Renewable Energy from Beach-Cast Seaweed: Calorific Power Heating Studies with Macroalgae
Abstract
1. Introduction
2. Results and Discussion
2.1. The Thermal Capacity of Macroalgae from HCV and LCV
2.1.1. Chemical Combustion Elements for Energy Production—Analysis of C, H, N, P, K, O
2.1.2. Analysis of Combustion Macroalgae Biomass to Get Bioenergy
2.2. Meaning Relation About Fibre Elements Energy in Macroalgae
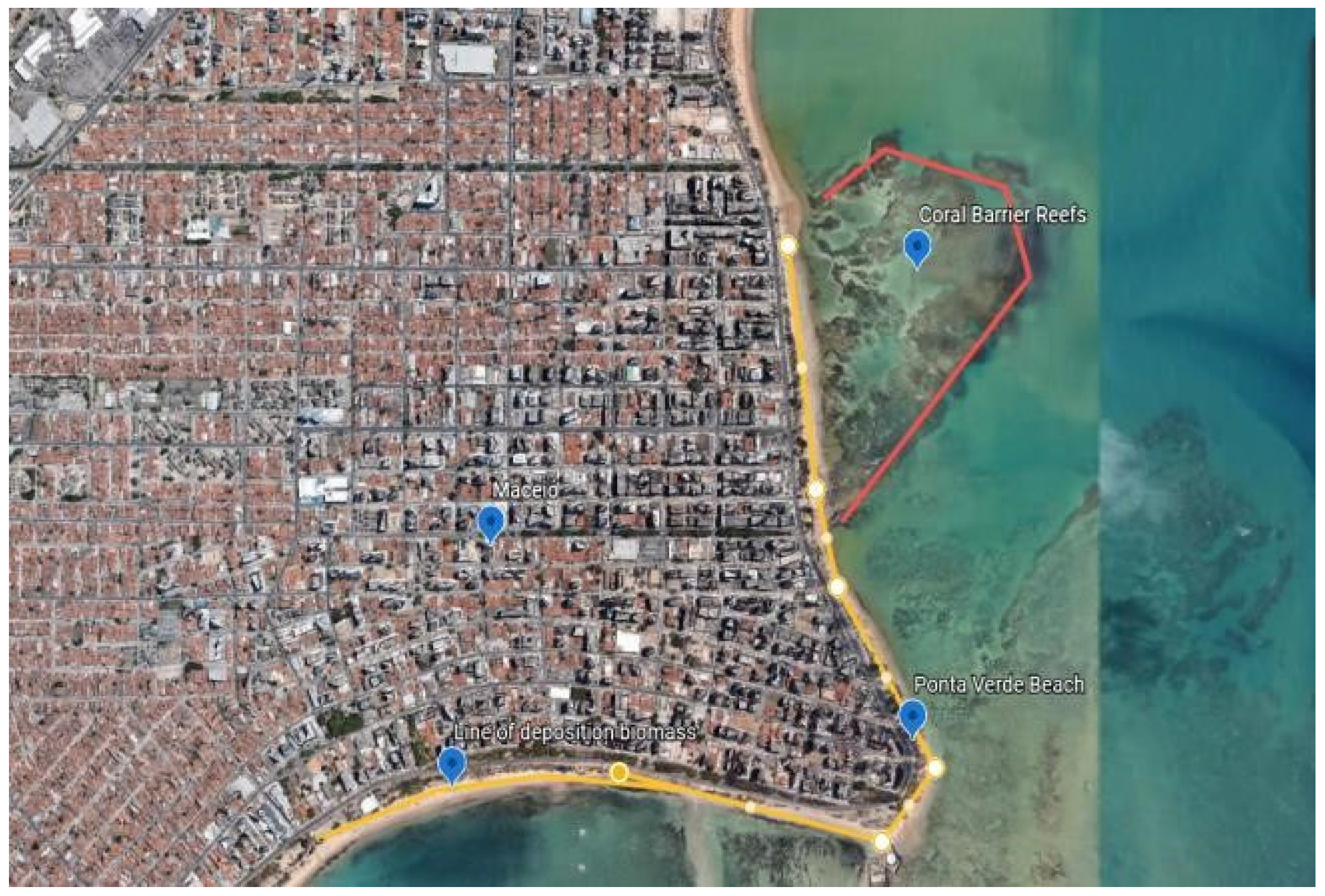
2.3. Sustainability of Macroalgae Biomass Deposition
2.4. Evaluation of Pellets Produced with Heat Capacity
| Types of Pellets | H.C.V. MJ/KG | References | Authors |
|---|---|---|---|
| Wood of Denmark | 20.08 | Bruhn et al. (2011) | [58] |
| Wood of Belgium | 20.31 | V.K. Verma et al. (2012) | [63] |
| Finland peat | 21.63 | V.K. Verma et al. (2012) | [63] |
| Reed canary grass pellets of Finland | 19.25 | V.K. Verma et al. (2012) | [63] |
| Poland apple juice from Industrial waste | 20.68 | V.K. Verma et al. (2012) | [63] |
| Pectin from citrus shell—Denmark | 19.24 | V.K. Verma et al. (2012) | [63] |
| Sunflower husks—Ukraine | 20.27 | V.K. Verma et al. (2012) | [63] |
| Belgium wheat straw | 18.25 | V.K. Verma et al. (2012) | [63] |
| Macroalgae—Brazil | 20.19 | Coelho, F. P. (2024) | [59] |
3. Material and Methods
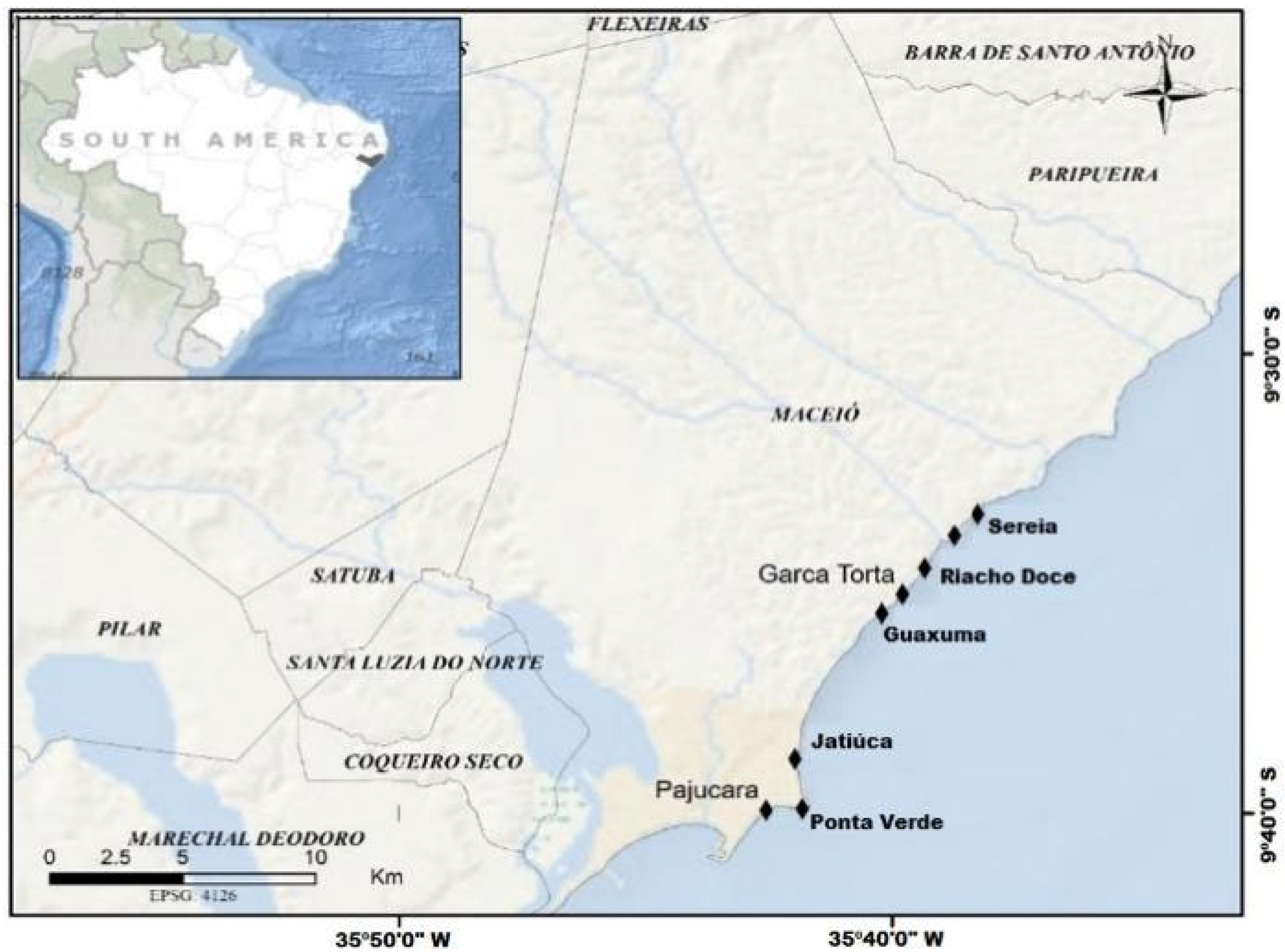
3.1. Collection Methodology: Description of Macroalgae Sampling Studies
Collection Methodology by Moon Tide Table
3.2. Macroalgae Characterization for Evaluation Capacity Energy Calorific Power
