The Role of FT/TFL1 Clades and Their Hormonal Interactions to Modulate Plant Architecture and Flowering Time in Perennial Crops
Abstract
1. Introduction
2. The FT/TFL1 Developmental Regulatory Pathways: From Model Species to Perennial Crops
3. Interaction Between TFL1 and Plant Hormones
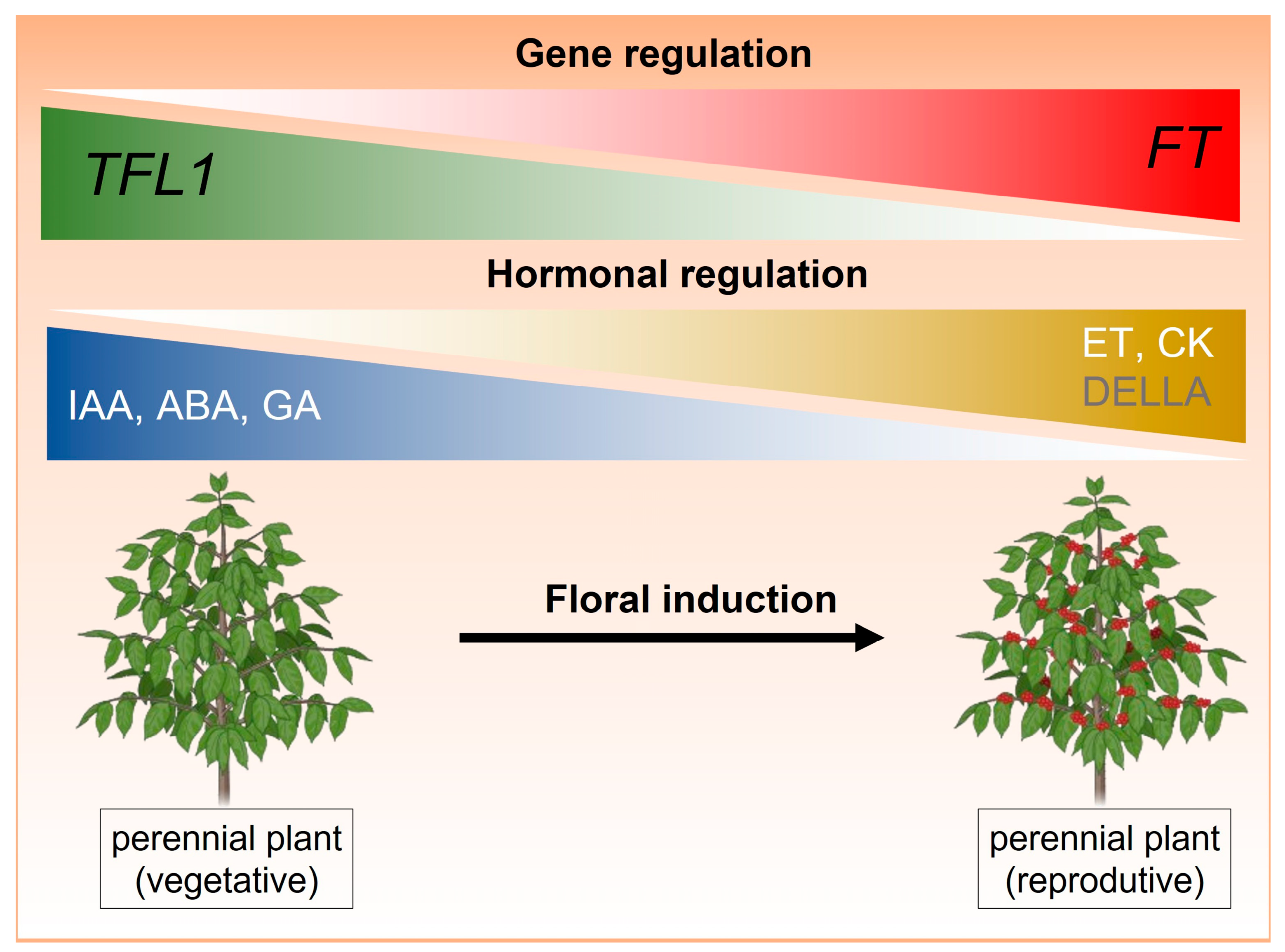
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Fang, C.; Liu, B.; Kong, F. Natural Variation and Artificial Selection of Photoperiodic Flowering Genes and Their Applications in Crop Adaptation. aBIOTECH 2021, 2, 156–169. [Google Scholar] [CrossRef]
- Wang, F.; Li, S.; Kong, F.; Lin, X.; Lu, S. Altered Regulation of Flowering Expands Growth Ranges and Maximizes Yields in Major Crops. Front. Plant Sci. 2023, 14, 1094411. [Google Scholar] [CrossRef]
- Alotaibi, M. Climate Change, Its Impact on Crop Production, Challenges, and Possible Solutions. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13020. [Google Scholar] [CrossRef]
- Keutgen, A.J. Climate Change: Challenges and Limitations in Agriculture. IOP Conf. Ser. Earth Environ. Sci. 2023, 1183, 012069. [Google Scholar] [CrossRef]
- Luo, N.; Mueller, N.; Zhang, Y.; Feng, P.; Huang, S.; Liu, D.L.; Yu, Y.; Wang, X.; Wang, P.; Meng, Q. Short-Term Extreme Heat at Flowering Amplifies the Impacts of Climate Change on Maize Production. Environ. Res. Lett. 2023, 18, 084021. [Google Scholar] [CrossRef]
- Phillip, K.; George, T.E.; Isabirye, M.; Assoua, J.E. Climate Variability and Arabica Coffee Production in Uganda. In Agricultural Transformation in Africa: Contemporary Issues, Empirics, and Policies; Odularu, G.O.A., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 87–114. ISBN 978-3-031-19527-3. [Google Scholar]
- Venancio, L.P.; Filgueiras, R.; Mantovani, E.C.; do Amaral, C.H.; da Cunha, F.F.; dos Santos Silva, F.C.; Althoff, D.; dos Santos, R.A.; Cavatte, P.C. Impact of Drought Associated with High Temperatures on Coffea canephora Plantations: A Case Study in Espírito Santo State, Brazil. Sci. Rep. 2020, 10, 19719. [Google Scholar] [CrossRef]
- Wagner, S.; Jassogne, L.; Price, E.; Jones, M.; Preziosi, R. Impact of Climate Change on the Production of Coffea arabica at Mt. Kilimanjaro, Tanzania. Agriculture 2021, 11, 53. [Google Scholar] [CrossRef]
- Peruta, R.D.; Mereu, V.; Spano, D.; Marras, S.; Trabucco, A. Coffee Agrosystems and Climate Change. In Proceedings of the 25th EGU General Assembly, Vienna, Austria, 23–28 April 2023. [Google Scholar]
- Dinh, T.L.A.; Aires, F.; Rahn, E. Statistical Analysis of the Weather Impact on Robusta Coffee Yield in Vietnam. Front. Environ. Sci. 2022, 10, 820916. [Google Scholar] [CrossRef]
- Bracken, P.; Burgess, P.J.; Girkin, N.T. Opportunities for Enhancing the Climate Resilience of Coffee Production through Improved Crop, Soil and Water Management. Agroecol. Sustain. Food Syst. 2023, 47, 1125–1157. [Google Scholar] [CrossRef]
