1. Introduction
Lepidoptera insects constitute predominant contributors to yield losses in maize cultivation, accounting for 6% of total production losses in North America and 13% in South America [
1,
2]. In China, lepidopteran-induced yield losses were estimated at 10% in spring maize and 20-30% in summer maize [
3]. The first genetically modified (GM) insect-resistant maize was successfully developed and commercially released in the United States in 1996. The earliest commercialized maize, MON810, expresses the CryIA(b) protein, which has a toxic effect on Lepidoptera insects, especially the European corn borer. After the larvae of the European corn borer feed on the MON810 maize, the CryIA(b) protein will bind to the specific receptors on the epithelial cells of the insect’s midgut, damaging the cell structure, causing the insect’s intestine to perforate, stopping it from feeding, and ultimately leading to its death [
4]. Since then, transgenic technology has emerged as the predominant method for controlling maize lepidopteran pests. Numerous transgenic maize varieties expressing diverse insecticidal proteins have been subsequently developed and commercialized. Initially adopted in USA, this innovation has been successfully implemented by major maize-producing nations like Brazil and Argentina, with subsequent global adoption expanding to more than five countries. This technological advancement is widely recognized as a seminal breakthrough in agricultural biotechnology [
5].
The Cry protein has a highly specific toxic effect on insect larvae, which can cause lethal effects on the larvae of different insect species, but it has no such effect on humans or other mammals [
6]. Generally, Bacillus thuringiensis secretes a series of insecticidal proteins, including toxins produced during the vegetative growth phase (such as secreted insecticidal protein, Sip; vegetative insecticidal proteins, Vip), parasporal crystalline δ-endotoxins produced during the stationary phase of vegetative growth (such as cytolytic toxin, Cyt, and crystal toxin, Cry), and β-exotoxins [
7]. The first product based on the toxins of Bacillus thuringiensis (
Bt) was commercialized in France in 1938 for the control of moths. In 1958, Bt products started to be commercially available in the United States [
8]. The main application of Bt toxins is to control agricultural pests, and this pest control work is carried out through genetically modified plants (
Bt plants). At present, a large number of Cry insecticidal toxin proteins have been discovered and applied to insect control. For example, proteins such as Cry1A, Cry2A, Cry3A, and Cry14A can be used to control insects of the order
Coleoptera, while proteins like Cry1A, Cry2A, Cry4A, and Cry10A can be used to control insects of the order
Diptera. Additionally, proteins including Cry3A, Cry5A, and Cry22A can be used to control insects of the order
Hymenoptera [
9].
Corn (Zea mays L.) is the biggest crop by acreage in China. With little doubt, transgenic insect resistance technology could be the most efficient and environment friendly method to control the lepidopteran insect pests in corn in China too. Therefore, it will be highly valuable to develop transgenic insect-resistant corn to control the lepidopteran pests, especially the Asian corn borer (ACB, Ostrinia furnacalis), cotton bollworm (CBW, Helicoverpa armigera), and oriental armyworm (OAW, Mythimna separata), which cause severe damage in China.
Cry1Ab is an effective
Bt toxin against major lepidopteran pests on maize. Transgenic maize Bt11 and Mon810 expressing Cry1Ab have been commercially released and demonstrated great effectiveness in controlling the European corn borer (ECB,
Ostrinia nubilalis) [
10,
11]. Cry1Ab was also found to be effective in controlling other lepidopteran species, such as corn earworm (CEW,
Helicoverpa zea) and western bean cutworm (WBC,
Striacosta albicosta) [
2]. According to relevant reports, Cry1Ab has a very strong activity against the ACB and the OAW [
12,
13]. Cry2Ab is another Bt toxin that is also quite effective against ECB, CBW, OAW, and fall armyworm (FAW,
Spodoptera frugiperda) [
14]. Cry2Ab was utilized to molecular stacking with Cry1.105 in transgenic corn event Mon89034 [
15].