3.3. Analysis of Algae Calorific Value
3.4. Energetic Pellets Condensates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alakangas, E.; Heikkinen, A.; Lensu, T.; Vestrsrinen, P. Summary Report; Intelligent Energy Europe: Jyväskylä, Finland, 2007; p. 57. ISSN VTT-R-03508-07. [Google Scholar]
- Bavisi, A.; Srinivas, B.A.; Shah, M.; Pritam, K.S.; Kumar, A. Sustainable Practices Through Carbon Offset Coins: Incentivizing Green Energy and Efficiency. In Proceedings of the 2024 International Conference on Sustainable Energy: Energy Transition and Net-Zero Climate Future (ICUE), Pattaya City, Thailand, 21–23 October 2024; pp. 1–4. [Google Scholar] [CrossRef]
- Balina, K.; Romagnoli, F.; Blumberga, D. Seaweed biorefinery concept for sustainable use of marine resources. Energy Procedia 2017, 128, 504–511. [Google Scholar] [CrossRef]
- Liu, H.; Yao, P.; Latif, S.; Aslam, S.; Iqbal, N. Impact of Green financing, FinTech, and financial inclusion on energy efficiency. Environ. Sci. Pollut. Res. 2022, 29, 18955–18966. [Google Scholar] [CrossRef] [PubMed]
- Roegen, N.G. The Entropy Law and the Economic Process in Retrospect. East. Econ. J. 1986, 12, 3–25. Available online: https://www.jstor.org/stable/40357380 (accessed on 19 February 2025).
- Hickel, J. The contradiction of the Sustainable Development Goals: Growth versus ecology on a finite planet. Sustain. Dev. 2019, 27, 873–884. [Google Scholar] [CrossRef]