- Bathiany, S.; Belleflamme, A.; Zohbi, J.E.; Ney, P.; Goergen, K.; Rechid, D. Increasing Interannual Climate Variability During Crop Flowering in Europe. Environ. Res. Lett. 2023, 18, 044037. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.D.C. Impacts of Drought and Temperature Stress on Coffee Physiology and Production: A Review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Bilen, C.; El Chami, D.; Mereu, V.; Trabucco, A.; Marras, S.; Spano, D. A Systematic Review on the Impacts of Climate Change on Coffee Agrosystems. Plants 2023, 12, 102. [Google Scholar] [CrossRef]
- Jawo, T.O.; Kyereh, D.; Lojka, B. The Impact of Climate Change on Coffee Production of Small Farmers and Their Adaptation Strategies: A Review. Clim. Dev. 2023, 15, 93–109. [Google Scholar] [CrossRef]
- Leijten, W.; Koes, R.; Roobeek, I.; Frugis, G. Translating Flowering Time from Arabidopsis thaliana to Brassicaceae and Asteraceae Crop Species. Plants 2018, 7, 111. [Google Scholar] [CrossRef]
- Kim, D.-H. Current Understanding of Flowering Pathways in Plants: Focusing on the Vernalization Pathway in Arabidopsis and Several Vegetable Crop Plants. Hortic. Environ. Biotechnol. 2020, 61, 209–227. [Google Scholar] [CrossRef]
- Bao, S.; Hua, C.; Shen, L.; Yu, H. New Insights into Gibberellin Signaling in Regulating Flowering in Arabidopsis. J. Integr. Plant Biol. 2020, 62, 118–131. [Google Scholar] [CrossRef]
- Khan, M.R.G.; Ai, X.-Y.; Zhang, J.-Z. Genetic Regulation of Flowering Time in Annual and Perennial Plants. WIREs RNA 2014, 5, 347–359. [Google Scholar] [CrossRef]
- Kaneko-Suzuki, M.; Kurihara-Ishikawa, R.; Okushita-Terakawa, C.; Kojima, C.; Nagano-Fujiwara, M.; Ohki, I.; Tsuji, H.; Shimamoto, K.; Taoka, K.-I. TFL1-Like Proteins in Rice Antagonize Rice FT-Like Protein in Inflorescence Development by Competition for Complex Formation with 14-3-3 and FD. Plant Cell Physiol. 2018, 59, 458–468. [Google Scholar] [CrossRef]
- Jin, S.; Nasim, Z.; Susila, H.; Ahn, J.H. Evolution and Functional Diversification of FLOWERING LOCUS T/TERMINAL FLOWER 1 Family Genes in Plants. Semin. Cell Dev. Biol. 2021, 109, 20–30. [Google Scholar] [CrossRef]
- Liu, L.; Xuan, L.; Jiang, Y.; Yu, H. Regulation by FLOWERING LOCUS T and TERMINAL FLOWER 1 in Flowering Time and Plant Architecture. Small Struct. 2021, 2, 2000125. [Google Scholar] [CrossRef]
- Matsoukas, I.G.; Massiah, A.J.; Thomas, B. Florigenic and Antiflorigenic Signaling in Plants. Plant Cell Physiol. 2012, 53, 1827–1842. [Google Scholar] [CrossRef] [PubMed]
- Moraes, T.S.; Dornelas, M.C.; Martinelli, A.P. FT/TFL1: Calibrating Plant Architecture. Front Plant Sci 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Teo, Z.W.N.; Song, S.; Wang, Y.-Q.; Liu, J.; Yu, H. New Insights into the Regulation of Inflorescence Architecture. Trends Plant Sci. 2014, 19, 158–165. [Google Scholar] [CrossRef]