Resistance development by insect pests to
Bt corn is a major threat for the long-term utilization of
Bt corn. The stacking of two
Bt toxins without cross resistance is regarded as an effective strategy to mitigate the development of resistance by insect pests to
Bt toxins [
16]. Considering that Cry1Ab and Cry2Ab are both active to the three most important corn lepidopteran pests (ACB, CBW, and OAW) in China. we decided to develop a transgenic event that simultaneously expresses these two
Bt toxins. An event named GAB-3 was selected, and its resistance to insect pest was evaluated primarily. In this study, we report the molecular characterization and evaluation of insect resistance and herbicide tolerance over multiple generations of GAB-3. This event was granted with a safety certificate in China under the name of ZDRF-8.
3. Materials and Methods
3.1. Vector Construction and Maize Transformation
The full-length coding sequences of the
Cry1Ab,
Cry2Ab, and
G10evo-epsps genes were optimized based on the codon bias of maize and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The
Hygromycin gene of the T-DNA vector pCAMBIA1300 was replaced with the glyphosate tolerance gene
G10evo-epsps. This modified pCAMBIA1300 vector was further inserted by two expression cassettes of
Cry1Ab and
Cry2Ab, respectively. The
Cry1Ab cassette consists of the maize ubiquitin promoter (pZmUbi), the
Cry1Ab coding sequence, and the maize PEPC terminator (PEPC) from the 5′ to 3′ end. The
Cry2Ab cassette consists of the rice actin-1 promoter (pActin), the
Cry2Ab coding sequence, and the 35S gene terminator of CaMV. The transformation construct was named as pZDRF, and its T-DNA region was illustrated in
Figure 1. The method of maize transformation generally follows Ishida’s Agrobacterium-mediated transgenic maize transformation method [
17]. However, in this study, we used 2 mM of glyphosate to screen for positive plants.
3.2. Trait Integration
The T0 transgenic events were first screened by bioassays with ACB and then by qPCR to select events with only single copy of T-DNA insertion. The selected events were backcrossed into elite inbred line Zheng58. After performing backcross and then self-pollination three times, the transgenic homozygous plants of Zheng58 genetic background were obtained. The segregated non-transgenic plants were also saved as the non-transgenic control, and named as Ruifeng-1.
3.3. Southern Blot Analysis
Genomic DNA was isolated from leaf tissues of ZDRF-8 and non-transgenic plants Ruifeng-1 using the cetyltrimethylammonium bromide (CTAB) method [
18], and was then digested overnight with restriction enzymes. After the digestion, genomic DNA was separated in 7 g/L agarose gel at 30 V over 8 h, and transferred onto a Hybond N
+ membrane (Amersham, UK). Hybridization probes, specific to
Cry1Ab,
Cry2Ab,
G10evo-epsps genes and vector backbone, respectively, were synthesized using the PCR DIG Probe Synthesis Kit (Roche, Basel, Switzerland). The probes were hybridized to DNA on blots on the membrane in the hybridization solution. After washing the unhybridized probe, the blots were visualized in a Gel Logic 2200 imaging system (Kodak, Rochester, NY, USA) through chemiluminescence. To have a better resolution for both large small size bands, the ZDRF-8 samples digested with restriction enzymes were loaded for two time points for a long run and a short run. The short run could prevent the loss of smaller fragment bands, and the long run could achieve higher band resolution of large size bands.
3.4. Determination of Insertion Sites by hiTAIL-PCR
Based on the sequences of the ends of the T-DNA, nested primers (LB-SPI, LB-SP2a, LB-SPIII, RB-0b, RB-1b, and RB-2b), random primers (LAD1/2/3/4), and the tag sequence AC1 were designed for high-efficiency TAIL-PCR (hiTAIL-PCR) (primers are listed in
Table 3). Following the method of hiTAIL-PCR described by Liu [
19]. The specific reaction systems and reaction steps are shown in
Table 4,
Table 5,
Table 6 and
Table 7. The actual maize genomic flanking sequences to the T-DNA were determined. The flanking maize genomic sequences were subjected to a BLAST analysis to the B73 RefGen_v2 (MGSC) database, and the insertion site of the T-DNA on the genome was determined.
