Temporal Variations in Photosynthesis and Leaf Element Contents of ‘Marselan’ Grapevines in Response to Foliar Fertilizer Application
Abstract
1. Introduction
2. Results
2.1. Effects of Different Treatments on Leaf Area, Leaf SPAD Value, New Shoot Base Thickness, and Internode Length of ‘Marselan’ Grape
2.2. Effects of Different Treatments on Photosynthetic Characteristics of ‘Marselan’ Grape Leaves
2.3. Effects of Different Treatments on Fluorescence Parameters of ‘Marselan’ Grape Leaves
2.4. Effects of Different Treatments on Mineral Element Content in Grape Leaves of ‘Marselan’
2.5. Comprehensive Evaluation of Shoot Growth, Leaf Photosynthetic Characteristics, and Mineral Element Content of ‘Marselan’ Grape Under Different Treatments
3. Discussion
4. Materials and Methods
4.1. Environmental Characteristics of the Test Area
4.2. Test Materials and Design
4.3. Determination of Indexes and Methods
4.3.1. Determination of Root Thickness, Internode Length, Leaf SPAD Value, and Leaf Area of Grape
4.3.2. Determination of Photosynthetic Parameters in Grape Leaves
4.3.3. Determination of Fluorescence Parameters of Grape Leaves
4.3.4. Determination of Element Content in Leaves
4.4. Data Processing
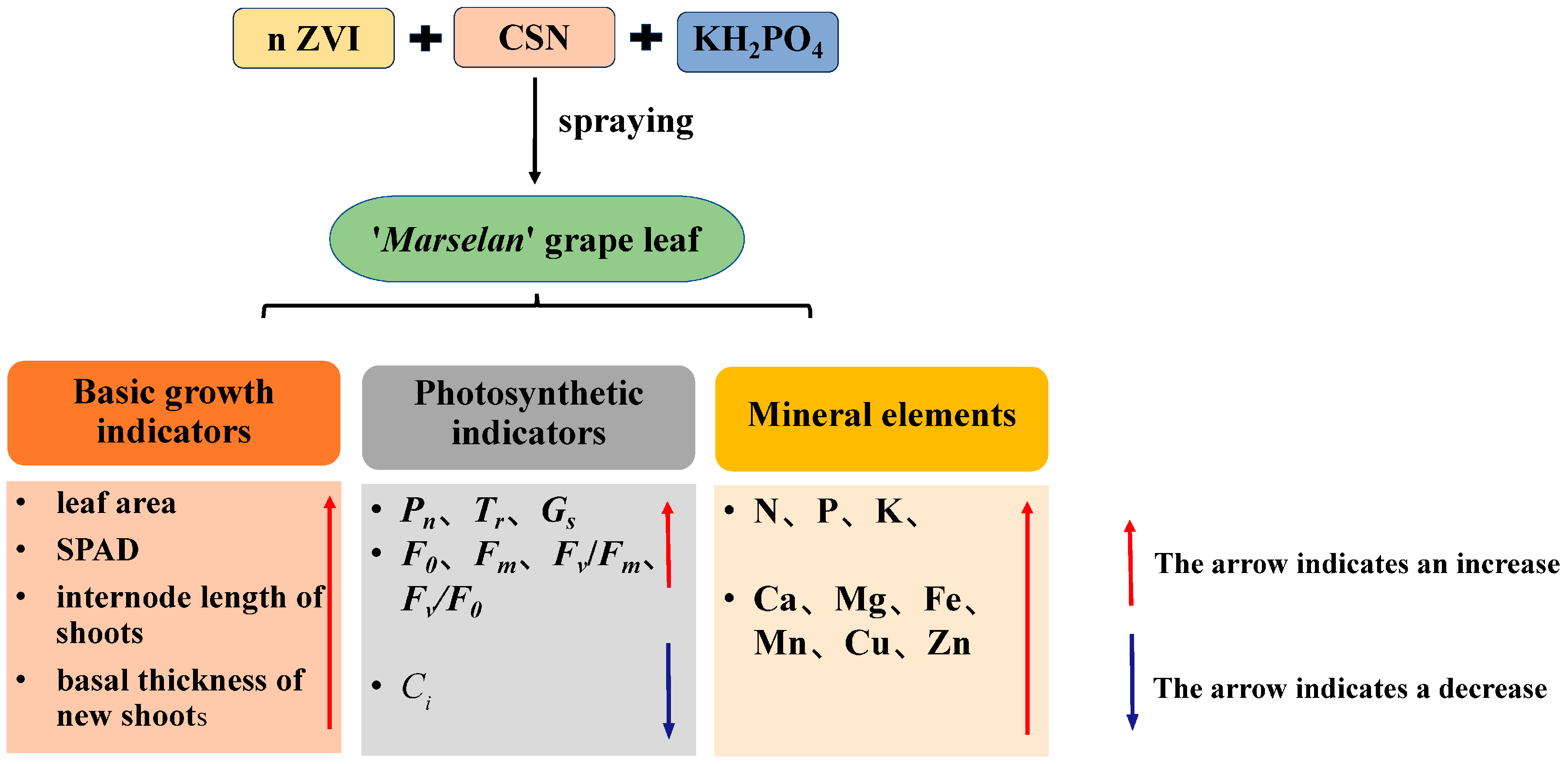
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, T.Y.; Hao, T.Y.; Zhang, P.; Hakeem, A.; Zhao, P.C.; Song, S.Y.; Ren, Y.H.; Chen, Y.H.; Jia, H.F.; Fang, J.G. A comprehensive evaluation of different responses of supplementary light qualities on physiological and biochemical mechanisms of ‘Kyoho’ grape. Sci. Hortic. 2024, 333, 113261. [Google Scholar] [CrossRef]
- Ren, R.H.; Shi, J.J.; Zeng, M.Y.; Tang, Z.Z.; Xie, S.; Zhang, Z.W. Inter- and intra-varietal genetic variations co-shape the polyphenol profiles of Vitis vinifera L. grapes and wines. Food Chem. X 2023, 20, 101030. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Ma, X.W.; Wang, F.C.; Li, H.Y.; Nie, X.; Fu, Q.F. Climatic drivers and temporal dynamics of sandstorms in the Hexi Corridor, China. Clim. Serv. 2025, 37, 100532. [Google Scholar] [CrossRef]