- Goretti, D.; Silvestre, M.; Collani, S.; Langenecker, T.; Méndez, C.; Madueño, F.; Schmid, M. TERMINAL FLOWER1 Functions as a Mobile Transcriptional Cofactor in the Shoot Apical Meristem. Plant Physiol. 2020, 182, 2081–2095. [Google Scholar] [CrossRef]
- Kim, W.; Park, T.I.; Yoo, S.J.; Jun, A.R.; Ahn, J.H. Generation and Analysis of a Complete Mutant Set for the Arabidopsis FT/TFL1 Family Shows Specific Effects on Thermo-Sensitive Flowering Regulation. J. Exp. Bot. 2013, 64, 1715–1729. [Google Scholar] [CrossRef]
- Colleoni, P.E.; van Es, S.W.; Winkelmolen, T.; Immink, R.G.H.; van Esse, G.W. Flowering Time Genes Branching Out. J. Exp. Bot. 2024, 75, 4195–4209. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.W.H.; Weigel, D. Structural Features Determining Flower-Promoting Activity of Arabidopsis FLOWERING LOCUS T. Plant Cell 2014, 26, 552–564. [Google Scholar] [CrossRef]
- Hanano, S.; Goto, K. Arabidopsis TERMINAL FLOWER1 Is Involved in the Regulation of Flowering Time and Inflorescence Development through Transcriptional Repression. Plant Cell 2011, 23, 3172–3184. [Google Scholar] [CrossRef]
- McGarry, R.C.; Ayre, B.G. Manipulating Plant Architecture with Members of the CETS Gene Family. Plant Sci. 2012, 188–189, 71–81. [Google Scholar] [CrossRef]
- Taoka, K.; Ohki, I.; Tsuji, H.; Furuita, K.; Hayashi, K.; Yanase, T.; Yamaguchi, M.; Nakashima, C.; Purwestri, Y.A.; Tamaki, S.; et al. 14-3-3 Proteins Act as Intracellular Receptors for Rice Hd3a Florigen. Nature 2011, 476, 332–335. [Google Scholar] [CrossRef]
- Bellinazzo, F.; Nadal Bigas, J.; Hogers, R.A.H.; Kodde, J.; van der Wal, F.; Kokkinopoulou, P.; Duijts, K.T.M.; Angenent, G.C.; van Dijk, A.D.J.; van Velzen, R.; et al. Evolutionary Origin and Functional Investigation of the Widely Conserved Plant PEBP Gene STEPMOTHER OF FT AND TFL1 (SMFT). Plant J. 2024, 120, 1410–1420. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Gu, M.; Shi, N.; Zhang, H.; Yang, X.; Osman, T.; Liu, Y.; Wang, H.; Vatish, M.; Jackson, S.; et al. Mobile FT mRNA Contributes to the Systemic Florigen Signalling in Floral Induction. Sci. Rep. 2011, 1, 73. [Google Scholar] [CrossRef]
- Li, C.; Zhang, K.; Zeng, X.; Jackson, S.; Zhou, Y.; Hong, Y. A Cis Element within Flowering Locus T mRNA Determines Its Mobility and Facilitates Trafficking of Heterologous Viral RNA. J. Virol. 2009, 83, 3540–3548. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, W.; Wang, Y.; Zhang, P.; Shi, N.; Hong, Y. Mobile Flowering Locus T RNA—Biological Relevance and Biotechnological Potential. Front. Plant Sci. 2022, 12, 792192. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Takahashi, Y.; Le Gourrierec, J.; Coupland, G. Photoperiodic and Thermosensory Pathways Interact Through CONSTANS to Promote Flowering at High Temperature Under Short Days. Plant J. 2016, 86, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, H. Molecular Function of Florigen. Breed. Sci. 2017, 67, 327–332. [Google Scholar] [CrossRef]