- French, D.; Kotzé, L.J. Sustainable Development Goals—Law, Theory and Implementation. In European Yearbook of International Economic Law 2019; Bungenberg, M., Krajewski, M., Tams, C.J., Terhechte, J.P., Ziegler, A.R., Eds.; Springer: Cham, Switzerland, 2019; Volume 10. [Google Scholar] [CrossRef]
- Moro, M.F.; de Souza Mendonça, A.K.; Barni, G.D.A.C.; Bornia, A.C. Transformação global da energia: A participação das energias renováveis na matriz elétrica das 50 maiores economias. MIX Sustentável 2019, 5, 115–123. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. Macroalgae Base; Worldwide Electronic Publication; National University of Ireland: Galway, Ireland, 2017; Available online: https://www.algaebase.org/ (accessed on 11 February 2025).
- Almeida, W.R.; Guimarães, S.M.P.B.; Moura, C.W.D.N. Novas adições à flora marinha bentônica da costa nordeste do Brasil. IHERINGIA Sér. Bot. 2014, 69, 97–105. [Google Scholar]
- Taşkin, E.; Güreşen, A.; Bilgiç, F. Implementation of a new Non-destructive Phytobenthic Index (NPI) with the Posidonia Biotic Index (BiPo) to evaluate the ecological status of the Turkish Aegean coasts—Eastern Mediterranean. Turk. J. Bot. 2024, 48, 296–307. [Google Scholar] [CrossRef]
- Vijay, K.; Balasundari, S.; Jeyashakila, R.; Velayathum, P.; Masilan, K.; Reshma, R. Proximate and Mineral Composition of Brown Seaweed from Gulf of Mannar. Int. J. Fish. Aquat. Stud. 2017, 5, 106–112. Available online: https://www.fisheriesjournal.com/ (accessed on 19 February 2025).