- Du, D.D.; Dong, B.; Zhang, R.; Cui, S.A.; Chen, G.R.; Du, F.F. Spatiotemporal dynamics of irrigated cropland water use efficiency and driving factors in northwest China’s Hexi Corridor. Ecol. Process. 2024, 13, 72. [Google Scholar] [CrossRef]
- Hao, Y.; Yan, H.; Wang, Y.J.; Li, Y.H.; Cui, K.X.; Xue, T.T.; Peng, S. Climate regionalization of various wine grape varieties in Hexi Corridor, China. IOP Conf. Ser. Earth Environ. Sci. 2020, 559, 012009. [Google Scholar] [CrossRef]
- Ferrari, M.; Bertin, V.; Bolla, P.K.; Valente, F.; Panozzo, A.; Giannelli, G.; Visioli, G.; Vamerali, T. Application of the full nitrogen dose at decreasing rates by foliar spraying versus conventional soil fertilization in common wheat. J. Agric. Food Res. 2025, 19, 101602. [Google Scholar] [CrossRef]
- Ghodke, P.B.; Prasanna, J.; Ubale, S.P.; Warpe, S.T.; Mirza, I.A.B. Impact of Organic Foliar Formulations on Growth and Yield of Okra (Abelmoschus esculentus). J. Adv. Biol. Biotechnol. 2024, 27, 637–642. [Google Scholar] [CrossRef]
- Hosseinabad, A.; Khadivi, A. The Effect of Microelements on Qualitative and Quantitative Characteristics of Vitis vinifera cv. Thompson Seedless. Erwerbs-Obstbau 2019, 61, 41–46. [Google Scholar] [CrossRef]
- Rustioni, L.; Grossi, D.; Brancadoro, L.; Failla, O. Characterization of iron deficiency symptoms in grapevine (Vitis spp.) leaves by reflectance spectroscopy. Plant Physiol. Biochem. 2017, 118, 342–347. [Google Scholar] [CrossRef]
- Jalali, M.; Ghanati, F.; ModarresSanavi, A.M.; Khoshgoftarmanesh, A.H. Physiological effects of repeated foliar application of magnetite nanoparticles on maize plants. J. Agron. Crop Sci. 2017, 203, 593–602. [Google Scholar] [CrossRef]
- Gattullo, C.E.; Pii, Y.; Allegretta, T.A.; Medici, L.; Cesco, S.; Mimmo, T.; Terzano, R. Iron Mobilization and Mineralogical Alterations Induced by Iron-Deficient Cucumber Plants (Cucumis sativus L.) in a Calcareous Soil. Pedosphere 2018, 28, 59–69. [Google Scholar] [CrossRef]
- Abbas, J.; Mehrnaz, H. Foliar-applied nanoscale zero-valent iron (n ZVI) and iron oxide (Fe3O4) induce differential responses in growth, physiology, antioxidative defense and biochemical indices in Leonurus cardiaca L. Environ. Res. 2022, 215 Pt 2, 114254. [Google Scholar]