- Ratcliffe, O.J.; Bradley, D.J.; Coen, E.S. Separation of Shoot and Floral Identity in Arabidopsis. Development 1999, 126, 1109–1120. [Google Scholar] [CrossRef]
- Liu, C.; Teo, Z.W.N.; Bi, Y.; Song, S.; Xi, W.; Yang, X.; Yin, Z.; Yu, H. A Conserved Genetic Pathway Determines Inflorescence Architecture in Arabidopsis and Rice. Dev. Cell 2013, 6, 612–622. [Google Scholar] [CrossRef]
- Myers, S.S.; Smith, M.R.; Guth, S.; Golden, C.D.; Vaitla, B.; Mueller, N.D.; Dangour, A.D.; Huybers, P. Climate Change and Global Food Systems: Potential Impacts on Food Security and Undernutrition. Annu. Rev. Public Health 2017, 38, 259–277. [Google Scholar] [CrossRef]
- Albani, M.C.; Coupland, G. Chapter Eleven—Comparative Analysis of Flowering in Annual and Perennial Plants. In Current Topics in Developmental Biology; Timmermans, M.C.P., Ed.; Plant Development; Academic Press: Cambridge, MA, USA, 2010; Volume 91, pp. 323–348. [Google Scholar]
- Lee, C.; Kim, S.-J.; Jin, S.; Susila, H.; Youn, G.; Nasim, Z.; Alavilli, H.; Chung, K.-S.; Yoo, S.J.; Ahn, J.H. Genetic Interactions Reveal the Antagonistic Roles of FT/TSF and TFL1 in the Determination of Inflorescence Meristem Identity in Arabidopsis. Plant J. 2019, 99, 452–464. [Google Scholar] [CrossRef]
- Freiman, A.; Shlizerman, L.; Golobovitch, S.; Yablovitz, Z.; Korchinsky, R.; Cohen, Y.; Samach, A.; Chevreau, E.; Le Roux, P.-M.; Patocchi, A.; et al. Development of a Transgenic Early Flowering Pear (Pyrus Communis L.) Genotype by RNAi Silencing of PcTFL1-1 and PcTFL1-2. Planta 2012, 235, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Kotoda, N.; Iwanami, H.; Takahashi, S.; Abe, K. Antisense Expression of MdTFL1, a TFL1-like Gene, Reduces the Juvenile Phase in Apple. J. Am. Soc. Hortic. Sci. 2006, 131, 74–81. [Google Scholar] [CrossRef]
- Mohamed, R.; Wang, C.-T.; Ma, C.; Shevchenko, O.; Dye, S.J.; Puzey, J.R.; Etherington, E.; Sheng, X.; Meilan, R.; Strauss, S.H.; et al. Populus CEN/TFL1 Regulates First Onset of Flowering, Axillary Meristem Identity and Dormancy Release in Populus. Plant J. 2010, 62, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.-C.; Luo, K.-R.; Yu, T.-S. Mobility of Antiflorigen and PEBP mRNAs in Tomato-Tobacco Heterografts. Plant Physiol 2018, 178, 783–794. [Google Scholar] [CrossRef]
- De Oliveira, R.R.; Noman, M.; Azevedo, L.M.; Santos, I.S.; Alvarenga, J.P.; Chalfun-Junior, A. Regulation of Coffea arabica Floral Development, Flowering and Fruit Maturation by Plant Growth Regulators. In Advances in Botanical Research (Chapter 12), Volume 114: Coffee—A Glimpse into the Future; Academic Press and Elsevier: Cambridge, MA, USA, 2024; p. S0065229624001320. [Google Scholar]