- Ashokkumar, V.; Salim, M.R.; Salam, Z.; Sivakumar, P.; Chong, C.T.; Elumalai, S.; Suresh, V.; Ani, F.N. Production of liquid biofuels (biodiesel and bioethanol) from brown marine macroalgae Padina Tetrastromatica. Energy Convers. Manag. 2017, 135, 351–361. [Google Scholar] [CrossRef]
- Jeyakumar, N.; Hoang, A.T.; Nižetić, S.; Balasubramanian, D.; Kamaraj, S.; Pandian, P.L.; Sirohi, R.; Nguyen, P.Q.P.; Nguyen, X.P. Experimental investigation on simultaneous production of bioethanol and biodiesel from macro-algae. Fuel 2022, 329, 125–362. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.; Nampoothiri, K.M.; Pandey, A. Micro and macroalgal biomass: A renewable source for bioethanol. Bioresour. Technol. 2011, 102, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Chae, S.W.; Park, D.H.; Sunwoo, C. Pretreatment of Gelidium amansii for the production of bioethanol. Bull. Korean Chem. Soc. 2010, 131, 511. [Google Scholar] [CrossRef][Green Version]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Kim, H.J.; Mahadevan, S.A.; Yang, D.J.; Bae, H.J. The potential value of the seaweed Ceylon moss (Gelidium amansii) as an alternative bioenergy resource. Bioresour. Technol. J. 2009, 100, 6658–6660. [Google Scholar] [CrossRef]
- Yoon, J.J.; Kim, S.H.; Ryu, H.J.; Choi, J.Y.; Kim, G.S.; Shin, M.K. Production of polysaccharides and corresponding sugars from red seaweed. Adv. Mater. Res. 2010, 93–94, 463–466. [Google Scholar] [CrossRef]
- Roesijadi, G.; Jones, S.B.; Snowden, L.J.S.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysis; Pacific Northwest National Laboratory: Richland, WA, USA; U.S. Department of Energy: Washington, DC, USA, 2010; pp. 1–50. ISSN PNNL-19944. [Google Scholar] [CrossRef]
- Silva Martins, L.O.; e Alex Santos de Andrade, D. Estimativa teórica do potencial de geração de energia elétrica a partir de cana-de-açúcar, capim elefante e coco da Bahia no Brasil. Textura 2021, 14, 38–58. [Google Scholar] [CrossRef]
- Brand, M.A. Energia de Biomassa Vegetal; Editora Interciência: Rio de Janeiro, Brazil, 2010; p. 131. ISBN 9788571932449. Available online: https://www.editorainterciencia.com.br/index.asp?pg=prodDetalhado.asp&idprod=79 (accessed on 11 February 2025).
- Soares, R.V.; Hakkila, P. Potencial energético dos resíduos de desbastes em plantações de Pinus Taeda no Estado do Paraná, Brasil. Rev. Floresta 1987, 17, 73–94. [Google Scholar] [CrossRef]
- Alburo, C.S.; Conje, R.H.; Pino, M.G.; Tan, E.P. Calorific Values and Proximate Analysis of Sargassum spp. and Ulva spp.; USC Chemical Engineering Student Research Annual; Department of Chemical Engineering, University of San Carlos: Cebu, Philippines, 2010; pp. 1–13. [Google Scholar]
- Roesijadi, G.; Coleman, A.; Judd, C.; Cleve, B.; Buenau, K.; Thom, R.; Ward, J.; Wigmosta, M. Macroalgae Analysis: A National GIS-Based Analysis of Macroalgae Production Potential; Summary Report and Project Plan; U.S. Department of Energy: Washington, DC, USA; Pacific Northwest National Laboratory Richland: Richland, WA, USA, 2011; pp. 1–69. [Google Scholar] [CrossRef]