- Yoon, H.; Kang, Y.G.; Chang, Y.S.; Kim, J.H. Effects of Zero valent Iron Nanoparticles on Photosynthesis and Biochemical Adaptation of Soil-Grown Arabidopsis thaliana. Nanomaterials 2019, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.S.; Sun, M.T.; Li, Y.S.; Feng, X.J.; Li, M.L.; Shi, A.K.; He, C.X.; Yan, Y.; Wang, J.; Yu, X.C. Sodium nitrophenolate mediates brassinosteroids signaling to enhance cold tolerance of cucumber seedling. Plant Physiol. Biochem. 2024, 206, 108317. [Google Scholar]
- Zarina, B.; Muhammad, I.; Nadeem, A.; Usman, Z.; Ahmad, A.S.; Muhammad, S.; Shahid, N.; Abida, A. Exogenous application of Atonik (sodium nitrophenolate) under skip irrigation regimes modulated the physiology, growth and productivity of Zea mays L. Arch. Agron. Soil Sci. 2023, 69, 2325–2339. [Google Scholar]
- Zhao, T.; Xie, S.; Zhang, Z.W. Effects of foliar-sprayed potassium dihydrogen phosphate on accumulation of flavonoids in Cabernet Sauvignon (Vitis vinifera L.). J. Sci. Food Agric. 2023, 103, 4838–4849. [Google Scholar] [CrossRef]
- SarmientoLópez, L.G.; LópezMeyer, M.; SepúlvedaJiménez, G.; Cárdenas, L.; RodríguezMonroy, M. Photosynthetic performance and stevioside concentration are improved by the arbuscular mycorrhizal symbiosis in Stevia rebaudiana under different phosphate concentrations. PeerJ 2020, 8, e10173. [Google Scholar]
- Ma, Q.H.; Wang, X.H.; Yuan, W.J.; Tang, H.L.; Luan, M.B. The Optimal Concentration of KH2PO4 Enhances Nutrient Uptake and Flower Production in Rose Plants via Enhanced Root Growth. Agriculture 2021, 11, 1210. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, Z.X.; Li, B.; Zhang, H.H.; Hu, J.; Zhao, J. Photosynthetic rate prediction model of newborn leaves verified by core fluorescence parameters. Sci. Rep. 2020, 10, 3013. [Google Scholar] [CrossRef]
- Chou, S.R.; Chen, B.; Chen, J.; Wang, M.M.; Wang, S.Q.; Holly, C.; Qin, S. Estimation of leaf photosynthetic capacity from the photochemical reflectance index and leaf pigments. Ecol. Indic. 2020, 110, 110105867. [Google Scholar] [CrossRef]
- Jin, L.Q.; Che, X.K.; Zhang, Z.S.; Li, Y.T.; Gao, H.Y.; Zhao, S.J. The mechanisms by which phenanthrene affects the photosynthetic apparatus of cucumber leaves. Chemosphere 2017, 168, 1498–1505. [Google Scholar] [CrossRef] [PubMed]