3.5. Event-Specific PCR for Transgenic Plant Identification
The genomic DNA samples were isolated from leaf tissues of ZDRF-8 (total of 5 generations, from 5th to 9th) and the non-transgenic recipient plants Ruifeng-1 by the cetyltrimethylammonium bromide method (CTAB) [
18], and were used as templates for event-specific PCR to identify transgenic plants. The genomic DNA of Ruifeng-1 was served as a negative control. The event-specific PCR primers were designed based on the flanking genomic sequences and their nearby T-DNA sequences (listed in
Table 8). The PCR was conducted using the following parameters: 94 °C for 2 min; 30 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 2 min.
3.6. Western Blot Analysis
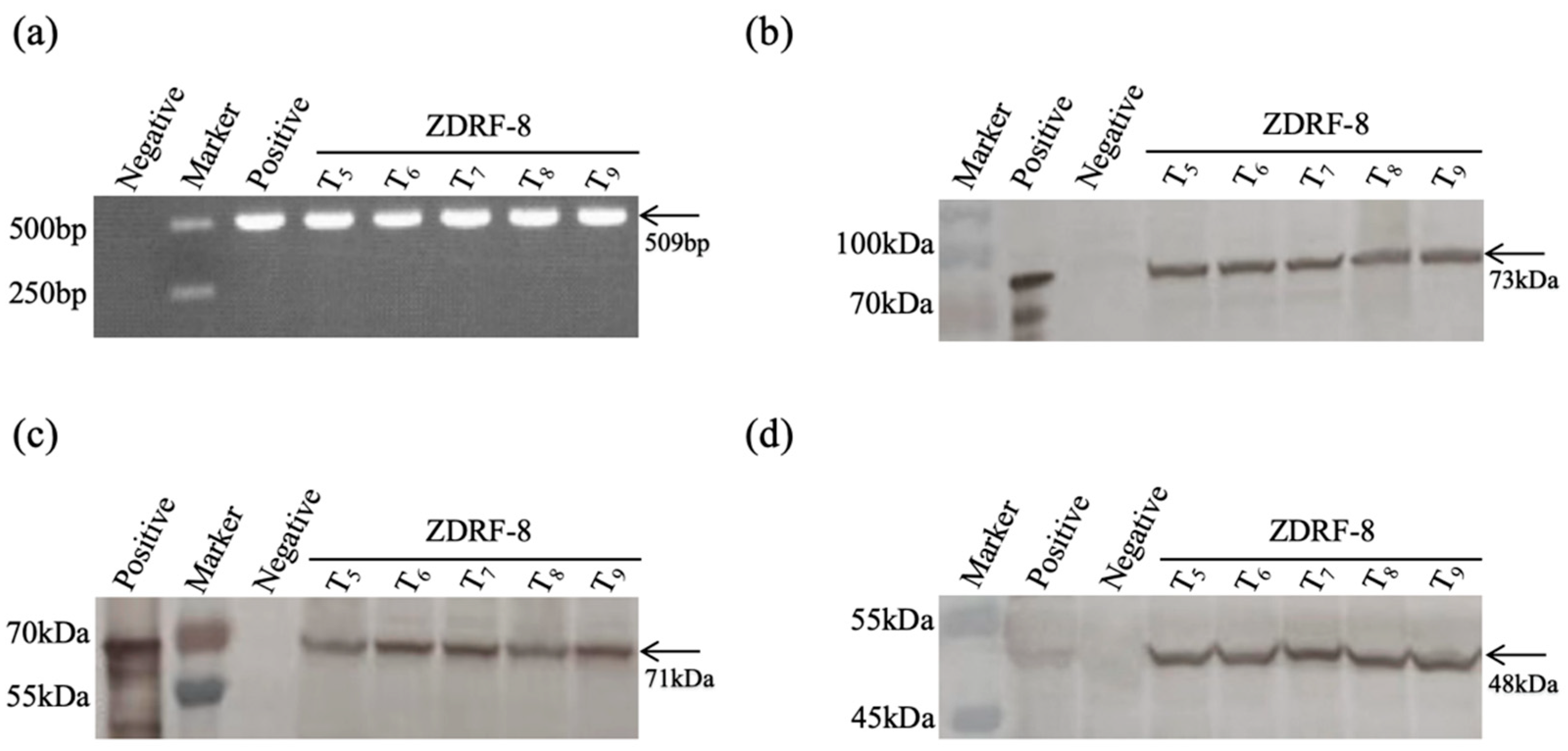
Fresh leaves of 5 generations of ZDRF-8 and non-transgenic control maize Ruifeng-1 were sampled for Western blot analysis. The sample leaves were added with steel balls and then subjected to sample crusher machine (45 Hz, 45 s) to grind into powder. The fine powders of the samples were then suspended into phosphate-buffered saline (PBS). Add 10 μL of 5× protein loading buffer (containing β-mercaptoethanol) and mix well. Boil in boiling water for 10 min and centrifuge at 12,000 rpm for 5 min. Take an appropriate amount of supernatant for electrophoresis. The protein samples were used to perform a Western blot analysis, following the protocol described by Liu [
20]. The polyclonal antibodies against insecticidal proteins Cry1Ab and Cry2Ab and the herbicide protein G10evo-epsps were diluted 250 times for incubation in a Western blot analysis, and they were prepared by the Zhejiang Chinese Medical University after four immunizations of New Zealand white rabbits. The goat anti-rabbit horseradish peroxidase (HRP) conjugate was used as secondary antibody, and 3,3′-diaminobenzidine (DAB) was used for the final chromogenic reaction. The expression of Cry1Ab, Cry2Ab, and G10evo-epsps in ZDRF-8 was detected by Western blot analysis.
3.7. Bioassay of ZDRF Resistance to Three Species of Lepidoptera Pests