- Ross, A.B.; Anastasakis, K.; Kubacki, M.; Jones, J.M. Investigation of the pyrolysis behaviour of brown algae before and after pre-treatment using PY-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2009, 85, 3–10. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Lane, C.E.; Mayes, C.; Druehl, L.D.; Saunders, G.W. A multi-gene molecular investigation of the kelps (Laminariales, Ochrophyta) supports substantial taxonomic re-organization. J. Phycol. 2006, 42, 493–512. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Marinho Soriano, E.; Fonseca, P.C.; Carneiro, M.A.A.; Moreira, W.S.C. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 18, 2402–2406. [Google Scholar] [CrossRef]
- Migliore, G.; Alisi, C.; Sprocati, A.R.; Massi, E.; Ciccoli, R.; Lenzi, M.; Wang, A.; Cremisini, C. Anaerobic digestion of macroalgal biomass and sediments sourced from the Orbetello lagoon, Italy. Biomass Bioenergy 2012, 42, 69–77. [Google Scholar] [CrossRef]
- Moreira, A.; Cruz, S.; Marques, R.; Cartaxana, P. The underexplored potential of green macroalgae in aquaculture. Rev. Aquac. 2021, 14, 526. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef]
- Lane, D.J.; Ashman, P.J.; Zevenhoven, M.; Hupa, M.; van Eyk, P.J.; de Nys, R.; Karlström, O.; Lewis, D.M. Combustion Behavior of Algal Biomass: Carbon Release, Nitrogen Release, and Char Reactivity. Energy Fuels 2014, 28, 41–51. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Zhang, M.; Chen, M.; Li, D.; Min, F.; Zang, S.; Ren, Z.; Yan, Y. A comparative study of termolysis characteristics and kinetics of seaweeds and fir wood. Process Biochem. 2006, 41, 1883–1886. [Google Scholar] [CrossRef]
- Kumar, G.; Sahoo, D. Effect of seaweed liquid extract on growth and yield of Triticum aestivum var. Pusa Gold. J. Appl. Phycol. 2011, 23, 251–255. [Google Scholar] [CrossRef]
- Reis, R.P.; Leal, M.C.R.; Yoneshigue-Valentin, Y.; Belluco, F. Efeito de fatores bióticos no crescimento de Hypnea musciformis (Rhodophyta—Gigartinales). Acta Bot. Bras. 2003, 17, 279–286. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, J.H. Ethanol production from Laminaria japonica: Effect of metal ion adsorption. J. Ind. Eng. Chem. 2012, 18, 1662–1665. [Google Scholar] [CrossRef]
- Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194–7222. [Google Scholar] [CrossRef]
- Krause-Jensen, D.; Duarte, C. Substantial role of macroalgae in marine carbon sequestration. Nat. Geosci. 2016, 9, 737–742. [Google Scholar] [CrossRef]
- Gonzalez Pelayo, A. Prospectus for Future Research: Temperature Effects on Green Macroalgae. Bachelor’s Thesis, Portland State University, Portland, OR, USA, 2016; p. 316. [Google Scholar] [CrossRef][Green Version]
- Habeebullah, S.F.K.; Alagarsamy, S.; Sattari, Z.; Al-Haddad, S.; Fakhraldeen, S.; Al-Ghunaim, A.; Al-Yamani, F. Enzyme-assisted extraction of bioactive compounds from brown seaweeds and characterization. J. Appl. Phycol. 2020, 32, 615–629. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Deshmukhe, G.V. Subtidal Marine Algae of the Dwaraka Coast (Gujarat). Indian J. Mar. Sci. 1996, 25, 297–301. Available online: http://drs.nio.org/drs/handle/2264/2157 (accessed on 11 February 2024).
- Wegeberg, S.; Felby, C. Algae biomass for bioenergy in Denmark. In Biological/Technical Challenges and Opportunities; Technical Report; Department of Biology, Science, University of Copenhagen: København, Danmark, 2010; p. 60. Available online: https://www.osti.gov/etdeweb/biblio/992674 (accessed on 11 February 2024).