- Amin, P.P.; Hassan, F.; Enayatolah, T. Nitrogen, phosphorous and potassium levels affected growth indices, leaf gas exchange parameters and biomass production of henna (Lawsonia inermis L.) ecotypes. Ind. Crops Prod. 2021, 163, 113297. [Google Scholar]
- Sun, K.W.; Zhang, J.; Wang, Y.; Qian, W.J.; Gong, S.T.; Li, Z.P.; Song, Y.J.; Yin, X.Y.; Ding, Z.T.; Fan, K. Foliar spraying KH2PO4 promotes shoot development by inducing stomatal opening and sugar transport in tea leaves. Sci. Hortic. 2024, 337, 113588. [Google Scholar] [CrossRef]
- Zait, Y.; Shemer, O.E.; Cochavi, A. Dynamic responses of chlorophyll fluorescence parameters to drought across diverse plant families. Physiol. Plant. 2024, 176, e14527. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Zhao, K. Root-exuded malic acid versus chlorophyll fluorescence parameters in four plant species under different phosphorus levels. J. Soil Sci. Plant Nutr. 2013, 13, 604–610. [Google Scholar] [CrossRef]
- Mozdzer, T.J.; Megonigal, J.P. Jack-and-master trait responses to elevated CO2 and N: A comparison of native and introduced Phragmites australis. PLoS ONE 2012, 7, e42794. [Google Scholar] [CrossRef]
- Gang, L.; Yang, Y.B.; Zhu, Z.H. Elevated nitrogen allows the weak invasive plant Galinsoga quadriradiata to become more vigorous with respect to inter-specific competition. Sci. Rep. 2018, 8, 3136. [Google Scholar]
- Olusegun, O.O.; Deanna, B.; Dane, P.F.; Vivian-Smith, G. Leaf trait co-ordination in relation to construction cost, carbon gain and resource-use efficiency in exotic invasive and native woody vine species. Ann. Bot. 2010, 106, 371–380. [Google Scholar]
- Zhang, H.Q.; Liu, X.W.; Nie, B.H.; Song, B.T.; Du, P.; Liu, S.X.; Li, L.; Zhao, Z.Q. Nitrogen management can inhibit or induce the sprouting of potato tubers: Consequences of regulation tuberization. Postharvest Biol. Technol. 2022, 183, 111722. [Google Scholar] [CrossRef]
- Yang, K.B.; Zhu, C.L.; Zhang, J.B.; Li, Z.Y.; Liu, Y.; Song, X.Z.; Gao, Z.M. Nitrogen fertilization in bamboo forest accelerates the shoot growth and alters the lignification process in shoots. Ind. Crops Prod. 2022, 187 Pt A, 115368. [Google Scholar] [CrossRef]