Tissue samples of various types—V5 leaves, R1 silks, and R2 kernels—were sampled from the ZDRF-8 maize and non-Bt maize Ruifeng-1 from a field trial and were taken immediately to the laboratory for bioassays against neonates larvae of ACB, CBW, and OAW. Maize tissue samples were placed in 24-well culture plates. Six neonates (0–24 h) were transferred to each well with a fine brush, and the plates were sealed with Para-film to prevent the larvae from escaping and to keep them moisturized. Each treatment was replicated five times for each maize variety, and total of 30 larvae were tested for each treatment. All plates were placed at a temperature of 28 °C and a relative humidity of 80%, with a photoperiod of 16 h/8 h (L/D). Mortality rates were calculated after 3 days. For better evaluation of the insecticidal effects, mortality rates were corrected by employing Abbott’s formula, which accounted for the natural mortality recorded in the control group [
21]. The larvae in the assays that exhibited no response even upon gentle brushing were deemed deceased.
The experiment involved a randomized complete block design for both the ZDRF-8 corn and conventional maize Ruifeng-1. After ZDRF-8 maize and non-Bt maize Ruifeng-1 were sown, each block (5 m × 6 m) was completely covered with 80-mesh gauze to isolate against interference from other lepidopteran larvae. The rows were spaced 60 cm apart, and the plants within each row were spaced 25 cm apart. Isolation belts were set up within the experimental blocks to prevent cross-contamination. Conventional cultivation methods and management practices were employed, and no insecticides were applied throughout the entire growth period. When maize plants reached the V5 stage, the second or sometimes third plants of a single spot were deselected due to multiple seeding, and 60 maize plants were retained in each block. Subsequently, 30 first-instar larvae were manually inoculated onto the whorl of each maize plant using a soft-bristled brush. After the inoculation, the leaf damage score was surveyed every two days. The leaf damage score was determined by following the standards outlined in the Ministry of Chinese Agriculture Bulletin [
22]. The tests were repeated three times.
