muthukumar, T.; Uma, E.; Pandey, R.R. Mycorrhizal association and morphology in orchids. J. Plant Interact. 2012, 7, 238–247. [Google Scholar] [CrossRef]
- Dearnaley, J.D.W.; Cameron, D.D. Nitrogen transport in the orchid mycorrhizal symbiosis—Further evidence for a mutualistic association. New Phytol. 2017, 213, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Fochi, V.; Falla, N.; Girlanda, M.; Perotto, S.; Balestrini, R. Cell-specific expression of plant nutrient transporter genes in orchid mycorrhizae. Plant Sci. 2017, 263, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Shah, B.; Sharma, R.; Rekadwad, B.; Shouche, Y.S.; Sharma, J.; Pant, B. Colonization with non-mycorrhizal culturable endophytic fungi enhances orchid growth and indole acetic acid production. BMC Microbiol. 2022, 22, 101. [Google Scholar] [CrossRef]
- Dearnaley, J.; Perotto, S.; Selosse, M. Structure and development of orchid mycorrhizas. In Molecular Mycorrhizal Symbiosis; Martin, F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 63–86. [Google Scholar] [CrossRef]
- Bender, S.F.; Valadares, R.B.d.S.; Taudiere, A. Mycorrhizas: Dynamic and complex networks of power and influence. New Phytol. 2014, 204, 15–18. [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Burghardt, B.; Gebauer, G.; Bruns, T.D.; Read, D.J. Changing partners in the dark: Isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees. Proc. R. Soc. Lond. B Biol. Sci. 2004, 271, 1799–1806. [Google Scholar] [CrossRef]
- Shan, T.; Zhou, L.; Li, B.; Chen, X.; Guo, S.; Wang, A.; Tian, L.; Liu, J. The plant growth-promoting fungus MF23 (Mycena sp.) increases production of Dendrobium officinale (Orchidaceae) by affecting nitrogen uptake and NH4+ assimilation. Front. Plant Sci. 2021, 12, 693561. [Google Scholar] [CrossRef]
- Bidartondo, M.I.; Read, D.J. Fungal specificity bottlenecks during orchid germination and development. Mol. Ecol. 2008, 17, 3707–3716. [Google Scholar] [CrossRef]
- Waud, M.; Brys, R.; Van Landuyt, W.; Lievens, B.; Jacquemyn, H. Mycorrhizal specificity does not limit the distribution of an endangered orchid species. Mol. Ecol. 2017, 26, 1687–1701. [Google Scholar] [CrossRef]
- Shao, S.C.; Luo, Y.; Jacquemyn, H. Co-cultures of mycorrhizal fungi do not increase germination and seedling development in the epiphytic orchid Dendrobium nobile. Front. Plant Sci. 2020, 11, 571426. [Google Scholar] [CrossRef]
- Xie, W.; Hao, Z.; Guo, L.; Zhang, X.; Zhao, S.; Wang, Y.; Chen, B. Research advances in terpenoids synthesis and accumulation in plants as influenced by arbuscular mycorrhizal symbiosis. Biotechnol. Bull. 2020, 36, 49. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Kaur, J.; Sharma, J. Co-occurring epiphytic orchids have specialized mycorrhizal fungal niches that are also linked to ontogeny. Mycorrhiza 2023, 33, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.K. Endophytic fungal resource of medicinal plants—A treasure need to be developed urgently. Mycosystema 2018, 37, 14–21. [Google Scholar] [CrossRef]
- Qian, S.; Xu, Y.; Zhang, Y.; Wang, X.; Niu, X.; Wang, P. Effect of AMF inoculation on reducing excessive fertilizer use. Microorganisms 2024, 12, 1550. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-H.; Chen, X.-G.; Li, N.-Q.; Li, T.-Q.; Anbazhakan, R.; Gao, J.-Y. Core Mycorrhizal Fungi Promote Seedling Growth in Dendrobium officinale: An Important Medicinal Orchid. Plants 2025, 14, 1024. https://doi.org/10.3390/plants14071024
Wu Y-H, Chen X-G, Li N-Q, Li T-Q, Anbazhakan R, Gao J-Y. Core Mycorrhizal Fungi Promote Seedling Growth in Dendrobium officinale: An Important Medicinal Orchid. Plants. 2025; 14(7):1024. https://doi.org/10.3390/plants14071024
Chicago/Turabian StyleWu, Yi-Hua, Xiang-Gui Chen, Neng-Qi Li, Tai-Qiang Li, Rengasamy Anbazhakan, and Jiang-Yun Gao. 2025. "Core Mycorrhizal Fungi Promote Seedling Growth in Dendrobium officinale: An Important Medicinal Orchid" Plants 14, no. 7: 1024. https://doi.org/10.3390/plants14071024
APA StyleWu, Y.-H., Chen, X.-G., Li, N.-Q., Li, T.-Q., Anbazhakan, R., & Gao, J.-Y. (2025). Core Mycorrhizal Fungi Promote Seedling Growth in Dendrobium officinale: An Important Medicinal Orchid. Plants, 14(7), 1024. https://doi.org/10.3390/plants14071024