- Barbot, Y.N.; Benz, R.; Thomsen, L. Thermo-Acidic Pre-treatment of Beach Macroalgae from Rügen to Optimize Biomethane Production—Double Benefit with Simultaneous Bioenergy Production and Improvement of Local Beach and Waste Management. Mar. Drugs 2015, 13, 5681–5705. [Google Scholar] [CrossRef]
- Chemodanov, A.; Jinjikhashvily, G.; Habiby, O.; Liberzon, A.; Israel, A.; Yakhini, Z.; Golberg, A. Net primary productivity, biofuel production and CO2 emissions reduction potential of Ulva sp. (Chlorophyta) biomass in a coastal area of the Eastern Mediterranean. Energy Convers. Manag. 2017, 148, 1497–1507. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. A Review of Seaweed Pre-Treatment Methods for Enhanced Biofuel Production by Anaerobic Digestion or Fermentation. Fermentation 2018, 4, 100. [Google Scholar] [CrossRef]
- Wei, N.; Quarterman, J.; Jin, Y.S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 71. [Google Scholar] [CrossRef]
- Sharma, S.; Hansen, L.D.; Hansen, J.Ø.; Mydland, L.T.; Horn, S.J.; Øverland, M.; Eijsink, V.G.H.; Vuoristo, K.S. Microbial Protein Produced from Brown Seaweed and Spruce Wood as a Feed Ingredient. J. Agric. Food Chem. 2018, 66, 8328–8335. [Google Scholar] [CrossRef]
- Nielsen, N.P.K.; Gardner, D.G.; Felby, C. Effect of extractives and storage on the pelletizing of sawdust. Fuel 2009, 89, 94–98. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulphated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Coelho, F.P.; Menezes, R.S.C.; Sampaio, E.V.d.S.B.; Barboza, M.G.; Soares, E.C.; Guedes-Coelho, E.A.C.; de França, E.J.; dos Santos, A.J.; de Lima, M.F.; Costa, M.M.d.S.; et al. Biorefinery of Beach Cast Seaweed in Brazil: Renewable Energy and Sustainability. Phycology 2024, 4, 394–413. [Google Scholar] [CrossRef]
- Ladeira, A.M.; Zaidan, L.B.P.; Figueiredo, R.C.L. Ageratum conyzoides L. Compositae: Germinação, floração e ocorrência de derivados fenólicos em diferentes estágios de desenvolvimento. Hoehnea 1987, 15, 53–62. [Google Scholar]
- Tabassum, M.R.; Xia, A.; Murphy, J.D. Comparison of pre-treatments to reduce salinity and enhance biomethane yields of Laminaria digitata harvested in different seasons. Energy 2017, 140, 546–551. [Google Scholar] [CrossRef]
- Guedes, E.A.C.; Moura, A.D.N. Estudo da Biomassa e composição mineral de “algas arribadas” em Praias do Litoral Norte de Alagoas. Bol. Estud. Ciênc. Mar. 1996, 9, 19–30. [Google Scholar]
- Allen, E.; Wall, D.M.; Herrmann, C.; Murphy, J.D. Investigation of the optimal percentage of green seaweed that may be co-digested with dairy slurry to produce gaseous biofuel. Bioresour. Technol. 2014, 170, 436–444. [Google Scholar] [CrossRef]
- Brown, L.M.; Zeiler, K.G. Aquatic biomass and carbon dioxide trapping. Energy Convers. Manag. 1993, 34, 1005–1013. [Google Scholar] [CrossRef]
- Bruhn, A.; Dahl, J.; Bangs, H.N.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of Ulva Lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef]
- Coelho, F.P.; Barboza, M.G.; Guedes-Coelho, É.A.C.; Costa, M.M.d.S.; dos Santos, A.J.; de Lima, M.F.; da Silva, E.C.S.; Carneiro, V.A.R.; Soares, B.M.; de França, E.J.; et al. Sustainability of beach cast seaweed biomass for biorefinery process: Sampling studies in the littoral of Alagoas/Brazil. Contemp. J. 2024, 4, e5306. [Google Scholar] [CrossRef]
- Kuokkanen, M.; Vilpo, T.; Kuokkanen, T.; Stoor, T.; Ninimaki, J. Additives in wood pellet production—A pilot scale study of binding agent usage. Bioresources 2011, 6, 4331–4355. [Google Scholar] [CrossRef]
- Lethikangas, P. Quality properties of pelletised sawdust, logging residues and bark. Biomass Bioenergy 2001, 19, 351–360. [Google Scholar] [CrossRef]
- Arromdee, P.; Ninduangdee, P. Combustion characteristics of pelletized-biomass fuels: A thermogravimetric analysis and combustion study in a fluidized bed combustor. Energy Ecol. Environ. 2023, 8, 69–88. [Google Scholar] [CrossRef]
- Verma, V.K.; Bram, S.; Delattin, F.; Laha, P.; Vandendael, I.; Hubin, A.; De Ruyck, J. Agro-pellets for domestic heating boilers: Standard laboratory and real-life performance. Appl. Energy 2012, 90, 17–23. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J. Heating values of wood pellets from different species. Biomass Bioenergy 2011, 35, 2634–2639. [Google Scholar] [CrossRef]
- Guimarães, S.M.P.B.; Pereira, A.P.V. Rodofíceas marinhas bentônicas do estado do Espírito Santo, Brasil: Gênero Calliblepharis (Cystocloniaceae, Gigartinales). Hoehnea 1993, 20, 35–46. [Google Scholar]
- Lopes, A.S. Estudos Taxonômicos da Ordem Dictyotales (Ochrophyta) da Praia de Serrambi (Município de Ipojuca—Estado de Pernambuco—Brasil). Master’s Thesis, Universidade Federal Rural de Pernambuco, Recife, Brazil, 1993; p. 110. [Google Scholar]
- Nunes, J.M.C.; Santos, A.C.C.; Minervino, A.; Brito, K.S. Algas marinhas bentônicas do município de Ilhéus, Bahia, Brasil. Acta Bot. Malacit. 1999, 24, 5–12. [Google Scholar]
- Nunes, J.M.C.; Paula, E.J. O gênero Dictyota Lamour. (Dictyotaceae-Ochrophyta) no Litoral do estado da Bahia. Acta Bot. Malacit. 2001, 26, 5–18. Available online: https://hdl.handle.net/10630/3999 (accessed on 19 February 2025).