- Cardon, C.; Lesy, V.; Fust, C.; Ribeiro, T.; Hebb, O.; de Oliveira, R.R.; Minow, M.; Chalfun-Junior, A.; Colasanti, J. A Leaf-Expressed TERMINAL FLOWER1 Ortholog from Coffee with Alternate Splice Forms Alters Flowering Time and Inflorescence Branching in Arabidopsis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cardon, C.H.; de Oliveira, R.R.; Lesy, V.; Ribeiro, T.H.C.; Fust, C.; Pereira, L.P.; Colasanti, J.; Chalfun-Junior, A. Expression of Coffee Florigen CaFT1 Reveals a Sustained Floral Induction Window Associated with Asynchronous Flowering in Tropical Perennials. Plant Sci. 2022, 325, 111479. [Google Scholar] [CrossRef]
- Zheng, J.; Ma, Y.; Zhang, M.; Lyu, M.; Yuan, Y.; Wu, B. Expression Pattern of FT/TFL1 and miR156-Targeted SPL Genes Associated with Developmental Stages in Dendrobium Catenatum. Int. J. Mol. Sci. 2019, 20, 2725. [Google Scholar] [CrossRef]
- de Oliveira, K.K.P.; de Oliveira, R.R.; de Campos Rume, G.; Ribeiro, T.H.C.; Fernandes-Brum, C.N.; do Amaral, L.R.; Kakrana, A.; Mathioni, S.; Meyers, B.C.; de Souza Gomes, M.; et al. Microsporogenesis and the Biosynthesis of Floral Small Interfering RNAs in Coffee Have a Unique Pattern among Eudicots, Suggesting a Sensitivity to Climate Changes. Plant Direct 2024, 8, e561. [Google Scholar] [CrossRef]
- Ribeiro, T.H.C.; Baldrich, P.; de Oliveira, R.R.; Fernandes-Brum, C.N.; Mathioni, S.M.; de Sousa Cardoso, T.C.; de Souza Gomes, M.; do Amaral, L.R.; Pimenta de Oliveira, K.K.; dos Reis, G.L.; et al. The Floral Development of the Allotetraploid Coffea arabica L. Correlates with a Small RNA Dynamic Reprogramming. Plant J. 2024, 118, 1848–1863. [Google Scholar] [CrossRef]
- Singh, P.; Dutta, P.; Chakrabarty, D. miRNAs Play Critical Roles in Response to Abiotic Stress by Modulating Cross-Talk of Phytohormone Signaling. Plant Cell Rep. 2021, 40, 1617–1630. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.; de Oliveira, R.R.; Ribeiro, T.H.C.; de Oliveira, K.K.P.; Silva, J.V.N.; Alves, T.C.; do Amaral, L.R.; de Souza Gomes, M.; de Souza Gomes, M.; Chalfun-Junior, A. Unveiling the Phenology and Associated Floral Regulatory Pathways of Humulus lupulus L. in Subtropical Conditions. Planta 2024, 259, 150. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Pullen, N.; Lamzin, S.; Morris, R.J.; Wigge, P.A. Interlocking Feedback Loops Govern the Dynamic Behavior of the Floral Transition in Arabidopsis. Plant Cell 2013, 25, 820–833. [Google Scholar] [CrossRef]
- Bai, S.; Tuan, P.A.; Saito, T.; Ito, A.; Ubi, B.E.; Ban, Y.; Moriguchi, T. Repression of TERMINAL FLOWER1 Primarily Mediates Floral Induction in Pear (Pyrus Pyrifolia Nakai) Concomitant with Change in Gene Expression of Plant Hormone-Related Genes and Transcription Factors. J. Exp. Bot. 2017, 68, 4899–4914. [Google Scholar] [CrossRef] [PubMed]