Using the SPSS 29.0 (IBM, Armonk, NY, USA) system, a variance analysis was performed on all data. The Duncan’s Test was employed for multiple comparisons of field insect resistance data, while a one-way ANOVA was used to analyze indoor insect resistance data.
3.8. Evaluation of Glyphosate Tolerance of ZDRF
In the field trial for glyphosate tolerance, the transgenic maize ZDRF-8 and non-GMO maize Ruifeng-1 were planted using a completely randomized block design (plots of rows spaced 0.6 m apart covered an area of 24 m2) with three replicates. The recommended dose of glyphosate was 100-fold dilution of glyphosate isopropylamine salt (41%, Roundup, Monsanto, Saint Louis, MO, USA), and three concentrations (0 g a.e. ha−1, 900 g a.e. ha−1, 1800 g a.e. ha−1) were applied to maize at the 3–4 leaf stage. Herbicide resistance assessments of ZDRF-8 and Ruifeng-1 were also conducted in the laboratory, in which the 3–4 leaf stage maize was pot-planted with three replicates in a greenhouse. The herbicide resistance and weed control efficacy were evaluated 14 days after the application of glyphosate in both field and laboratory.
3.9. ELISA Detection of ZDRF
The expression levels of Cry1Ab, Cry2Ab, and G10evo-EPSPS in transgenic maize ZDRF-8 were measured using the enzyme-linked immunosorbent assay (ELISA). The kits for measuring Cry1Ab and Cry2Ab were purchased from EnviroLogix (Portland, ME, USA) (Catalog Numbers AP-003-CRBS and AP-005-CT, respectively); the ELISA kit for measuring G10evo-EPSPS was developed and prepared by Youlong Biotech (Shanghai, China) The specific methods and procedures for the measurements followed the protocols provided with the kits. The transgenic maize ZDRF-8 samples were sourced from a transgenic trial base of Zhejiang University Agricultural Experiment Station, planted in Xinfeng Village, Sian Town, Changxing County, Zhejiang Province. The trial used a randomized block design with four replicates. Test samples were collected from five different plants at the trial site, and the average and standard deviation were calculated after measurement. The collected plant tissues mainly included leaves (V3, V6, V12, VT, and R3), roots (V3, V6, V12, VT, and R3), stems (V3, V6, V12, VT, and R3), pollen (VT), styles (R1), and grains (R6). All collected samples were thoroughly ground under liquid nitrogen and stored as powdered samples at −80 °C before measurement. The protein expression levels in each plant tissue were measured based on micrograms of protein per gram of fresh weight (μg/g fw). By calculating the moisture content in each tissue, the dry weight conversion factor was obtained, and the measured exogenous protein content was converted to micrograms of protein per gram of dry weight (μg/g dw).
4. Discussions
The T0 transgenic plants generated by Agrobacterium-mediated transformation were initially screened based on three key parameters: insect resistance efficacy, expression levels of insecticidal proteins, and T-DNA insertion copy numbers. Events exhibiting both strong insect resistance and a single T-DNA insertion copy were prioritized for comprehensive characterization. This included the identification of flanking genomic sequences. Ultimately, the ZDRF-8 event was selected as the lead candidate based on two decisive factors: (1) demonstration of superior insecticidal activity compared to other events, and (2) confirmation through a genomic analysis that the T-DNA insertion did not disrupt any known or predicted gene sequences in the host genome.