- Nunes, J.M.C.; Barros-Barreto, M.B.; Guimarães, S.M.P.B. A família Ceramiaceae (Ceramiales, Rhodphyta) no estado da Bahia, Brasil. Monogr. Ficológicas 2008, 3, 75–159. [Google Scholar]
- Bast, F. Ancestors of land plants with rich diversity. Resonance 2014, 1, 1032–1043. [Google Scholar] [CrossRef]
- Van Soest, P.J. Use of detergent in analysis of fibrous feeds; Study of effects of heating and drying on yield of fiber and lignin in forages. J. Assoc. Off. Agric. Chem. 2020, 48, 785–790. [Google Scholar] [CrossRef]
- ASTM D-240-64; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter. Annual Book of ASTM Standards. ASTM: West Conshohocken, PA, USA, 2013.



| Species | Phylum | HCV/MJ/Kg | LCV/MJ/Kg |
|---|---|---|---|
| Cryptonemia luxurians | Rhodophyta | 11.43 | 10.04 |
| Sargassum sp. | Ochrophyta | 10.68 | 9.67 |
| Ulva fasciata | Chlorophyta | 8.21 | 6.76 |
| Cryptonemia crenulata | Rhodophyta | 12.02 | 10.87 |
| Lobophora variegata | Ochrophyta | 10.58 | 9.58 |
| Gracilaria sp. | Rhodophyta | 11.14 | 9.91 |
| Cryptonemia seminervis | Rhodophyta | 9.67 | 8.99 |
| Ulva lactuca | Chlorophyta | 11.43 | 9.38 |
| Hydropuntia cornea | Rhodophyta | 11.42 | 9.86 |
| Padina sp. | Ochrophyta | 8.32 | 7.31 |
| Caulerpa microphysa | Chlorophyta | 6.37 | 5.97 |
| Hypnea pseudomusciformis | Rhodophyta | 8.73 | 6.51 |
| Sargassum vulgare | Ochrophyta | 11.19 | 9.79 |
| Average | - | 10.09 | 8.82 |
| Species/Biomass | Weight/g | Carbon % | Hydrogen % | Nitrogen % | Phosphorus g/Kg | Potassium g/Kg |
|---|---|---|---|---|---|---|
| Cryptonemia sp. | 3.1 | 37.94 | 6.54 | 3.74 | 0.730 | 3.113 |
| Sargassum sp. | 3.1 | 28.99 | 4.78 | 1.30 | 0.048 | 19.869 |
| Ulva fasciata | 3.1 | 29.51 | 6.86 | 1.62 | 0.236 | 5.691 |
| Cryptonemia crenulata | 2.8 | 30.42 | 5.44 | 3.03 | 0.582 | 8.022 |
| Lobophora variegata | 3.1 | 30.62 | 4.72 | 1.19 | 0.470 | 7.606 |
| Gracilaria sp. | 2.9 | 33.62 | 5.8 | 2.55 | 0.225 | 34.872 |
| Cryptonemia seminervis | 3 | 20.60 | 3.22 | 2.35 | 1.255 | 6.197 |
| Ulva lactuca | 2.9 | 50.08 | 9.64 | 4.18 | 0.301 | 4.819 |
| Hydropuntia cornea | 3.1 | 41.97 | 7.33 | 0.95 | ND | 34.014 |
| Padina sp. | 3.1 | 28.21 | 4.79 | 1.59 | 0.284 | 9.181 |
| Caulerpa microphysa | 3 | 16.58 | 1.88 | 0.98 | 1.186 | 1.466 |
| Hypnea pseudomusciformis | 3 | 61.87 | 10.44 | 4.79 | 0.658 | 50.068 |
| Sargassum vulgare | 2.9 | 38.60 | 6.6 | 3.33 | 0.595 | 9.412 |
| Aggregate biomass | 2.9 | 43.97 | 6.73 | 4.53 | 0.593 | 8.795 |
| Seaweed Species | Phylum | Lignin (%) | Cellulose (%) | Ashes (%) |
|---|---|---|---|---|
| Sargassum sp. | Ochrophyta | 9.41 | 11.76 | 8.06 |
| Cryptonemia crenulata | Rhodophyta | 2.44 | 9.27 | 2.46 |