- Hu, W.J.; Wang, J.; Deng, Q.X.; Liang, D.; Xia, H.; Lin, L.J.; Lv, X.L. Effects of Different Types of Potassium Fertilizers on Nutrient Uptake by Grapevine. Horticulturae 2023, 9, 470. [Google Scholar] [CrossRef]
- Arrobas, M.; Ferreira, I.Q.; Freitas, S.; Verdial, J.; Rodrigues, M.A. Guidelines for fertilizer use in vineyards based on nutrient content of grapevine parts. Sci. Hortic. 2014, 17, 2191–2198. [Google Scholar] [CrossRef]
- Kodur, S.; Tisdall, J.-M.; Tang, C.; Walker, R.R. Accumulation of potassium in grapevine rootstocks (Vitis) grafted to ‘Shiraz’ as affected by growth, root-traits and transpiration. Vitis J. Grapevine Res. 2010, 49, 7–13. [Google Scholar]
- Mónica, R.C.; Arthur, W.; Karl, J.N. Spatiotemporal distribution of essential elements through Populus leaf ontogeny. J. Exp. Bot. 2016, 67, 2777–2786. [Google Scholar]
- Gajdanowicz, P.; Michard, E.; Sandmann, M.; Rocha, M.; Guedes Corrêa, L.G.; Ramírez-Aguilar, S.J.; Gomez-Porras, J.L.; González, W.; Thibaud, J.B.; van Dongen, J.T.; et al. Potassium (K+) gradients serve as a mobile energy source in plant vascular tissues. Proc. Natl. Acad. Sci. USA 2011, 108, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Yuliya, V.; Susanne, I.S.; Olga, E.K.; Yury, V.P.; Nikolay, I.K.; Nina, N.K. Species-dependent effect of cover cropping on trace elements and nutrients in vineyard soil and Vitis. J. Sci. Food Agric. 2020, 100, 885–890. [Google Scholar]
- Gąstol, M.; Domagała Świątkiewicz, I. Trace Element Partitioning in ‘Sibera’ Grapevines as Affected by Nitrogen Fertilisation. S. Afr. J. Enol. Vitic. 2016, 35, 217–225. [Google Scholar] [CrossRef]
- Merle, T.; Ershad, T.; Bálint, J. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar]
- Ye, X.; Chen, X.F.; Deng, C.L.; Yang, L.T.; Lai, N.W.; Guo, J.X.; Chen, L.S. Magnesium-Deficiency Effects on Pigments, Photosynthesis and Photosynthetic Electron Transport of Leaves, and Nutrients of Leaf Blades and Veins in Citrus sinensis Seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef]