- Coelho, C.P.; Minow, M.A.A.; Chalfun-Júnior, A.; Colasanti, J. Putative Sugarcane FT/TFL1 Genes Delay Flowering Time and Alter Reproductive Architecture in Arabidopsis. Front. Plant Sci. 2014, 5, 221. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, N.; Sasabe, M.; Endo, M.; Machida, Y.; Araki, T. Calcium-Dependent Protein Kinases Responsible for the Phosphorylation of a bZIP Transcription Factor FD Crucial for the Florigen Complex Formation. Sci. Rep. 2015, 5, 8341. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, J.-W. Perenniality: From Model Plants to Applications in Agriculture. Mol. Plant 2024, 17, 141–157. [Google Scholar] [CrossRef]
- Sun, L.; Nie, T.; Chen, Y.; Yin, Z. From Floral Induction to Blooming: The Molecular Mysteries of Flowering in Woody Plants. Int. J. Mol. Sci. 2022, 23, 10959. [Google Scholar] [CrossRef]
- Izawa, T. What Is Going on with the Hormonal Control of Flowering in Plants? Plant J. 2021, 105, 431–445. [Google Scholar] [CrossRef]
- Vanstraelen, M.; Benková, E. Hormonal Interactions in the Regulation of Plant Development. Annu. Rev. Cell Dev. Biol. 2012, 28, 463–487. [Google Scholar] [CrossRef]
- Yang, M.; Jiao, Y. Regulation of Axillary Meristem Initiation by Transcription Factors and Plant Hormones. Front. Plant Sci. 2016, 7, 183. [Google Scholar] [CrossRef]
- Haberman, A.; Ackerman, M.; Crane, O.; Kelner, J.-J.; Costes, E.; Samach, A. Different Flowering Response to Various Fruit Loads in Apple Cultivars Correlates with Degree of Transcript Reaccumulation of a TFL1-Encoding Gene. Plant J. 2016, 87, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Mimida, N.; Komori, S.; Suzuki, A.; Wada, M. Functions of the Apple TFL1/FT Orthologs in Phase Transition. Sci. Hortic. 2013, 156, 106–112. [Google Scholar] [CrossRef]
- Renau-Morata, B.; Nebauer, S.G.; García-Carpintero, V.; Cañizares, J.; Gómez Minguet, E.; de los Mozos, M.; Molina, R.V. Flower Induction and Development in Saffron: Timing and Hormone Signalling Pathways. Ind. Crops Prod. 2021, 164, 113370. [Google Scholar] [CrossRef]
- Zhang, S.; Gottschalk, C.; van Nocker, S. Genetic Mechanisms in the Repression of Flowering by Gibberellins in Apple (Malus x domestica Borkh.). BMC Genom. 2019, 20, 747. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Xing, L.; Zhang, S.; Zhao, C.; Han, M. Effect of Exogenous 6-Benzylaminopurine (6-BA) on Branch Type, Floral Induction and Initiation, and Related Gene Expression in ‘Fuji’ Apple (Malus domestica Borkh). Plant Growth Regul. 2016, 79, 65–70. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Zhang, L.; Zuo, X.; Fan, S.; Zhang, X.; Shalmani, A.; Han, M. Identification and Expression Analysis of Cytokinin Response-Regulator Genes During Floral Induction in Apple (Malus domestica Borkh). Plant Growth Regul. 2017, 83, 455–464. [Google Scholar] [CrossRef]