| Lobophora variegata | Ochrophyta | 5.27 | 23.29 | 4.04 |
| Gracilaria sp. | Rhodophyta | 1.98 | 7.17 | 3.16 |
| Cryptonemia seminervis | Rhodophyta | 4.11 | 8.33 | 3.86 |
| Ulva lactuca | Chlorophyta | 9.13 | 9.57 | 4.15 |
| Hydropuntia cornea | Rhodophyta | 0.31 | 5.13 | 1.86 |
| Padina sp. | Ochrophyta | 12.72 | 10.42 | 10.56 |
| Caulerpa microphysa | Chlorophyta | 13.74 | 13.92 | 9.79 |
| Hypnea pseudomusciformis | Rhodophyta | 8.58 | 8.23 | 7.32 |
| Sargassum vulgare | Ochrophyta | 7.13 | 17.72 | 4.33 |
| Aggregate biomass | - | 7.29 | 12.01 | 3.75 |
| Overall average | 6.81 | 11.28 | 5.30 |
| Date | Hours | Tide Height/m | Moon Phases | |
|---|---|---|---|---|
| 12 March 2015 | 13.47 m | 0.6 | full moon |  |
| 4 April 2015 | 9.47 m | 0.2 | full moon |  |
| 5 April 2015 | 10.15 m | 0.2 | full moon |  |
| 5 March 2016 | 7.11 m | 0.6 | waning moon |  |
| 12 March 2016 | 12.09 m | 0.1 | new moon |  |
| 17 September 2016 | 10.00 m | 0.0 | full moon |  |
| 15 October 2016 | 8.56 m | 0.0 | waxing moon |  |
| 16 October 2016 | 9.38 m | 0.0 | full moon |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, F.P.; Sampaio, E.V.d.S.B.; Barboza, M.G.; Guedes-Coelho, E.A.C.; Costa, M.M.d.S.; Silva, E.C.S.d.; Carneiro, V.A.R.; Soares, B.M.; França, E.J.d.; Menezes, R.S.C.; et al. Renewable Energy from Beach-Cast Seaweed: Calorific Power Heating Studies with Macroalgae. Plants 2025, 14, 1005. https://doi.org/10.3390/plants14071005
Coelho FP, Sampaio EVdSB, Barboza MG, Guedes-Coelho EAC, Costa MMdS, Silva ECSd, Carneiro VAR, Soares BM, França EJd, Menezes RSC, et al. Renewable Energy from Beach-Cast Seaweed: Calorific Power Heating Studies with Macroalgae. Plants. 2025; 14(7):1005. https://doi.org/10.3390/plants14071005
Chicago/Turabian StyleCoelho, Fernando Pinto, Everardo Valadares de Sá Barreto Sampaio, Márcio Gomes Barboza, Elica Amara Cecília Guedes-Coelho, Manoel Messias da Silva Costa, Emerson Carlos Soares da Silva, Victor Andrei Rodrigues Carneiro, Bruno Moreira Soares, Elvis Joacir de França, Rômulo Simões Cezar Menezes, and et al. 2025. "Renewable Energy from Beach-Cast Seaweed: Calorific Power Heating Studies with Macroalgae" Plants 14, no. 7: 1005. https://doi.org/10.3390/plants14071005
APA StyleCoelho, F. P., Sampaio, E. V. d. S. B., Barboza, M. G., Guedes-Coelho, E. A. C., Costa, M. M. d. S., Silva, E. C. S. d., Carneiro, V. A. R., Soares, B. M., França, E. J. d., Menezes, R. S. C., & Abreu, C. A. M. d. (2025). Renewable Energy from Beach-Cast Seaweed: Calorific Power Heating Studies with Macroalgae. Plants, 14(7), 1005. https://doi.org/10.3390/plants14071005