Cry1Ab and Cry2Ab are widely commercialized
Bt toxins in maize for their high activity to major corn lepidopteran insect pests. However, the development of resistance in pests to Cry1Ab has significantly compromised its field effectiveness [
23]. Field-evolved resistance to Cry2Ab was also first reported in Australian
Helicoverpa punctigera populations (2002), followed by subsequent confirmation in Chinese
H. punctigera populations (2004). Practical resistance to Cry2Ab has been confirmed in Indian
Pectinophora gossypiella populations infesting
Bt cotton fields [
24]. These documented resistance cases highlight the urgent need for the deployment of pest resistance management strategies, such as toxin pyramiding.
Theoretical model studies indicate that “pyramided” plants expressing two distinct
Bt toxin genes can delay pest resistance more effectively than those employing a single toxin [
25]. There are reports indicating that plants containing two different Bt toxin genes, Cry1Ac and Cry1C (“pyramided” plants), compared with single-toxin plants used sequentially or in a mosaic planting pattern, can significantly delay the time when the diamondback moth (
Plutella xylostella) develops resistance to the pyramided plants with two genes after 24 generations of screening [
26]. This strategy also broadens the spectrum of pest insect and enhances the efficacy [
27]. Therefore, we used both Cry1Ab and Cry2Ab to develop the transgenic maize ZDRF-8.
Bacillus thuringiensis is an invertebrate pathogen that can infect a variety of insects, mainly mediated by the activity of crystal Cry toxins. These toxins are produced simultaneously during the process of spore formation. The host range of a specific Bacillus thuringiensis strain is highly specific and largely depends on the specific Cry toxins it produces [
28].
Many proteins of the Cry family have been used to cultivate transgenic insect-resistant maize. The transgenic maize events OE1 and OE3 expressing the Cry1Ah-1 protein are expressed in different tissues during the six-leaf stage, heading stage, and grain-filling stage, which can ensure that the maize is protected from corn borers throughout the entire growth cycle. Indoors, the mortality rate of corn borers that feed on the leaves of OE1 and OE3 maize reaches 100% after 3 days. In the field, they also have a strong insecticidal activity against corn borers, reaching a high resistance level [
29]. The transgenic maize events CVC-1 and CVC-2 expressing the exogenous proteins of Cry2Ab and Vip3A showed strong insecticidal toxicity against
Mythimna separata,
H. punctigera, and FAW. Six days after the occurrence of the insect infestation, the mortality rates of these insects exceeded 96%, 100%, and 100%, respectively [
30].
Numerous experiments and practices have shown that Cry proteins can effectively control pests such as Lepidoptera or Coleoptera. Transgenic Bt crops can bring benefits to the environment and the economy by reducing the environmental pollution caused by chemical pesticides and improving the quality and yield of crops. However, due to concerns such as the evolution of pest resistance and other existing issues, the cultivation of Bt crops still remains controversial [
31]. To evaluate the safety of insect-resistant transgenic crops for non-target organisms, it is not feasible to test every arthropod species in the field due to their extreme diversity [
32]. Therefore, it is necessary to select representative species for testing. In the following research, the safety assessment of the transgenic maize event ZDRF-8 for non-target organisms will continue.
The molecular characterization of ZDRF-8 demonstrated the single-copy T-DNA integration of three transgenes (
Cry1Ab,
Cry2Ab, and
G10evo-epsps) into the maize genome. A border sequence analysis revealed that the T-DNA was not inserted into a known or predicated gene, and thus, it is unlikely to exert unintended biological effects on maize [
33]. Our analysis of the stability of transgene expression and insect resistance across multiple generations suggested that the T-DNA insertion site of ZDRF-8 is favorable.
Our previous study demonstrated that transgenic maize ZDRF-8 exhibits potent insecticidal activity against four major lepidopteran corn pests: OAW, ACB, CBW, and FAW [
34]. Data from this study further showed that the strong resistance of ZDRF-8 to lepidopteran insects was stable for multiple generations, suggesting that there was no significant gene silencing of the transgenes of this event. The combination of Cry1Ab and Cry2Ab not only provided high resistance to major lepidopteran pests in corn but also effectively expanded the insecticidal spectrum to include earworm and cutworm [
30].