- López, M.E.; de Oliveira, R.R.; Azevedo, L.M.; Santos, I.S.; Ribeiro, T.H.C.; Zhang, D.; Chalfun-Junior, A. The Contrasting Flowering-Time Among Coffee Genotypes Is Associated with Ectopic and Differential Expressions of Genes Related to Environment, Floral Development, and Hormonal Regulation. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Ye, T.; Lu, Y.; Chen, X.; Wu, Y. The Inhibitory Effect of ABA on Floral Transition Is Mediated by ABI5 in Arabidopsis. J. Exp. Bot. 2013, 64, 675–684. [Google Scholar] [CrossRef]
- Yao, C.; Finlayson, S.A. Abscisic Acid Is a General Negative Regulator of Arabidopsis Axillary Bud Growth. Plant Physiol. 2015, 169, 611–626. [Google Scholar] [CrossRef]
- Wuriyanghan, H.; Zhang, B.; Cao, W.-H.; Ma, B.; Lei, G.; Liu, Y.-F.; Wei, W.; Wu, H.-J.; Chen, L.-J.; Chen, H.-W.; et al. The Ethylene Receptor ETR2 Delays Floral Transition and Affects Starch Accumulation in Rice. Plant Cell 2009, 21, 1473–1494. [Google Scholar] [CrossRef]
- Randoux, M.; Jeauffre, J.; Thouroude, T.; Vasseur, F.; Hamama, L.; Juchaux, M.; Sakr, S.; Foucher, F. Gibberellins Regulate the Transcription of the Continuous Flowering Regulator, RoKSN, a Rose TFL1 Homologue. J. Exp. Bot. 2012, 63, 6543–6554. [Google Scholar] [CrossRef] [PubMed]
- Arro, J.; Yang, Y.; Song, G.-Q.; Zhong, G.-Y. RNA-Seq Reveals New DELLA Targets and Regulation in Transgenic GA-Insensitive Grapevines. BMC Plant Biol. 2019, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Elsysy, M.A.; Hirst, P.M. Molecular Basis of Flower Formation in Apple Caused by Defoliation and Gibberellins. J. Am. Soc. Hortic. Sci. 2019, 144, 414–419. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, L.M.; de Oliveira, R.R.; Chalfun-Junior, A. The Role of FT/TFL1 Clades and Their Hormonal Interactions to Modulate Plant Architecture and Flowering Time in Perennial Crops. Plants 2025, 14, 923. https://doi.org/10.3390/plants14060923
Azevedo LM, de Oliveira RR, Chalfun-Junior A. The Role of FT/TFL1 Clades and Their Hormonal Interactions to Modulate Plant Architecture and Flowering Time in Perennial Crops. Plants. 2025; 14(6):923. https://doi.org/10.3390/plants14060923
Chicago/Turabian StyleAzevedo, Lillian Magalhães, Raphael Ricon de Oliveira, and Antonio Chalfun-Junior. 2025. "The Role of FT/TFL1 Clades and Their Hormonal Interactions to Modulate Plant Architecture and Flowering Time in Perennial Crops" Plants 14, no. 6: 923. https://doi.org/10.3390/plants14060923
APA StyleAzevedo, L. M., de Oliveira, R. R., & Chalfun-Junior, A. (2025). The Role of FT/TFL1 Clades and Their Hormonal Interactions to Modulate Plant Architecture and Flowering Time in Perennial Crops. Plants, 14(6), 923. https://doi.org/10.3390/plants14060923