- Abou ElNasr, M.K.; ElHennawy, H.M.; Samaan, M.S.F.; Salaheldin, T.A.; Abou ElYazied, A.; ElKereamy, A. Using Zinc Oxide Nanoparticles to Improve the Color and Berry Quality of Table Grapes Cv. Crimson Seedless. Plants 2021, 10, 1285. [Google Scholar] [CrossRef]
- Juliane, M.; Sabrina, G.; Adriana, G.; Henrique, N.; Tomas, F.D.; Alacimar, G.; Aleixo, I.; Plínio, C.; Jéssica, C.; Amanda, D.; et al. Changes in leaf functional traits with leaf age: When do leaves decrease their photosynthetic capacity in Amazonian trees? Tree Physiol. 2021, 42, 922–938. [Google Scholar]
- Hu, M.M.; Dou, Q.H.; Cui, X.M.; Lou, Y.H.; Zhuge, Y.P. Polyaspartic acid mediates the absorption and translocation of mineral elements in tomato seedlings under combined copper and cadmium stress. J. Integr. Agric. 2019, 18, 1130–1137. [Google Scholar] [CrossRef]
- Brodowska, M.S.; Wyszkowski, M.; Bujanowicz-Haraś, B. Mineral Fertilization and Maize Cultivation as Factors Which Determine the Content of Trace Elements in Soil. Agronomy 2022, 12, 286. [Google Scholar] [CrossRef]
- Krejsa, J.; Šíma, J.; Křížek, M.; Šeda, M.; Svoboda, L. Selected detrimental and essential elements in fruiting bodies of culinary and toxic medicinal macroscopic fungi growing in the Bohemian Forest, the Czech Republic. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2024, 59, 483–496. [Google Scholar] [CrossRef]
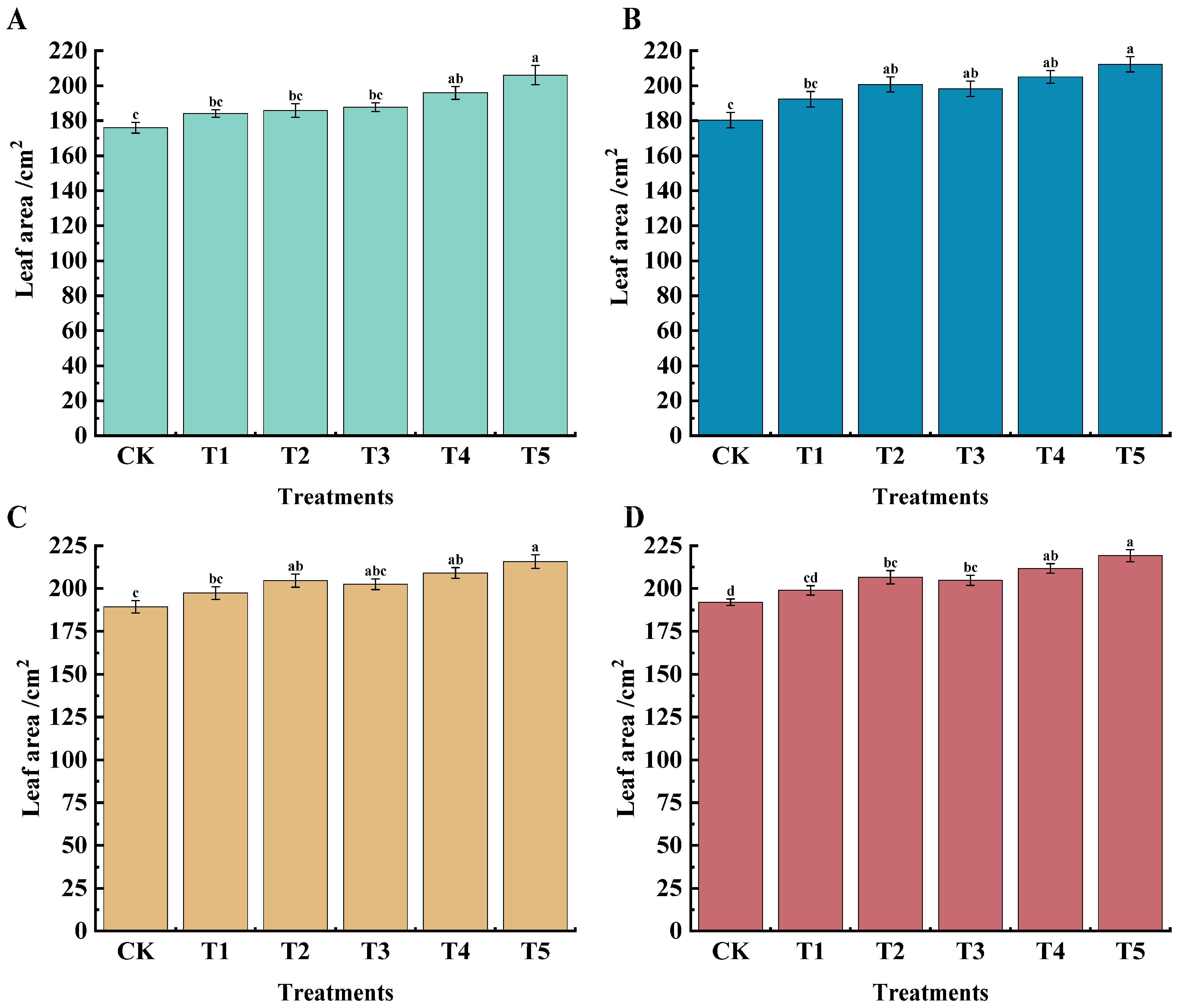
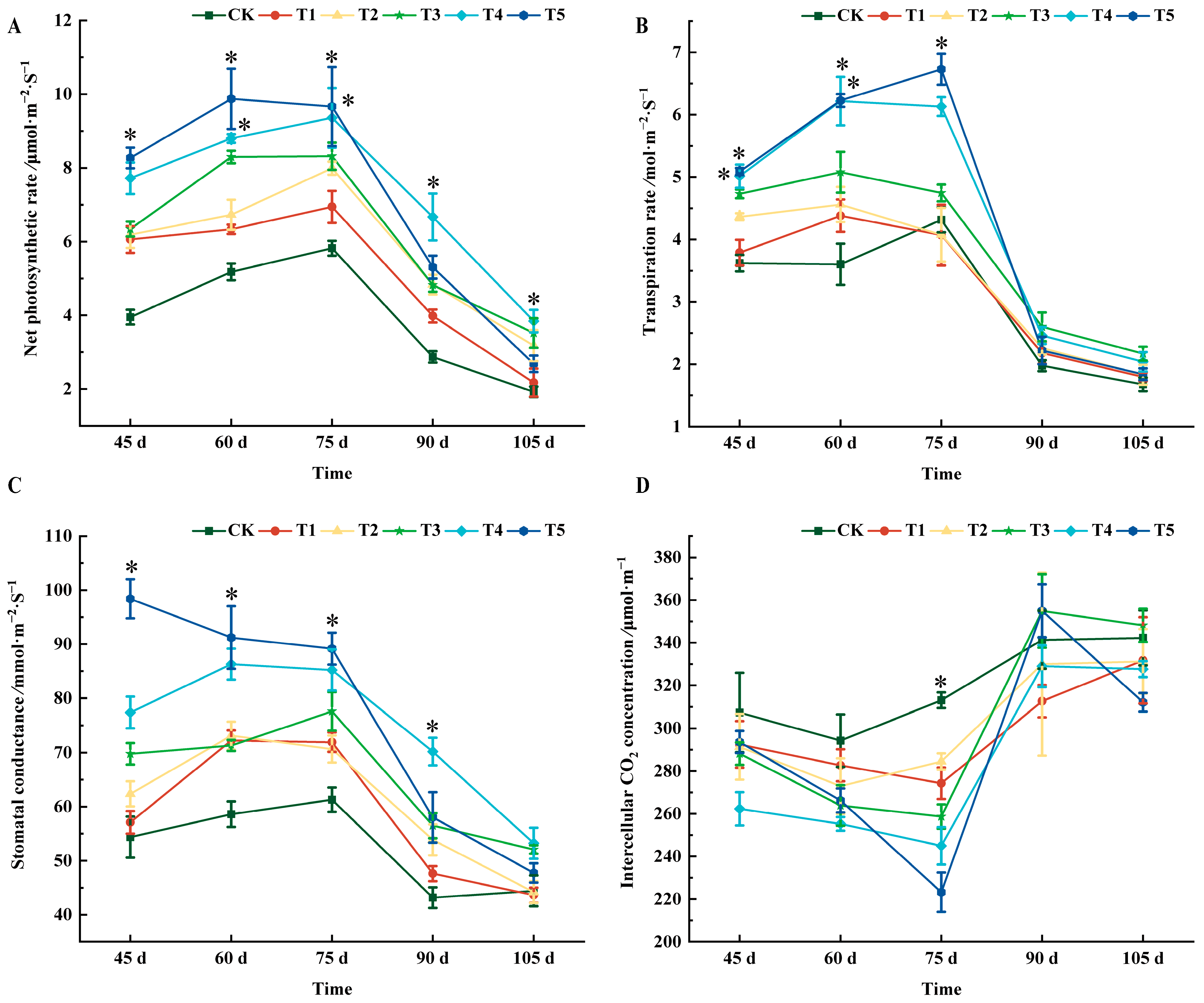
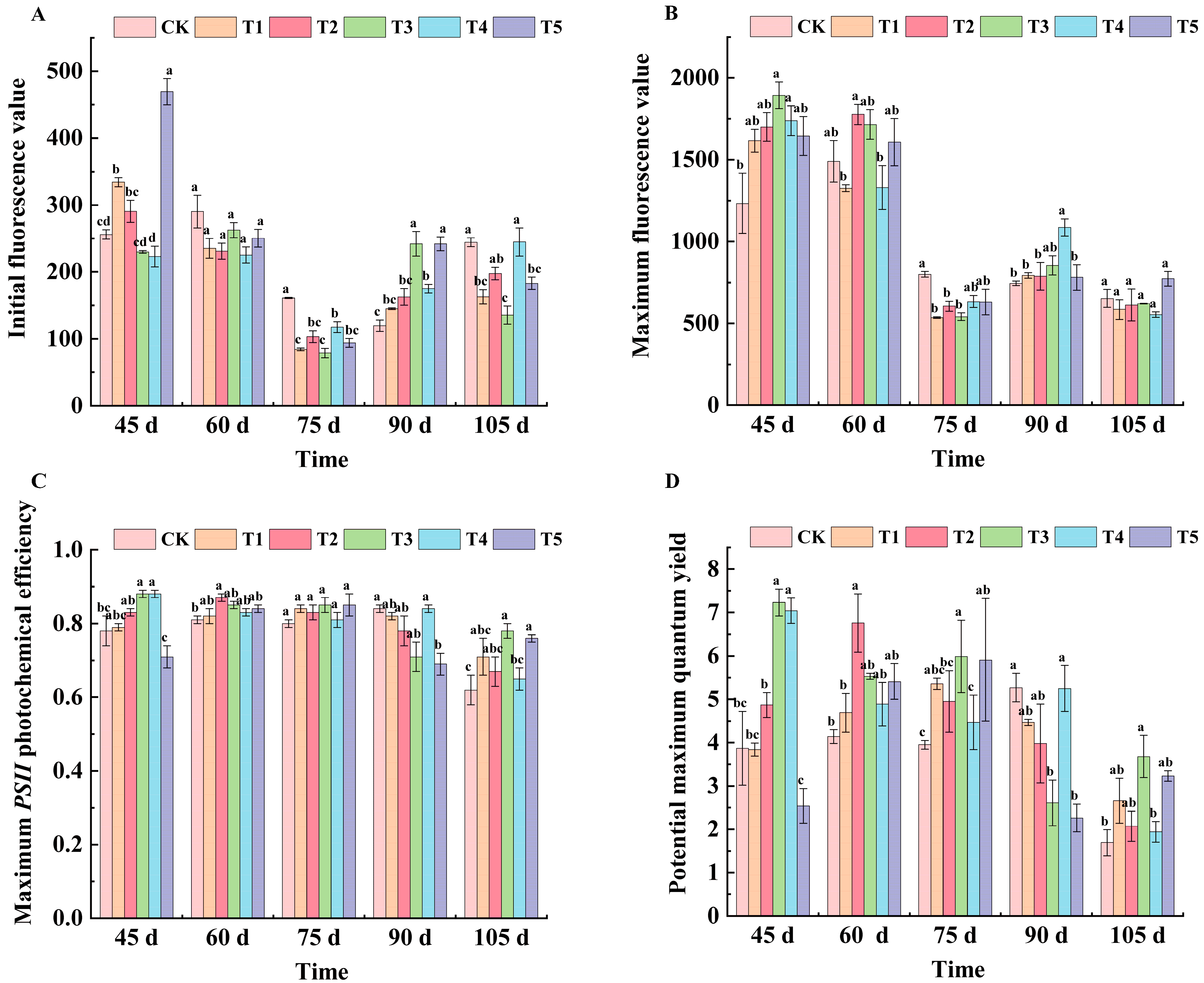
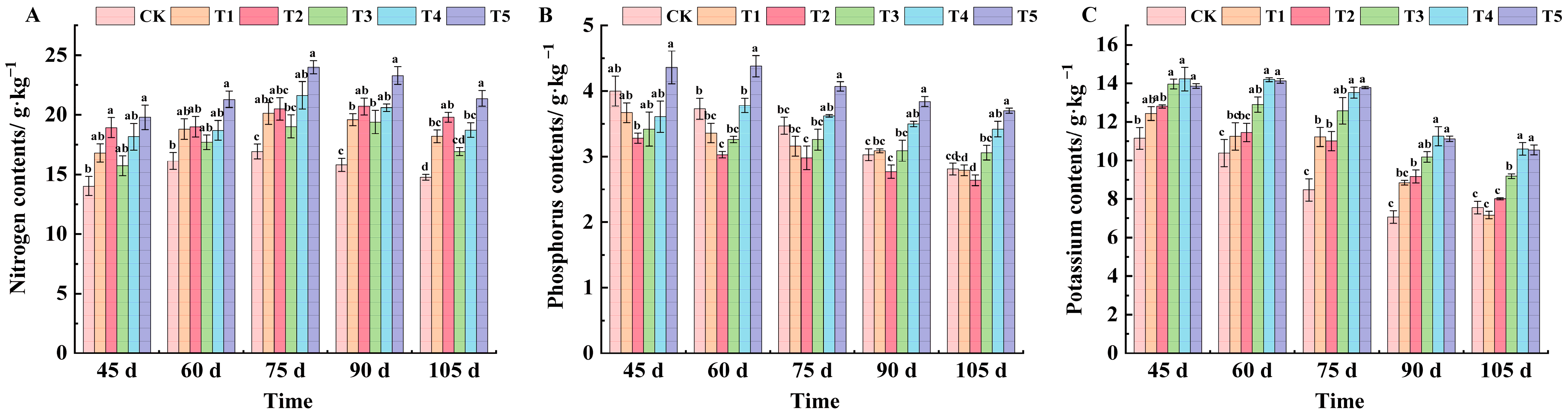
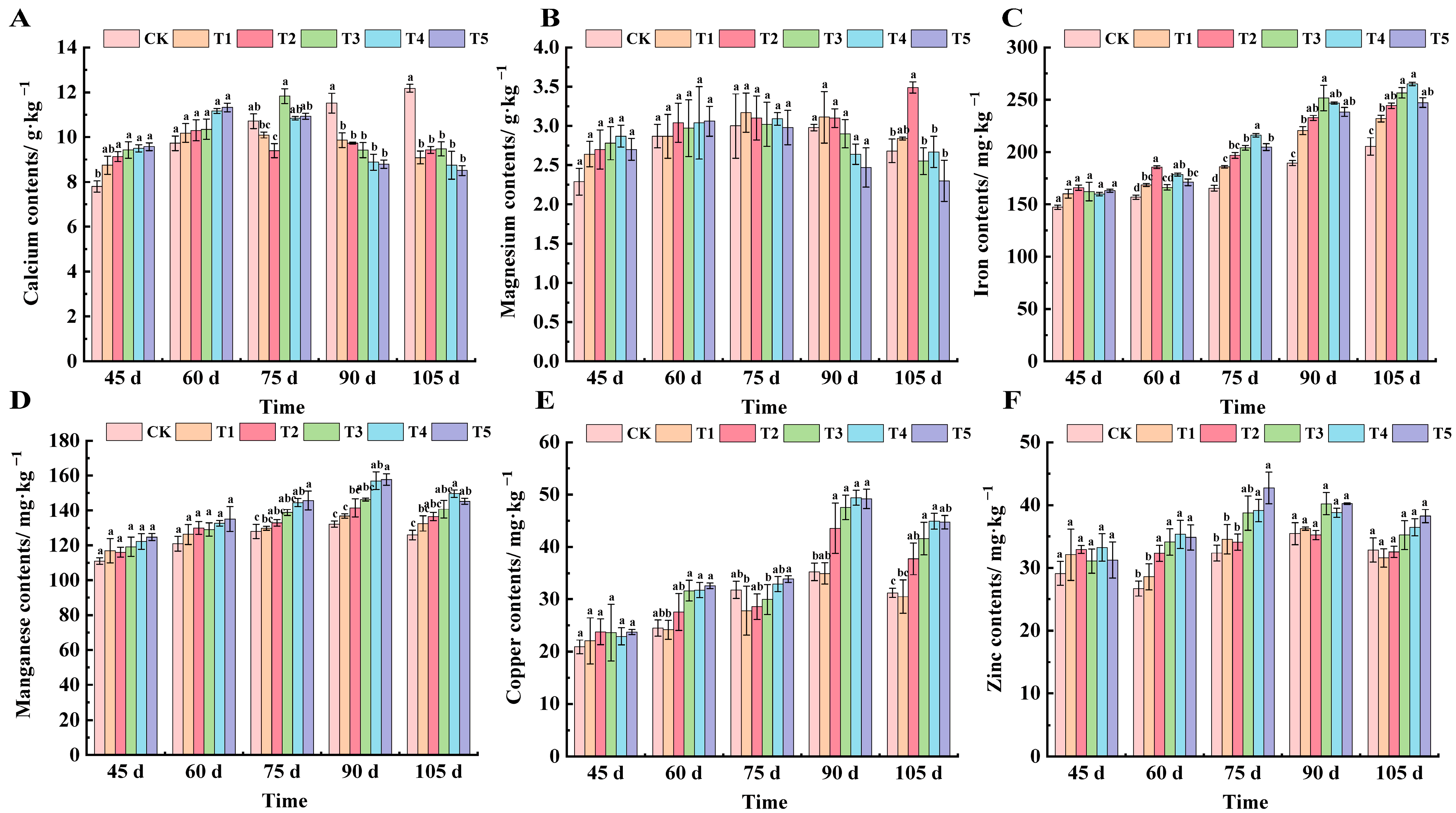
| Treatments | 45 d | 60 d | 75 d | 90 d | 105 d |
|---|---|---|---|---|---|
| CK | 38.46 ± 0.65 c | 41.92 ± 0.52 b | 43.82 ± 0.92 c | 43.94 ± 0.44 c | 40.42 ± 0.59 d |
| T1 | 41.52 ± 0.29 b | 44.48 ± 0.68 a | 46.22 ± 0.80 ab | 46.54 ± 0.56 b | 41.20 ± 0.29 cd |
| T2 | 42.04 ± 0.506 ab | 44.22 ± 0.66 a | 46.32 ± 0.74 bc | 46.98 ± 0.43 ab | 43.32 ± 0.63 ab |
| T3 | 42.64 ± 0.68 ab | 45.66 ± 0.60 a | 46.12 ± 0.95 ab | 47.36 ± 0.83 ab | 43.00 ± 0.99 bc |
| T4 | 42.52 ± 0.73 a | 46.12 ± 0.95 a | 48.28 ± 0.72 a | 48.66 ± 0.67 a | 42.92 ± 0.40 abc |
| T5 | 43.74 ± 0.79 a | 45.66 ± 0.60 a | 48.86 ± 0.60 a | 49.02 ± 0.84 a | 45.10 ± 0.58 a |
| Treatments | 45 d | 60 d | 75 d | 90 d | 105 d |
|---|---|---|---|---|---|
| CK | 37.08 ± 2.60 a | 46.16 ± 2.13 c | 50.02 ± 1.85 c | 51.90 ± 1.75 b | 52.64 ± 1.67 c |
| T1 | 39.20 ± 1.99 a | 49.59 ± 2.32 bc | 54.80 ± 2.30 bc | 56.50 ± 2.43 b | 57.75 ± 2.35 bc |
| T2 | 41.64 ± 0.70 a | 51.53 ± 1.02 abc | 54.17 ± 1.34 bc | 55.46 ± 1.53 b | 56.72 ± 1.53 c |
| T3 | 43.05 ± 0.91 a | 55.20 ± 1.13 ab | 61.34 ± 1.01 a | 62.69 ± 1.23 a | 63.63 ± 1.39 a |
| T4 | 42.49 ± 1.97 a | 57.84 ± 0.60 a | 61.55 ± 0.80 a | 63.08 ± 0.79 a | 64.72 ± 0.58 a |
| T5 | 43.71 ± 1.54 a | 56.39 ± 0.81 a | 60.56 ± 1.02 a | 63.22 ± 0.91 a | 65.28 ± 0.87 a |
| Treatments | 45 d | 60 d | 75 d | 90 d | 105 d |
|---|---|---|---|---|---|
| CK | 8.75 ± 0.69 a | 10.20 ± 0.56 a | 10.84 ± 0.58 a | 11.35 ± 0.62 b | 11.77 ± 0.63 c |
| T1 | 9.14 ± 0.67 a | 10.17 ± 0.68 a | 10.88 ± 0.63 a | 11.52 ± 0.69 b | 12.02 ± 0.65 bc |
| T2 | 9.42 ± 0.33 a | 10.50 ± 0.33 a | 11.33 ± 0.39 a | 11.83 ± 0.38 ab | 12.21 ± 0.38 bc |
| T3 | 9.47 ± 0.63 a | 10.46 ± 0.47 a | 12.16 ± 0.35 a | 12.91 ± 0.29 ab | 13.34 ± 0.27 abc |
| T4 | 9.86 ± 0.43 a | 11.41 ± 0.47 a | 12.94 ± 0.47 a | 13.62 ± 0.34 ab | 13.91 ± 0.28 ab |
| T5 | 10.29 ± 0.94 a | 11.81 ± 0.77 a | 12.66 ± 0.54 a | 13.92 ± 0.71 a | 14.52 ± 0.55 a |
| Treatments | CK | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| Leaf area | 0.00 | 0.28 | 0.53 | 0.48 | 0.73 | 1.00 |
| SPAD | 0.00 | 0.39 | 0.61 | 0.64 | 0.77 | 1.00 |
| Internode length of new shoots | 0.00 | 0.40 | 0.35 | 0.92 | 0.98 | 1.00 |
| Roughness at the base of new shoots | 0.00 | 0.08 | 0.19 | 0.57 | 0.84 | 1.00 |
| Rate of transpiration | 0.00 | 0.24 | 0.37 | 0.89 | 1.00 | 0.82 |
| Intercellular CO2 concentration | 0.96 | 0.04 | 0.35 | 1.00 | 0.00 | 0.25 |
| Net photosynthetic rate | 0.00 | 0.28 | 0.56 | 0.65 | 1.00 | 0.71 |
| Stomatal conductance | 0.00 | 0.17 | 0.33 | 0.59 | 1.00 | 0.74 |
| Initial fluorescence value | 0.45 | 0.00 | 0.33 | 0.39 | 0.68 | 1.00 |
| Maximum fluorescence value | 0.09 | 0.00 | 0.40 | 0.68 | 1.00 | 0.79 |
| Maximum photochemical efficiency of PSII | 0.19 | 1.00 | 0.32 | 0.00 | 0.71 | 0.10 |
| Potential maximum quantum yield | 0.54 | 0.77 | 0.48 | 0.76 | 1.00 | 0.00 |
| Nitrogen content | 0.00 | 0.51 | 0.69 | 0.39 | 0.62 | 1.00 |
| Phosphorus content | 0.28 | 0.23 | 0.00 | 0.31 | 0.66 | 1.00 |
| Potassium content | 0.00 | 0.24 | 0.37 | 0.69 | 1.00 | 0.98 |
| Calcium content | 0.16 | 0.19 | 0.21 | 1.00 | 0.04 | 0.00 |
| Magnesium content | 0.43 | 0.64 | 1.00 | 0.37 | 0.32 | 0.00 |
| Iron content | 0.00 | 0.50 | 0.72 | 0.94 | 1.00 | 0.78 |
| Manganese content | 0.00 | 0.24 | 0.42 | 0.61 | 1.00 | 0.95 |
| Copper content | 0.05 | 0.00 | 0.54 | 0.82 | 1.00 | 1.00 |
| Zinc content | 0.00 | 0.05 | 0.10 | 0.72 | 0.75 | 1.00 |
| Global affiliation function | 0.15 | 0.30 | 0.42 | 0.64 | 0.77 | 0.72 |
| Comprehensive ranking | 6 | 5 | 4 | 3 | 1 | 2 |
| Treatments | Nano Zero-Valent Iron (mg·L−1) | Compound Sodium Nitrophenolate (g·L−1) | Potassium Dihydrogen Phosphate (g·L−1) |
|---|---|---|---|
| T1 (Treatment 1) | 15 | 0 | 0 |
| T2 (Treatment 2) | 15 | 0.4 | 0 |
| T3 (Treatment 3) | 15 | 0.4 | 1.25 |
| T4 (Treatment 4) | 15 | 0.4 | 1.67 |
| T5 (Treatment 5) | 15 | 0.4 | 2.50 |
| CK (Control) | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, H.-J.; Wang, X.; Ma, W.-F.; Gou, H.-M.; Liang, G.-P.; Mao, J. Temporal Variations in Photosynthesis and Leaf Element Contents of ‘Marselan’ Grapevines in Response to Foliar Fertilizer Application. Plants 2025, 14, 946. https://doi.org/10.3390/plants14060946
Zheng H-J, Wang X, Ma W-F, Gou H-M, Liang G-P, Mao J. Temporal Variations in Photosynthesis and Leaf Element Contents of ‘Marselan’ Grapevines in Response to Foliar Fertilizer Application. Plants. 2025; 14(6):946. https://doi.org/10.3390/plants14060946
Chicago/Turabian StyleZheng, Hai-Ju, Xin Wang, Wei-Feng Ma, Hui-Min Gou, Guo-Ping Liang, and Juan Mao. 2025. "Temporal Variations in Photosynthesis and Leaf Element Contents of ‘Marselan’ Grapevines in Response to Foliar Fertilizer Application" Plants 14, no. 6: 946. https://doi.org/10.3390/plants14060946
APA StyleZheng, H.-J., Wang, X., Ma, W.-F., Gou, H.-M., Liang, G.-P., & Mao, J. (2025). Temporal Variations in Photosynthesis and Leaf Element Contents of ‘Marselan’ Grapevines in Response to Foliar Fertilizer Application. Plants, 14(6), 946. https://doi.org/10.3390/plants14060946






